Abstract
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterized by lymphocytic infiltration, destruction of lacrimal and salivary glands and the presence of serum autoantibodies. Most women that suffer from SS are post-menopausal however, not all post-menopausal women develop SS, suggesting that other factors, in addition to the decrease in ovarian hormones, are necessary for the development of SS. The purposes of this study were to investigate a) the time course of lymphocytic infiltration and apoptosis in the lacrimal gland after ovariectomy, b) if a predisposed genetic background for SS aggravates the effects of decreasing levels of sex hormones in the lacrimal glands and c) if physiological doses of estrogen or androgen prevent the effects observed after ovariectomy. Six weeks old mice that are genetically predisposed to SS (NOD.B10.H2b) and control (C57BL/10) mice were either sham-operated, ovariectomized (OVX), OVX + 17β estradiol (E2) or OVX + Dihydrotestosterone (DHT). Lacrimal glands were collected at 3, 7, 21 or 30 days after surgery and processed for immunohistochemistry to measure CD4+ , CD8+ T cells, B220+ B cells, nuclear DNA degradation and cleaved caspase-3 activity. Quantification of the staining was done by light microscopy and Image Pro Plus software. The results of our study show that lymphocytic infiltration preceded lacrimal gland apoptosis after ovariectomy. Moreover, removal of ovarian sex hormones accelerated these effects in the genetically predisposed animal and these effects were more severe and persistent compared to control animals. In addition, sex hormone replacement at physiological levels prevented these symptoms. The mechanisms by which decreased levels of sex hormones caused lymphocytic infiltration and apoptosis and the interaction of lack of sex hormones with the genetic elements remain to be elucidated.
Keywords: lymphocytic infiltration, apoptosis, caspase 3, lacrimal gland, sjögren's syndrome, sex hormone, androgen, estrogen
1. Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease that targets primarily lacrimal and salivary glands resulting in dry eye and dry mouth disease. One of the main hallmarks of SS is mononuclear cell infiltration of the lacrimal and salivary glands. The lymphocytic infiltrates consist of CD4+ T cells, CD8+ T cells, B220+ B cells and macrophages (Nguyen et al., 2007; Nguyen et al., 2009). Although the majority of the infiltrate seems to be composed of T cells (Mitsias et al., 2002), and the increase in CD4+ T lymphocyte population has been recognized as an integral part of the pathogenesis of SS, new evidence suggests that B cell hyperactivity also plays an important role in both the pre-clinical and clinical phases of the disease (Nguyen et al., 2007; Tobon et al., 2010).
Another major characteristic of SS is glandular epithelial cell death. Acinar and ductal epithelial cells in the lacrimal and salivary glands of SS patients have been shown to undergo apoptosis or programmed cell death (PCD). Caspase-3, a prominent apoptosis-associated molecule, plays a major role by cleaving proteins that are essential for cell survival (Masago et al., 2001; Elmore, 2007).
SS primarily affects postmenopausal women, suggesting a role for sex hormones in the prevention of the pathogenesis of this disease. However, the fact that not all post-menopausal women develop SS, suggests that other elements, such as a genetic component are also necessary for the development of this disease.
In the present studies we used genetically predisposed NOD.B10.H2b and control C57BL/10 mice to investigate the time course of lymphocytic infiltration and apoptosis that occur in the lacrimal gland after ovariectomy, and to determine whether decreased levels of ovarian sex hormones can accelerate and aggravate the characteristic symptoms of this disease in the genetically predisposed mice. In addition, we treated these mice with physiological doses of E2 or DHT to investigate if hormone replacement can prevent the symptoms observed after ovariectomy.
2. Materials and Methods
2.1. Animals and Treatments
NOD.B10.H2b breeders were obtained from the animal facilities at the University of Florida, Gainesville, FL., and were bred and maintained under veterinary services at Florida Atlantic University, Boca Raton, FL. Sexually mature female C57BL/10 mice, aged 5 weeks, were purchased from Jackson Laboratory (Bar Harbor, Maine, USA), and housed for one week prior to surgery. All mice were housed in constant temperature rooms with fixed light/dark intervals of 12 hr length. All animal experiments were approved by the Florida Atlantic University Animal Care Committee and were in accord with the NIH Guiding Principles for the Care and Use of Animals and the ARVO Resolution on the Use of Animals in Ophthalmic and Vision Research.
