Abstract
House dust mite (HDM) is a major source of allergen in house dust and has been suggested to be involved in the pathogenesis of asthma. In this study, we aimed to investigate whether HDM can modulate the sensitivity of pulmonary sensory neurons, and if so, to elucidate the underlying mechanism. Fura-2 based ratiometric Ca2+ imaging was carried out to determine the effect of HDM extract on the capsaicin-evoked Ca2+ transient in mouse vagal pulmonary sensory neurons. Pretreatment with HDM (50 μg/ml, 5 min) significantly enhanced the Ca2+ transient evoked by capsaicin in these neurons isolated from wildtype mice. This potentiating effect of HDM was not antagonized by E-64, a selective cysteine protease inhibitor, but was completely prevented by AEBSF, a specific serine protease inhibitor. In addition, the potentiating effect of HDM on capsaicin-evoked Ca2+ transient was absent in the pulmonary sensory neurons isolated from protease-activated receptor-2 (PAR2) knockout mice. Further, the sensitizing effect of HDM was completely abolished by U73122, a PLC inhibitor, or chelerythrine, a PKC inhibitor. In summary, our results demonstrate that HDM, mainly through its serine protease activity, potentiates capsaicin-evoked Ca2+ transient in mouse pulmonary sensory neurons via the activation of PAR2 and PLC-PKC intracellular transduction cascade.
Keywords: House dust mite, protease-activated receptor, pulmonary chemosensitivity
Introduction
Protease activated receptor-2 (PAR2) belongs to a family of G-protein coupled receptors that are activated by specific proteases (Macfarlane et al. 2001; Ossovskaya & Bunnett, 2004). PAR2 activation occurs after proteolytic cleavage of its extracellular N-terminal domain by proteases, which reveals a tethered ligand domain that binds to and activates the cleaved receptor (Macfarlane et al. 2001; Ossovskaya & Bunnett, 2004; Sokolova & Reiser, 2007). Expression of PAR2 has been demonstrated in a variety of cells including vagal sensory nerves in the lung and airways (Howells et al. 1997; D’Andrea et al. 1998; Akers et al. 2000; Chambers et al. 2001; Reed & Kita, 2004; Gu & Lee, 2006, 2009). Compelling evidence has indicated that activation of PAR2 by endogenous or exogenous agonists contributes to the pathogenesis of airway inflammation and airway hyperresponsiveness, the hallmarks of allergic asthma (Ricciardolo et al. 2000; Chambers et al. 2001; Schmidlin et al. 2002; Barrios et al. 2003; Ebeling et al. 2005). Mast cell tryptase, trypsin, trypsin-like proteases, and coagulation factors VIIa and Xa are considered as the endogenous agonists of PAR2 (Reed & Kita, 2004; Sokolova & Reiser, 2007).
House dust mite (HDM) is a major source of allergen in house dust. HDM extracts contain many allergens of known and unknown properties which have been strongly associated with asthma, asthma severity and morbidity (Squillace et al. 1997; Langley et al. 2005; Kovac et al. 2007; Gaffin & Phipatanakul, 2009). It has been reported that some airborne allergens including HDM allergens can activate PAR2 in certain experimental conditions (Sun et al. 2001; Kauffman et al. 2006; Kato et al. 2009). In the present study, we aimed to investigate whether HDM can modulate the sensitivity of pulmonary sensory neurons, and if so, to determine a potential involvement of PAR2 activation. Since intracellular Ca2+ is an important signal transduction molecule and plays a critical role in the regulation of neuronal membrane excitability, fura-2 based ratiometric Ca2+ imaging was carried out to determine the effect of HDM extract on the capsaicin-evoked Ca2+ transient in mouse vagal pulmonary sensory neurons.
Methods
Animals
Wildtype mice (PAR2+/+; strain: C57BL/6J) and the homozygote PAR2 knockout mice (PAR2−/−; strain: B6.Cg-F2rl1tm1Mslb/J) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Experimental procedures were approved by the Mercer University and University of Kentucky Institutional Animal Care and Use Committees.
Retrograde labeling of vagal pulmonary sensory neurons
Cell bodies of vagal sensory nerves arising from lungs and airways reside in nodose and jugular ganglia. These sensory neurons were identified by retrograde labeling with a fluorescent tracer 3,3-dioctadecylindocarbocyanine (DiI); the method was modified from those previously described (Nassenstein et al. 2008; Gu & Lin, 2010). Briefly, mice were anesthetized with isoflurane inhalation (2% in O2) via a nose cone connected to a vaporizing machine. A small mid-line incision was made on the ventral neck skin to expose the trachea. DiI (0.15 mg/ml, 20 μl) was instilled into the lungs via a 30-gauge needle inserted into the lumen of the trachea; the skin incision was then closed. Animals were kept undisturbed for 7–10 days until they were euthanized for the cell culture.