Six weeks old female mice from each strain were anesthetized with a mixture of xylazine (0.011 mg/g) and ketamine (1.0 mg/g) i.p. and either sham operated, OVX, OVX and treated with E2 or DHT. A small incision of the skin in the back of the neck was made and a pellet containing sex hormones or a matching placebo (Innovative Research of America, Sarasota, FL) was placed under the skin. The OVX mice treated with E2 or DHT received a pellet that provided a physiological concentration of the desired hormone previously determined for these mice. Mice treated with E2 received a 0.005 mg pellet (0.16 μg/day) and mice treated with DHT received a 0.10 mg pellet (3.3 μg/day).
Each animal received 0.05 mg/kg of the analgesic buprenorphine intramuscularly 12 and 24 hr after surgery. Animals were monitored daily for eating, drinking, wound healing and incision status. At each experimental time point, mice were anesthetized as described above, lacrimal glands were removed, placed in OCT, rapidly frozen in liquid nitrogen and processed for immunohistochemistry analyses as described below. Lacrimal glands were collected from the sham group during the diestrus phase when the estrogen levels in serum are the lowest (Nelson et al., 1982). The diestrus phase was chosen so that the least amount of estrogen difference would be compared between sham and OVX groups. Blood was obtained by cardiac puncture which resulted in the death of the animal.
2.2. Vaginal Smears
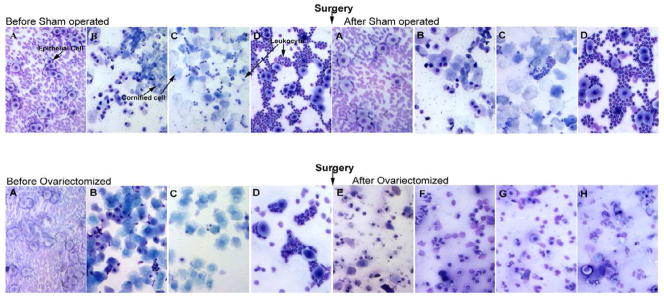
Vaginal smears were examined daily before and after surgery to classify the phases of the estrous cycle and to determine whether ovariectomy was successful. The sexual cycle of female rodents is a well-defined period characterized by distinct phases of hormonal fluctuation and corresponding changes in the vaginal lining: (A) Proestrus stage – mostly nucleated epithelial cells; (B) Estrus stage – significant increase in estradiol, cornified cells are predominant, (C) Metestrus stage – progesterone level rises, many cornified cells which are larger and more clumped than estrus, (D) Diestrus stage – decrease in estradiol, leukocytes are predominant with some epithelial cells. The estrous cycle lasts around 4 to 5 days, and the stages are not of equal duration. Thus, in a cycle of 4 days, the combined proestrous-estrus stage last only about 24 hrs (Champlin et al., 1973, DeLeon et al., 1990).
To obtain a vaginal smear, one drop of sterile water was gently expelled into the vagina using a sterile tip, aspirated back into the tip twice, and then transferred to a microscope slide. Dry smears were stained with the Jorvet Dip Quick Stain (Jorgensen Lab. Inc, Loveland, CO) and examined by light microscopy with an Olympus Provis AX70 microscope (Olympus America Inc., Melville, NY) equipped with a digital camera.
2.3. Ovarian Hormone Levels
Serum levels of androstenedione, testosterone (T), DHT and E2 were measured in the C57BL/10 and NOD.B10.H2b mice using an immunoassay test kit (Diagnostic Automation Inc. Calabasas, CA), following the manufacturer’s instructions. The lowest levels of detectable hormone using this assay are: E2 1 pg/ml; T 0.05 ng/ml, DHT 6 pg/ml and androstenedione 5 pg/ml.
2.4. Detection of CD4+T, CD8+T and B220+B lymphocytes by immunohistochemistry
To detect CD4+T, CD8+T and B220+B cells, frozen lacrimal glands were serially sectioned at 5 μm on a Cryostat (Leica Microsystem, Inc., IL, USA) and placed on Superfrost-Plus (Fischer Scientific, Pittsburgh, PA, USA) glass slides. Sections were fixed in cold acetone for 10 minutes, blocked with Background Terminator (Bio Care Medical, CA, USA) for 20 minutes at room temperature and incubated overnight at 4ºC with primary antibody, either purified monoclonal rat anti-mouse CD4, -CD8 or -B220 (BD Pharmigen, Minneapolis, MA) in phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Sections were then quenched in 0.3% H2O2, 40% methanol in PBS for 30 minutes at room temperature. Sections were incubated for 30 minutes at room temperature with goat anti-rat-biotin secondary antibody (Millipore, MA, USA), followed by incubation with Vectastain ABC reagent (Vector Laboratories, Inc., Burlingame, CA) for 30 minutes at room temperature and then with Sigma Fast 3,3:-Diaminobenzidine (DAB Peroxidase Substrate) (Sigma St. Louis, MO). The reaction was stopped by rinsing the sections with PBS followed by distilled water and then counter-stained with methyl green. The sections were then rinsed in distilled water, dehydrated with 95% and 100% alcohol, cleared in xylene and mounted in Permount (Fisher Scientific, Pittsburg, PA, USA). Immunohistochemistry negative controls were obtained by omitting the primary antibody.
2.5. Detection of nuclei DNA degradation by the TUNEL assay
Lacrimal gland sections obtained as described under section 2.4 were stained using the in situ apoptosis detection kit ApopTag® (Millipore, MA, USA) as recommended by the manufacturer. For negative controls the TdT enzyme was substituted with distilled water.
2.6. Detection of cleaved caspase-3 by immunohistochemistry
Lacrimal gland sections obtained as described under Section 2.4 were fixed in 1% paraformaldehyde for 10 minutes at room temperature, followed by post-fixation in precooled ethanol: acetic acid (2:1) at −20ºC for 5 minutes. Sections were then quenched with 3% H2O2 for 5 minutes at room temperature and blocked for 20 minutes with Background Terminator (BioCare Medical, CA, USA) at room temperature. After incubation overnight at 4ºC with rabbit anti-cleaved caspase-3 primary antibody (BioCare Medical, CA, USA), sections were incubated for 30 minutes with MACH-2 goat anti-rabbit HRP polymer secondary antibody (BioCare Medical, CA, USA) at room temperature. The sections were then stained with Cardassian diaminobenzidine chromagen (CDC) (BioCare Medical, CA, USA), and counterstained with methyl green (Sigma-Aldrich Corporation, St. Louis, MI). Sections were rinsed in distilled water, dehydrated with 95% and 100% alcohol, cleared in xylene and mounted in Permount (Fisher Scientific, Pittsburg, PA, USA). Immunohistochemistry negative controls were obtained by omitting the primary antibody.
2.7. Quantitation of Staining
All sections were visualized by light microscopy using an Olympus Provis AX70 microscope (Olympus America INC., Melville, NY) equipped with a digital camera. Quantification of the staining was done by using an Image Pro Plus analysis system (Media Cybernetics, Inc. Bethesda, MD). For each immunohistochemistry experiment, lacrimal glands from six animals per experimental group and time and three sections per gland were used. Staining in each section was measured in at least 6 different fields. The relative areas of comparable sections from control and experimental groups were measured. The areas analyzed for each parameter from the different experimental groups and times were similar in both strains. Therefore, for each strain and parameter measured, the total areas from all of the experimental groups and times were averaged (see below).
2.7.1. CD4+, CD8+ and B220+ cells
The numbers of CD4+ and CD8+ cells (used as a marker of T cells) and B220+ cells (used as a marker of B cells) were counted. The average of the total areas of lacrimal gland analyzed for CD4+, CD8+ and B220+ cells for the 4 experimental groups were 7.0 ± 0.6 × 107 μm2, 6.5 ± 0.05 × 107 μm2 and 6.7 ± 1.2 × 107 μm2 respectively for the NOD.B10.H2b mice, and 6.5 ± 0.3 × 107 μm2, 6.1 ± 0.5 × 107 μm2 and 6.3 ± 0.7 × 107 μm2 respectively for the C57BL/10 mice.
2.7.2. Nuclear DNA degradation
Areas of brown staining (apoptotic nuclei) and green staining (non-apoptotic nuclei) in the lacrimal glands were measured. The area of apoptotic nuclei was expressed as a percentage of a total nuclear area. The average of the total nuclear area analyzed for the 4 experimental groups was 6.1 ± 0.6 × 107 μm2 for the NOD.B10.H2b mice and 6.0 ± 0.2 × 107μm2 for the C57BL/10 mice.