Isolation and culture of nodose-jugular ganglion complex neurons
Methods were adapted from those described previously (Gu & Lin, 2010; Hu et al. 2010). Since nodose and jugular ganglia in adult mice often present as a single ganglionic structure anatomically (Carr & Undem, 2003), the nodose-jugular ganglion complex was isolated and treated as one ganglion origin in our study. In brief, mice were killed after isoflurane inhalation. The nodose-jugular complex was extracted, cut into small pieces, then placed in the combination of 0.04% type IV collagenase and 0.02% dispase II, and incubated in 5% CO2 in air at 37°C for 45 min. The ganglion suspension was centrifuged (150 g, 5 min) and supernatant aspirated. The cell pellet was then resuspended in a modified DMEM/F12 solution (DMEM/F12 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids) and gently triturated. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of 15% bovine serum albumin to separate the cells from myelin debris. The cell pellets were resuspended in the modified DMEM/F12 solution, plated onto poly-L-lysine-coated glass coverslips, and incubated at 37°C in 5% CO2 in air. Isolated neurons were used within 24 h of culture.
Ca2+ imaging recording
For all experiments, cells were washed or maintained in an extracellular solution (ECS; in mM): 5.4 KCl, 136 NaCl, 1 MgCl2, 1.8 CaCl2, 0.33 NaH2PO4, 10 glucose, 10 HEPES; pH adjusted to 7.4 with NaOH and osmolarity to 300 mOsm with sucrose. Ca2+ transients were measured in these cells with a Zeiss digital fluorescence microscope unit, equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and a digital CCD camera (Princeton Instruments, Trenton, NJ), as previously described (Gu et al. 2003; Hu et al. 2010). In brief, nodose-jugular ganglion complex neurons were incubated with 5 μM Ca2+ indicator fura-2 AM for 30 min at 37°C in tissue culture medium, then rinsed (×3) with ECS, and allowed to de-esterify for at least 30 min before use. Dual images (340- and 380-nm excitation, 510-nm emission) were collected and pseudocolor ratiometric images were monitored during the experiments by using the software Axon Imaging Workbench (Axon Instruments, Union City, CA). The imaging system was standardized with a two-point calibration using a Ca2 +-free standard (−) and a Ca2 +-saturated standard (+). Both standards contained 11 μM fura-2 [44 μl of 10 mM fura-2 penta K+ salt (Molecular Probes), 8 ml of 20 mM HEPES-Na (pH 7.4), 32 ml H2O] and were prepared as follows: (− standard) 18 ml fura-2, 1.98 ml of 10 mM EGTA-Na (pH 7.6); (+ standard) 18 ml fura-2, 1.98 ml of 10 mM CaCl2. The parameters used for the two-point calibration included the dissociation constant of fura-2 (Kd: 285), the ratio values for the (−) and (+) concentration standards (Rmin and Rmax), and the denominator wavelength intensities for the (−) and (+) concentration standards (Denmin and Denmax). The intracellular concentration of Ca2+ ([Ca2+]i; in nM) was calculated according to the following equation: [Ca2+]i = Kd (R − Rmin)/(Rmax − R) (Denmin/Denmax). Typical Rmin and Rmax values were 0.225 and 1.45, respectively.
Following the incubation period with fura-2 AM, the coverslip containing cells was mounted into a chamber placed on the stage of microscope. Each cell, viewed under light field and selected on the basis of its regular, symmetrical shape and no attachment to other cells, was closely outlined within a circular or elliptic region of interest for the measurement of [Ca2+]i. Cells were continuously perfused with the ECS at a constant flow rate of 2 ml/min; a complete change of the bath solution occurred in ~6 s. Pharmacological agents were perfused through the chamber by a gravity-fed valve-control system (VC-6 Valve Controller, Warner Instruments, Hamden, CT).
Chemicals
The standardized lyophilized extract of HDM (Dermatophagoides pteronyssinus) was obtained from Greer Laboratories (Lenoir, NC). Dispase II was purchased from Roche (Indianapolis, IN). All other chemicals were purchased from Sigma (St. Louis, MO). The stock solution of capsaicin (1 mM) was prepared in a vehicle of 1% Tween 80, 1% ethanol, and 98% ECS. The stock solution of HDM (protein: 5 mg/ml) was prepared with PBS (pH 7.4); those of E-64 (10 mM) and AEBSF (0.1 M) were in distilled H2O; and U73122 (3 mM) and chelerythrine (20 mM) in DMSO. These stock solutions were divided into small aliquots and kept at −80°C. Solutions of these chemicals at desired concentrations were prepared daily by dilution with ECS before use.
Statistical analysis
Data were analyzed with one-way repeated-measure ANOVA and Newman–Keuls post hoc analysis. A value of P < 0.05 was considered to be significant. Data are reported as means ± S.EM.
Results
Cells in our study were selected based upon two criteria: 1) Only the neurons innervating the lung structure (labeled with DiI) were selected. 2) A high concentration of KCl (60 mM, 20-s perfusion) was applied at the end of each experimental run to test for cell viability. A cell was considered capsaicin-sensitive if capsaicin (0.5 μM, 30 s) evoked a change in [Ca2+]i exceeding 20% of its maximal response to KCl. Cells that did not respond to KCl were considered non-excitable, and were not included for analysis. All appropriate control experiments were carried out, including those using the vehicles of chemical agents tested in our study.