2.7.3. Cleaved caspase-3 positive activity
The numbers of cells positive for cleaved caspase-3 were counted. The average of the total area analyzed for the 4 experimental groups was 6.8 ± 0.6 × 107μm2 for the NOD.B10.H2b mice and 6.2 ± 0.2 × 107μm2 for the C57BL/10 mice.
2.8. Statistical Analysis
A Student’s t-test for unpaired values was used to analyze statistical significance between two groups. Significance among all groups was determined by ANOVA followed by Duncan’s new multiple range test. Values of p < 0.05 were considered to be statistically significant.
3. Results
3.1 Vaginal smears
A successful ovariectomy was confirmed by the results of vaginal smears and the measurement of ovarian hormone levels (see below). Animals which were ovariectomized showed no cycling after the removal of ovaries, while sham operated animals continued to show normal cycling after surgery (Fig.1).
Fig. 1.
Representative vaginal smears taken from sham operated and OVX NOD.B10.H2b mice showing the Estrous cycle before and after surgery. After OVX, mice show no signs of Estrous cycle, as cell type does not change. (A) Proestrus stage- mostly nucleated epithelial cells, (B) Estrus-cornified cells are predominant, (C) Metestrus-many cornified cells which are larger and more clumped than in estrus, some leukocytes are also present, (D) Diestrus-predominantly leukocytes with some epithelial cells. (E) – (H) all smears show cornified, nucleated epithelial and leukocytes with no changes of Estrus cycle. Similar findings were observed with the C57BL/10 mice.
3.2 Ovarian Hormone Levels
The serum levels of androstenedione in both strains after ovariectomy were undetectable. The serum levels of DHT, E2 and T were also significantly decreased in both strains after ovariectomy compared to sham operated group. Hormonal replacement with E2 or DHT at the time of ovariectomy brought the serum levels of these hormones back to the physiological levels (Table 1A & B).
Table 1.