HDM potentiated the Ca2+ transient evoked by capsaicin in pulmonary sensory neurons isolated from wildtype mice
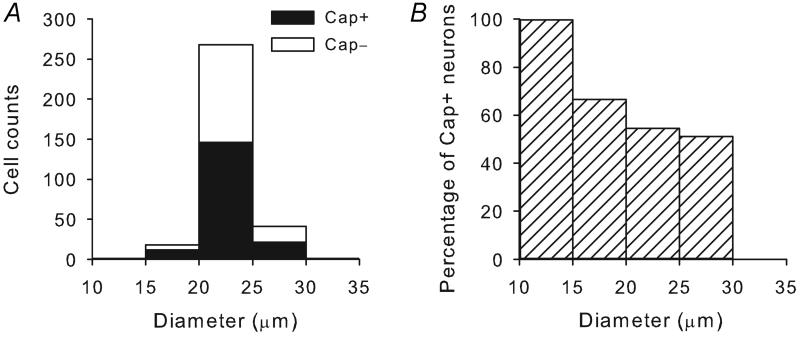
Application of capsaicin (0.5 μM, 30 s) evoked a rapid and transient increase in the [Ca2+]i in a subset (129 out of 238) of mice pulmonary sensory neurons. Figure 1 showed the size distribution of capsaicin-sensitive neurons. Similar to that found in rat pulmonary sensory neurons in our previous studies (Chung et al. 2002; Hu et al. 2010), capsaicin sensitivity was predominately found in the small- and medium-sized neurons. However, the pulmonary sensory neurons in C57BL/6J mice seemed less widely-distributed in size and exhibited a relative lower percentage of capsaicin sensitivity compared to that in Sprague-Dawley rats (Chung et al. 2002): approximately 99% and 67% of these neurons were in the diameter range of 15-30 μm in mice and rats, respectively; a total of 54.7% and 57.5% of them were capsaicin-sensitive in mice and rats, respectively.
Figure 1. Size distribution of capsaicin-sensitive pulmonary sensory neurons isolated from wildtype (C57BL/6) mice.
A, cells were grouped based upon their size (in 5-μm increments of cell diameter); filled bars represent cells that responded to capsaicin (Cap+) at the concentration of 0.5 μM, while open bars represent capsaicin-insensitive (Cap−) neurons. B, capsaicin-sensitive neurons were depicted as percentage of all pulmonary sensory neurons in each size category.
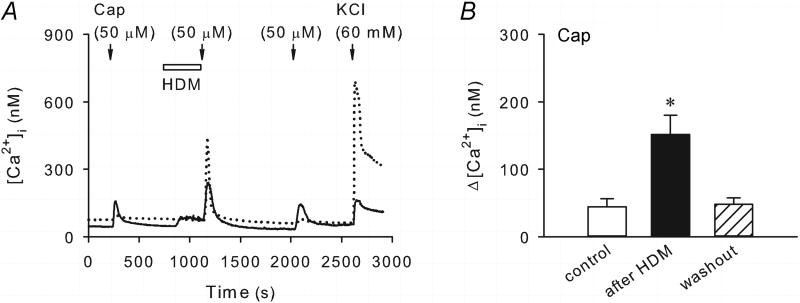
Interesting, the capsaicin-evoked Ca2+ transient was markedly potentiated after a pretreatment with HDM (50 μg/ml, 5 min) (Figure 2). The peak Ca2+ transient evoked by capsaicin was significantly increased from 44.3 ± 11.9 nM at control to 151.6 ± 28.6 nM after HDM (P < 0.05, n = 23); this enhanced capsaicin responses returned to the control level (47.9 ± 9.9 nM; P > 0.05, n = 23) after 15 min washout. Although the size distribution of the capsaicin-sensitive neurons after HDM pretreatment was not analyzed because of the small sample size, capsaicin was able to evoke significant Ca2+ transient after HDM in two neurons which did not show capsaicin sensitivity at control. In a subset of pulmonary sensory neurons tested (16/85), perfusion with HDM induced a relatively small increase in the [Ca2+]i (10.9 ± 3.6 nM; n = 16), which showed a gradual and mild desensitization but remained elevated during the 5-min of HDM incubation (e.g., Figure 2A).
Figure 2. Pretreatment with HDM potentiated the capsaicin-evoked Ca2+ transient in pulmonary sensory neurons isolated from wildtype mice.
A, Ca2+ transients evoked by capsaicin (Cap, 0.5 μM, 30 s) before, during and 15 min after the HDM treatment (50 μg/ml, 5 min) in two different neurons during the same experimental run. KCl (60 mM, 20 s) was applied at the end to test for cell viability. B, group data showing that the capsaicin-evoked Ca2+ transient was significantly enhanced after the pretreatment with HDM (P < 0.05, n = 23).
Sensitizing effect of HDM on capsaicin-evoked Ca2+ transient seems mainly dependent on its serine protease but not cysteine protease activities
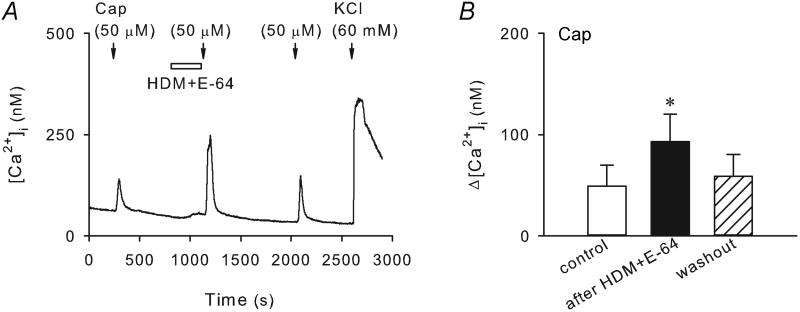
It is known that HDM has proteolytic activities including cysteine and serine protease activities (Thomas et al. 2002). This study series was carried out to determine whether blocking proteolytic activities using a cysteine or serine protease inhibitor affects the potentiation of capsaicin-evoked Ca2+ transient by HDM. As shown in the Figure 3, co-application of HDM (50 μg/ml) with E-64 (10 μM), a selective cysteine protease inhibitor, did not abolish the potentiating effect of HDM on the capsaicin (0.5 μM, 30 s) -evoked Ca2+ transient. The peak Ca2+ transient evoked by capsaicin was increased from 49.3 ± 20.4 nM at control to 92.9 ± 26.9 nM after the pretreatment with HDM and E-64 (P < 0.05, n = 17). In addition, the small gradual increase in [Ca2+]i during HDM perfusion in a subset of pulmonary sensory neurons was not abolished either in the presence of E-64 (e.g., Figure 3A).
Figure 3. Pretreatment with E-64 did not abolish the sensitizing effect of HDM on capsaicin-evoked Ca2+ transient in wildtype pulmonary sensory neurons.
A, Ca2+ transients evoked by capsaicin (0.5 μM, 30 s) before, during and 15 min after 5-min treatment of HDM (50 μg/ml) and E-64 (10 μM), a selective cysteine protease inhibitor. B, group data showing that the capsaicin-evoked Ca2+ transient was significantly enhanced by HDM in the presence of E-64 (P < 0.05, n = 17).
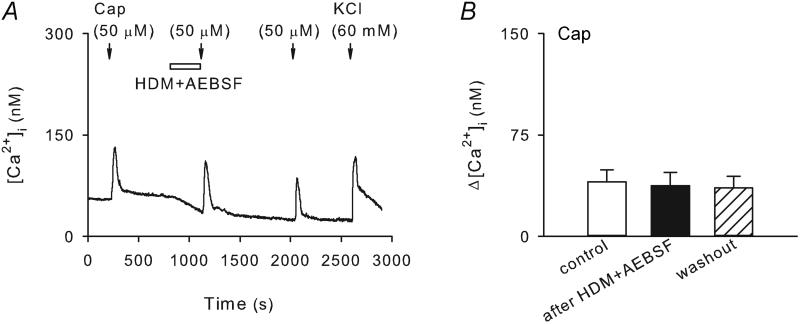
On the other hand, co-incubation with AEBSF (37.5 μM, 5 min), a specific serine protease inhibitor, completely prevented the sensitizing effect of HDM (50 μg/ml, 5 min) (Figure 4). The Ca2+ transients evoked by capsaicin (0.5 μM, 30 s) were 40.2 ± 9.1 nM and 37.4 ± 9.8 nM before and after the pretreatment with HDM + AEBSF (P < 0.05, n = 19), respectively. Interestingly, AEBSF induced a reduction of baseline level of [Ca2+]i and eliminated the elevation of [Ca2+]i evoked by HDM (e.g., Fig. 4A). The effect of AEBSF was concentration dependent; of the three concentrations we tested (25 μM, 37.5 μM and 50 μM), 37.5 μM AEBSF was able to produce a sufficient blockade of the sensitizing effect of HDM, whereas not reducing the baseline [Ca2+]i in a more dramatic manner as the higher concentration (50 μM) of AEBSF did (not shown).
Figure 4. The sensitizing effect of HDM was completely prevented by the pretreatment with AEBSF in wildtype pulmonary sensory neurons.
A, Ca2+ transients evoked by capsaicin (0.5 μM, 30 s) before, during and 15 min after 5-min treatment of HDM (50 μg/ml) and AEBSF (37.5 μM), a specific serine protease inhibitor. B, group data showing that the capsaicin-evoked Ca2+ transient was not significantly affected by HDM in the presence of AEBSF (P > 0.05, n = 19).