Serum levels of Androstenedione, DHT, T and E2 in C57BL/10 (A) and NOD.B10.H2b (B) mice after sham operation, OVX, OVX plus E2 or OVX plus DHT. N=10. (NA=Non applicable).
| A | ||||
|---|---|---|---|---|
| C57BL/10 | ||||
| Androstenedione | DHT | T | E2 | |
| Pg/ml | Pg/ml | Pg/ml | Pg/ml | |
| Sham operated | 43±9 | 57±3.5 | 49±3 | 13±0.7 |
| OVX | 0 | 36.5±0.5 | 28±6 | 9.14±0.4 |
| OVX+ E2 | 0 | NA | NA | 12±1.2 |
| OVX+DHT | 0 | 51.8±9.6 | NA | NA |
| B | ||||
|---|---|---|---|---|
| NOD.B10.H2b | ||||
| Androstenedione | DHT | T | E2 | |
| Pg/ml | Pg/ml | Pg/ml | Pg/ml | |
| Sham operated | 47±12 | 46.7±5.8 | 44±6 | 14.7±1.3 |
| OVX | 0 | 29.7±1.2 | 10±4 | 8.7±0.9 |
| OVX+ E2 | 0 | NA | NA | 15.9±2.9 |
| OVX+ DHT | 0 | 41.3±4.6 | NA | NA |
3.3 Effects of Ovariectomy on lymphocytic infiltration in the lacrimal gland
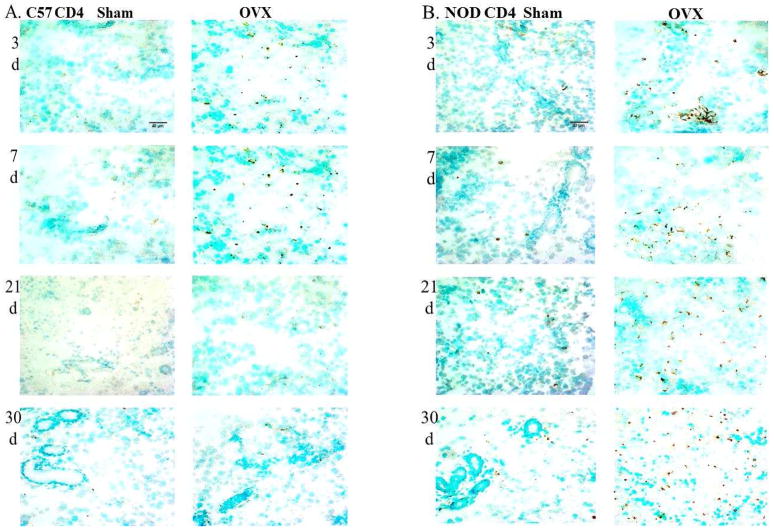
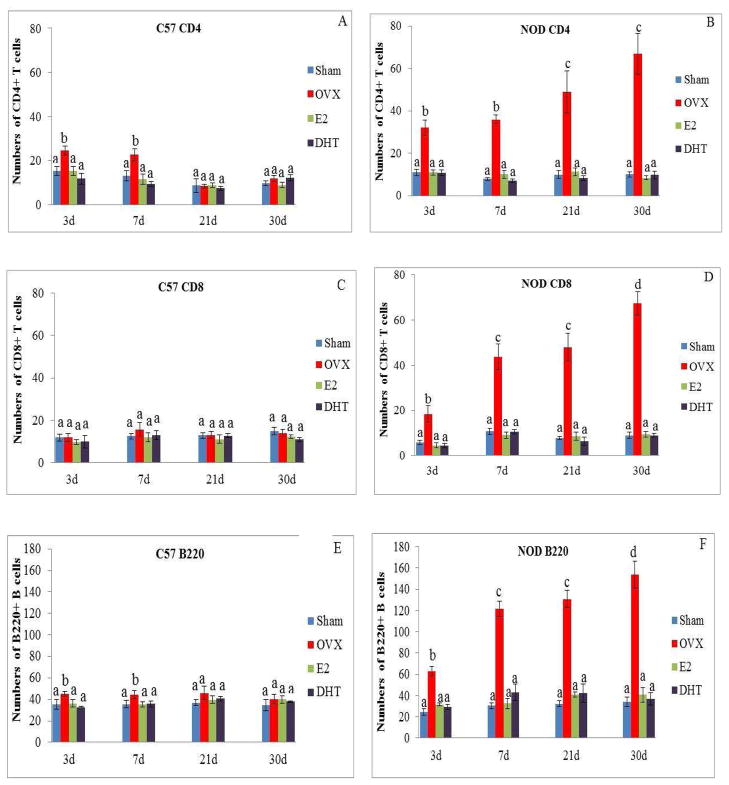
Quantitation of staining for CD4+T cells in the lacrimal glands of OVX C57BL/10 showed approximately1.6 fold increase in the numbers of these cells at 3 and 7 days after surgery compared to the sham operated group, while no significant differences were found at 21 or 30 days after OVX (Fig. 2A and Fig. 3A). On the other hand, quantitation of staining for CD4+T cells in the lacrimal glands of OVX NOD.B10.H2b mice showed significant increases in the numbers of CD4+ cells at all four experimental times studied compared to the sham operated group (Fig. 2B and Fig. 3B). The numbers of CD4+ T cells in the lacrimal gland significantly increased at 3 days (32 ± 3.7) after ovariectomy compared to sham operated group (10.9 ± 1.4) and continued to increase over time.
Fig. 2.
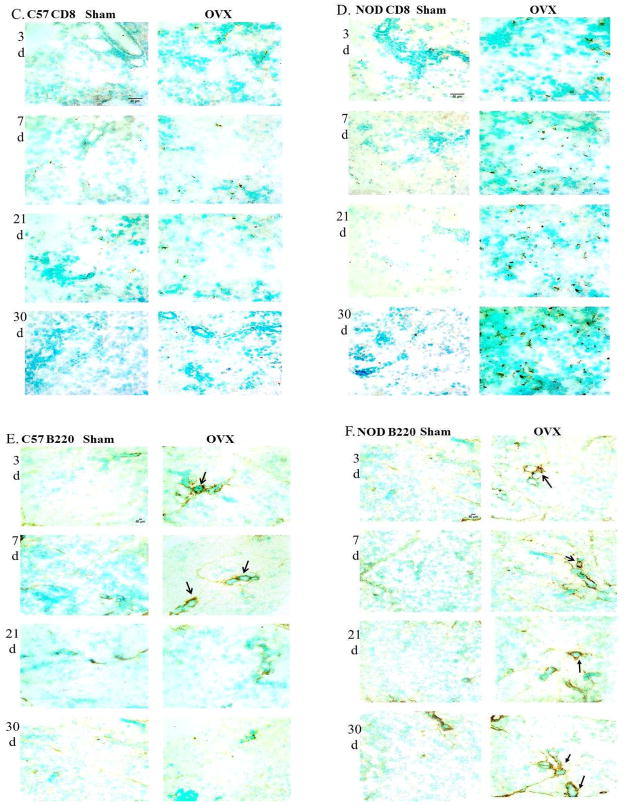
Representative cryostat sections of lacrimal glands from C57BL/10 and NOD.B10.H2b mice showing CD4+ T cells (A and B), CD8+ T cells (C and D) and B220+ B cells (E and F) (brown staining) at 3, 7, 21 and 30 days (d) after sham operation and OVX. Arrows show periductal distribution of B lymphocytes (E and F). Total magnification for CD4+ and CD8+= 400X. Total magnification for B220+ =200X. N=6.