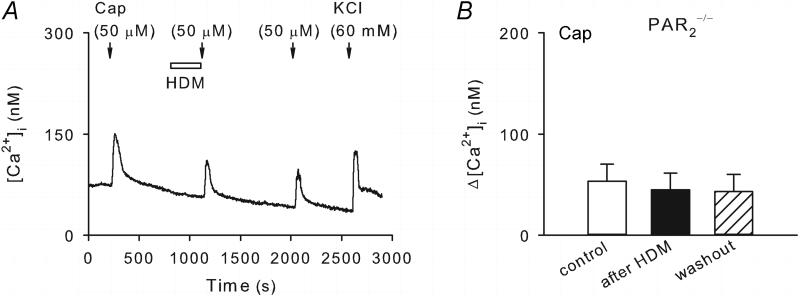
Genetic deletion of PAR2 abolished the potentiating effect of HDM on capsaicin-evoked Ca2+ transient
Ca2+ imaging was performed in pulmonary sensory neurons isolated from PAR2−/− mice in this study series. The Ca2+ transient evoked by capsaicin (0.5 μM, 30 s) in these neurons was comparable to that in neurons from wildtype mice. However, as shown in Figure 5, the potentiation of capsaicin-evoked Ca2+ transient by HDM (50 μg/ml, 5 min) was absent in these PAR2-deficient neurons. The peak Ca2+ transients evoked by capsaicin were 53.4 ± 16.6 nM and 44.8 ± 16.7 nM before and after HDM pretreatment (P > 0.05, n = 16), respectively.
Figure 5. The potentiating effect of HDM on capsaicin-evoked Ca2+ transient was absent in pulmonary sensory neurons isolated from PAR2−/− mice.
A, Ca2+ transients evoked by capsaicin (0.5 μM, 30 s) before, during and 15 min after 5-min treatment of HDM (50 μg/ml). B, group data showing that the HDM pretreatment did not significantly affect the capsaicin-evoked Ca2+ transient in these PAR2-deficient neurons (P > 0.05, n = 16).
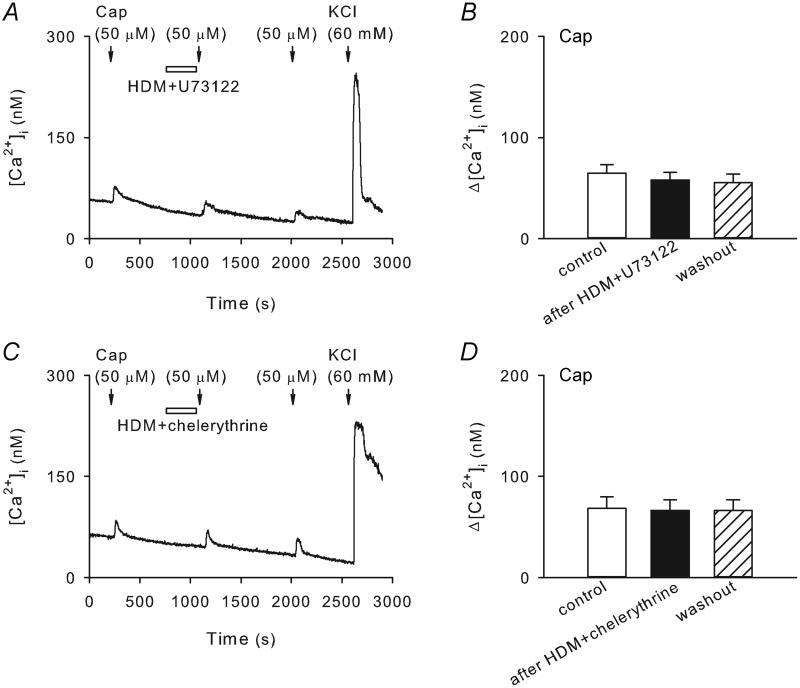
Potentiating effect of HDM on capsaicin-evoked Ca2+ transient is PLC and PKC dependent
The PLC-PKC intracellular transduction cascade has been known to account for many of the PAR2 activation-induced cellular processes (Macfarlane et al. 2001; Ossovskaya & Bunnett, 2004). Here we aimed to determine whether this transduction pathway is also responsible for the potentiating effect of HDM observed in our present study. Recordings were carried out in pulmonary sensory neurons isolated from wildtype mice. As shown in Figure 6, pretreatment with U73122 (1 μM, 5 min), a PLC inhibitor, or chelerythrine (10 μM, 5 min), a PKC inhibitor, completely abolished the potentiating effect of HDM on capsaicin (0.5 μM, 30 s) responses in these neurons. The Ca2+ transients evoked by capsaicin were 64.8 ± 8.2 nM and 58.1 ± 7.3 nM before and after pretreatments with HDM + U73122, respectively (P > 0.05, n = 19; Figure 6A and B); these values were 68.3 ± 11.7 nM and 66.3 ± 10.4 nM before and after pretreatment with HDM + chelerythrine, respectively (P > 0.05, n = 15; Figure 6C and D).
Figure 6. Pretreatment with U73122, a PLC inhibitor, or chelerythrine, a PKC inhibitor, abolished the potentiating effect of HDM on capsaicin-evoked Ca2+ transient in wildtype pulmonary sensory neurons.
A and C, Ca2+ transients evoked by capsaicin (0.5 μM, 30 s) before, during and 15 min after 5-min treatments of HDM (50 μg/ml) + U73122 (1 μM) and HDM (50 μg/ml) + chelerythrine (10 μM), respectively. B and D, group data showing that the effect of HDM was completely prevented by U73122 (P > 0.05, n = 19) and chelerythrine (P > 0.05, n = 15), respectively.
Discussion
It has been well recognized that exposure to HDM allergens is strongly associated with the development of allergic asthma (Grunstein et al. 2005; De Alba et al. 2010). More than 20 groups of HDM proteins have been identified as allergens (Thomas et al. 2002). Of all these HDM allergens, at least four groups are proteases: group 3, 6 and 9 allergens (e.g., Der p 3, p 6, and p 9) belong to serine protease family whereas group 1 allergens (e.g., Der p 1) seem mainly display papain-like cysteine protease activities (Thomas et al. 2002; Kato et al. 2009). The effect of HDM allergens has been studied extensively in airway epithelial cells. Sun and coworkers (2001) demonstrated that Der p 3 and Der p 9 induce the mobilization of Ca2+ via the activation of PAR2 and the release of proinflammatory cytokines GM-CSF and eotaxin, in a human airway epithelial cell line A549. Der p 1 has been reported to induce epithelial cell desquamation, release of cytokines and facilitate transport of allergens across cultured epithelial cell layers (King et al. 1998; Wan et al. 2000). A recent study showed that HDM decreases epithelial resistance and promotes junction disassembly through the PAR2 and epidermal growth factor receptor signaling (Heijink et al. 2010). In the present study, our results showed that acute pretreatment with HDM significantly potentiated the capsaicin-evoked Ca2+ transient in mouse vagal pulmonary sensory neurons. The effect was completely prevented by AEBSF but not E-64, indicating the serine protease but not cysteine protease activities of HDM are mainly responsible for its sensitizing effect observed in our study. In addition, our data showed that the effect of HDM was abolished by the genetic deletion of PAR2, indicating that the sensitizing effect of HDM was mediated through the activation of PAR2.
In respiratory system, PAR2 is distributed in various cells in the lung and airways including epithelial cells, airway smooth muscles, endothelial cells, neurons, and fibroblasts, as well as inflammatory cells such as mast cells, neutrophils, and macrophages (Howells et al. 1997; D’Andrea et al. 1998; Akers et al. 2000; Chambers et al. 2001; Reed & Kita, 2004; Gu & Lee, 2006, 2009). Although PAR2 may also be anti-inflammatory under certain conditions (De Campo & Henry, 2005), the proinflammatory role of PAR2 has been consistently observed in the airways of mouse, guinea pig and human (Chambers et al. 2001; Ebeling et al. 2005; Sokolova & Reiser, 2007), and confirmed by studies with PAR2-deficient mice (Su et al. 2005). Capsaicin-evoked Ca2+ transient is known to be mediated through the activation of TRPV1, a polymodal transducer responsible for detecting chemical, thermal and mechanical nociceptive stimuli. Immunohistochemical evidence shows that TRPV1 channels are often co-expressed with certain neuropeptides, such as tachykinins and CGRP, in the same axon (Watanabe et al. 2006). These TRPV1-expressing C-fiber sensory nerves innervate the entire respiratory tract, from upper airways (nose, larynx, trachea) to lung parenchyma (alveolar wall) (Coleridge & Coleridge, 1994). They are found in the mucosa as well as in the deeper regions of the airway structure (e.g., near smooth muscles). Activation of these C-fiber afferents is known to evoke dyspneic sensation, airway irritation and cough, and to elicit reflex responses such as pulmonary chemoreflexes, bronchoconstriction and hypersecretion of mucus, which are mediated through the central nervous system and cholinergic pathway. In addition, tachykinins and CGRP released from these nerve endings upon activation can produce additional local effects such as airway constriction, protein extravasation, mucosal edema and inflammatory cell chemotaxis (Coleridge & Coleridge, 1994; Lee & Pisarri, 2001; Fisher, 2009). Therefore, it is believed that these C-fiber sensory nerves play an important part in the manifestation of various symptoms of airway hypersensitivity in patients with airway inflammatory diseases (Lee & Pisarri, 2001; Carr & Undem, 2003; Geppetti et al. 2006; Lee & Gu, 2009). Unfortunately, nerve endings of C-fibers are diffuse structures in the lungs and airways, it is not yet possible to record from them directly. A variety of experimental approaches have been developed to study the excitability of these afferents. As it is well established that ion channels involved in the regulation of neuronal excitability are present not only at their peripheral terminals but also in their cell bodies, characterization of afferent nerves has been studied extensively in isolated sensory neurons (Carr & Ellis, 2002). This approach has proven invaluable especially in some species such as mice with which even the in-vivo extracellular recording of impulses from vagal axons becomes a challenge technically because of the small animal size. In the present study, our data analysis was performed selectively in the DiI labeled capsaicin-sensitive neurons, the latter presumably give rise to pulmonary C-fiber afferents as proposed in our recent studies (Gu & Lee, 2010). Our data showed some differences in the size distribution and capsaicin sensitivity of pulmonary sensory neurons in mice as they were compared to those in rats (e.g., Figure 1). It is not yet clear whether the disparity is due to the species difference, the cell culture protocol, or both. In addition, bronchopulmonary C-fibers, but not rapidly adapting (RAR) and slowly adapting (SAR) stretch receptors, display rather selective capsaicin sensitivity in rats (Ho et al. 2001). Whether stretch receptors in mouse airway/lung have afferent properties similar to the RAR and SAR described in other species is not known. Furthermore, whether they are all myelinated and uniformly capsaicin-insensitive also remains to be determined. Nonetheless, results from our study suggest that the sensitizing effect of HDM on pulmonary sensory neurons may lead to the hypersensitivity of these neurons and therefore contribute, at least in part, to the pathogenesis of allergic asthma.