Fig. 3.
Summary of quantitative data of the number of CD4+ T cells, CD8+ T cells and B220+ B cells from lacrimal glands of C57BL/10 (A, C and E) and NOD.B10.H2b (B, D and F) mice after 3, 7, 21 and 30 days of sham operation, OVX, OVX plus treatment with E2 or OVX plus treatment with DHT. Values (mean± SE), with different letter superscripts differ from each other at P < 0.05 (by ANOVA and Duncan’s new multiple range test) N=6.
No significant changes in the numbers of CD8+T cells were observed in the lacrimal glands of OVX C57BL/10 mice at any of the experimental times studied as compared to their respective sham operated groups (Fig. 2C and Fig. 3C). However, the numbers of CD8+ T cells in the lacrimal glands of OVX NOD.B10.H2b mice increased significantly compared to the sham operated groups at all the experimental times studied (Fig. 2D and Fig. 3D). The numbers of CD8+T cells also increased with time in this animal group, compared to their respective sham operated groups. The distribution of CD4+ T and CD8+ T cells were evenly dispersed throughout the glands in both strains.
A slight but significant increase in the numbers of B220+B cells was observed at 3 and 7 days after OVX in the C57BL/10 mice compared to the sham operated group. However, no significant changes in the numbers of B220+B cells were observed at the later experimental times (Fig. 2E and Fig. 3E). Staining of OVX NOD.B10.H2b lacrimal glands on the other hand, showed a significant increase in the numbers of B220+ B cells at all 4 experimental times compared to the sham operated animals (Fig. 2F and Fig. 3F). The numbers of B220+ B cells in the OVX NOD.B10.H2b also increased significantly with time as compared to their respective sham operated groups.
B lymphocyte infiltration was found mostly periductal (Fig. 2E and Fig. 2F). These results are similar to those reported for the salivary glands of SS patients (Thomas et al., 1974).
3.4. Effects of Ovariectomy on nuclear DNA degradation and cleaved caspase-3 activity in the lacrimal gland
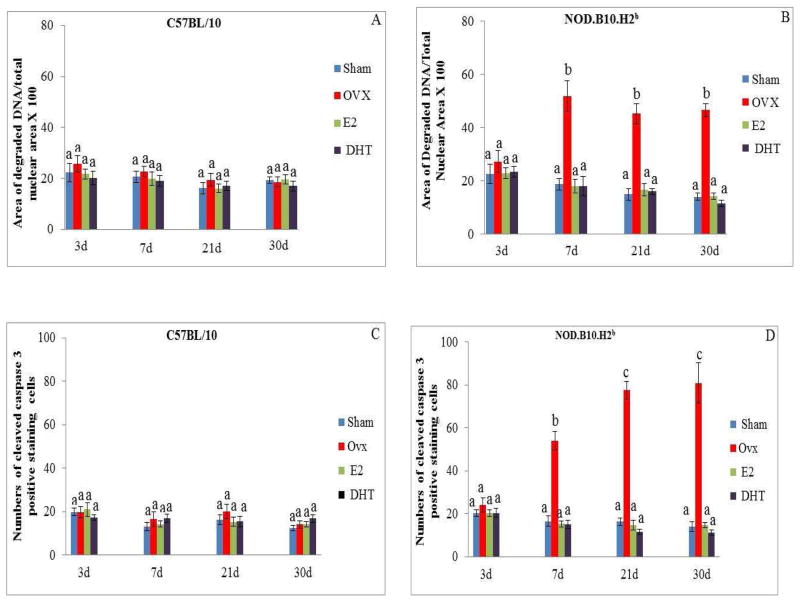
No significant changes in the amount of nuclear DNA degradation (Fig. 4A and Fig. 5A), or in the numbers of cells that stained positive for cleaved caspase-3 (Fig. 4C and Fig. 5C) were observed in the lacrimal glands of OVX C57BL/10 mice compared to the sham operated group at all of the 4 experimental times studied.