Our study showed that the potentiation of capsaicin responses induced by HDM pretreatment was completely abolished by pretreatment with U73122 or chelerythrine. These results demonstrated that the sensitizing effect of HDM is mediated through the PLC-PKC intracellular transduction cascade. This finding is in consistency with the signaling mechanisms of PAR2 in a number of cell systems. PAR2 has been reported to be coupled to Gq/11, resulting in activation of PLC and generation of 1,4,5-inositol trisphosphate and diacylglycerol, which is expected to mobilize intracellular Ca2+ and activate PKC (Macfarlane et al. 2001; Ossovskaya & Bunnett, 2004). By using a combination of confocal microscopy, subcellular fractionation and Western blotting, Amadesi et al. (2006) demonstrated that, in both dorsal root ganglion neurons and HEK 293 cells, activation of PAR2 promoted the translocation of PKCε from the cytosol to plasma membrane. Indeed, activation of PKC with phorbol 12-myristate 13-acetate has been demonstrated to mimic the effect of PAR2 agonist and potentiate the capsaicin-evoked Ca2+ transient in rat dorsal root ganglion neurons (Amadesi et al. 2004). PAR2 activation has been shown to exaggerate the TRPV1-dependent tussive response in guinea pigs (Gatti et al. 2006). Recent studies from our laboratory have demonstrated that activation of PAR2 by its specific activator PAR2-activating peptide significantly potentiates capsaicin-evoked whole-cell and single-channel TRPV1 activities in isolated rat vagal pulmonary sensory neurons (Gu & Lee, 2006, 2009). All the above mentioned PAR2 effects have been shown to be mediated through the intracellular PLC-PKC transduction pathway. Whether HDM can also sensitize the responses of pulmonary sensory neurons to certain endogenous substances participating in an allergic or asthmatic reaction (e.g., histamine) through the activation of this intracellular transduction cascade remains to be determined.
In addition to its sensitizing effect on capsaicin-evoked Ca2+ transient, HDM also showed a stimulatory effect in a subset of mouse vagal pulmonary sensory neurons. Interestingly, our recent studies have shown that PAR2-activating peptide also displayed both stimulatory and sensitizing effects in isolated rat pulmonary sensory neurons (Gu & Lee, 2006). At the comparable concentration used in our present study, HDM and some of its allergens have been reported to directly activate airway epithelial cells and cause the release of proinflammatory cytokines (King et al. 1998; Heijink et al. 2010). HDM has also been shown to induce the intracellular mobilization of Ca2+ in keratinocytes and upregulate the expression of proinflammatory cytokines IL-8 and GM-CSF in both protein and mRNA levels (Kato et al. 2009). In the present study, our results showed that a small but sustained increase in [Ca2+]i during HDM incubation was effectively abolished by AEBSF but not E-64, indicating this stimulatory effect of HDM seems also predominately mediated through its serine protease activities.
It should be noticed that PAR2 is widely expressed in a variety of cell types, and activation of this receptor is associated with, at least in epithelial cells as we described earlier, the release of many proinflammatory mediators including prostanoids such as prostaglandin E2, and cytokines such as IL-6 and IL-8 (Sun et al. 2001; Asokananthan et al. 2002). These mediators are known to have potential regulatory effects on the sensitivity of TRPV1 (Gu et al. 2003; Schaible et al. 2010). Therefore, even in the cultured neuron preparation, we cannot completely dismiss the possibility that HDM may interact with satellite glial or other non-neuronal cells present in the culture, and trigger the release of chemical mediators that can sensitize these neurons.
In summary, our study demonstrated that HDM pretreatment significantly enhanced the capsaicin-evoked Ca2+ transient in mouse vagal pulmonary sensory neurons. This sensitizing effect of HDM appears predominately resulted from its serine protease but not cysteine protease activities. In addition, our data showed that the effect of HDM was mediated through the activation of PAR2 and the subsequent intracellular PLC-PKC transduction cascade. Further studies are required to investigate the importance of chronic exposure to HDM allergens on the hypersensitivity and plasticity of bronchopulmonary sensory nerves.
Acknowledgements
We thank Marcus J. Geer and Carolyn A. Gilbert for the technical assistance. This study was partially supported by AHA SDG-0835320N, NIH AI076714 and HL058686.
References
- Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, McAnulty RJ. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278:L193–201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol. 2003;66:519–525. doi: 10.1016/s0006-2952(03)00292-2. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Ellis JL. The study of airway primary afferent neuron excitability. Curr Opin Pharmacol. 2002;2:216–219. doi: 10.1016/s1471-4892(02)00147-9. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- Chambers LS, Black JL, Poronnik P, Johnson PR. Functional effects of protease-activated receptor-2 stimulation on human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1369–1378. doi: 10.1152/ajplung.2001.281.6.L1369. [DOI] [PubMed] [Google Scholar]
- Chung E, Gu Q, Kwong K, Arden WA, Lee LY. Comparison of capsaicin-evoked calcium transients between rat nodose and jugular ganglion neurons. Auton Neurosci. 2002;97:83–88. doi: 10.1016/s1566-0702(02)00045-0. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- D’Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, Knowles RG, Belvisi MG, Birrell MA. House dust mite induces direct airway inflammation in vivo: implications for future disease therapy? Eur Respir J. 2010;35:1377–1387. doi: 10.1183/09031936.00022908. [DOI] [PubMed] [Google Scholar]
- De Campo BA, Henry PJ. Stimulation of protease-activated receptor-2 inhibits airway eosinophilia, hyperresponsiveness and bronchoconstriction in a murine model of allergic inflammation. Br J Pharmacol. 2005;144:1100–1108. doi: 10.1038/sj.bjp.0706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Fisher JT. The TRPV1 ion channel: implications for respiratory sensation and dyspnea. Respir Physiol Neurobiol. 2009;67:45–52. doi: 10.1016/j.resp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9:128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol. 2006;101:506–511. doi: 10.1152/japplphysiol.01558.2005. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- Grunstein MM, Veler H, Shan X, Larson J, Grunstein JS, Chuang S. Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J Allergy Clin Immunol. 2005;116:94–101. doi: 10.1016/j.jaci.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- Gu Q, Lee LY. Hypersensitivity of pulmonary chemosensitive neurons induced by activation of protease-activated receptor-2 in rats. J Physiol. 2006;574:867–876. doi: 10.1113/jphysiol.2006.110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Lee LY. Effect of PAR2 activation on single TRPV1 channel activities in rat vagal pulmonary sensory neurons. Exp Physiol. 2009;94:928–936. doi: 10.1113/expphysiol.2009.047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Lee LY. Regulation of acid signaling in rat pulmonary sensory neurons by protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2010;298:L454–461. doi: 10.1152/ajplung.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Lin RL. Heavy metals zinc, cadmium and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol. 2010;108:891–897. doi: 10.1152/japplphysiol.01371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijink IH, van Oosterhout A, Kapus A. Epidermal growth factor receptor signaling contributes to house dust mite-induced epithelial barrier dysfunction. Eur Respir J. 2010;36:1016–1026. doi: 10.1183/09031936.00125809. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Howells GL, Macey MG, Chinni C, Hou L, Fox MT, Harriott P, Stone SR. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- Hu YM, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor alpha in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2010;299:L483–492. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. 2009;64:1366–1374. doi: 10.1111/j.1398-9995.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:645–651. [PubMed] [Google Scholar]
- Kovac K, Dodig S, Tjesic-Drinkovic D, Raos M. Correlation between asthma severity and serum IgE in asthmatic children sensitized to Dermatophagoides pteronyssinus. Arch Med Res. 2007;38:99–105. doi: 10.1016/j.arcmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Langley SJ, Goldthorpe S, Craven M, Woodcock A, Custovic A. Relationship between exposure to domestic allergens and bronchial hyperresponsiveness in non-sensitised, atopic asthmatic subjects. Thorax. 2005;60:17–21. doi: 10.1136/thx.2004.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol. 2009;9:243–249. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, Creminon C, Bertrand C, Bunnett NW, Fabbri LM, Salvadori S, Geppetti P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- Schaible HG, von Banchet GS, Boettger MK, Bräuer R, Gajda M, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Reiser G. A novel therapeutic target in various lung diseases: airway proteases and protease-activated receptors. Pharmacol Ther. 2007;115:70–83. doi: 10.1016/j.pharmthera.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Squillace SP, Sporik RB, Rakes G, Couture N, Lawrence A, Merriam S, Zhang J, Platts-Mills AE. Sensitization to dust mites as a dominant risk factor for asthma among adolescents living in central Virginia. Multiple regression analysis of a populationbased study. Am J Respir Crit Care Med. 1997;156:1760–1764. doi: 10.1164/ajrccm.156.6.9704026. [DOI] [PubMed] [Google Scholar]
- Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen der p 1. Clin Exp Allergy. 2000;30:685–698. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience. 2006;141:1533–1543. doi: 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]