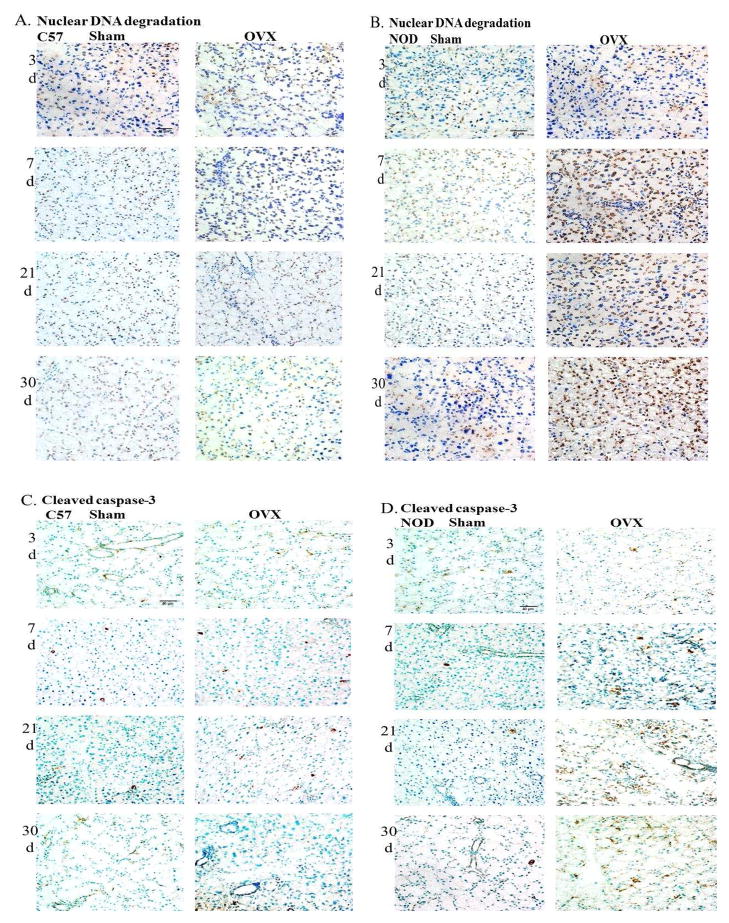
Fig. 4.
Representative cryostat sections of lacrimal glands from C57BL/10 mice and NOD.B10.H2b mice showing nuclear fragmented DNA (A and B) and cleaved caspase-3 positive cells (C and D) (brown staining) after 3, 7, 21 and 30 days of sham operation and OVX. Total magnification= 400X. N=6.
Fig.5.
Summary of quantitative data of the area of nuclear fragmented DNA and the number of cleaved caspase-3 in lacrimal glands of C57BL/10 (A and C) and NOD.B10.H2b (B and D) mice after 3, 7, 21 and 30 days of sham operation, OVX, OVX plus treatment with E2 or OVX plus treatment with DHT. Values (mean±SE), with different letter superscripts differ from each other at P < 0.05 (by ANOVA and Duncan’s new multiple range test) N=6.
A significant increase in nuclear DNA degradation in the lacrimal epithelial cells of OVX NOD.B10.H2b mice was found at 7 (51.7 ± 5.7%), 21 (45.1 ± 3.7%) and 30 (46.5 ± 2.5%) days after surgery compared to the sham operated groups (18.6 ± 3.6%, 14.7 ± 2.2%, 13.8 ± 1.3% respectively) (Fig. 4B and Fig. 5B).
In addition, a significant increase in the number of cells that stained positive for cleaved caspase-3 was observed in the lacrimal glands of the OVX NOD.B10.H2b mice at 7 (54 ± 4.4), 21 (77.5 ± 4.2) and 30 (80.9 ± 9.3) days after surgery compared to the sham operated mice (16.5 ± 2.4, 16.4 ± 1.7, 14.0 ± 2.2 respectively) (Fig. 4D and Fig. 5D). No changes in the amount of nuclear DNA degradation or in the number of cells staining positive for cleaved caspase-3 were observed at 3 days after surgery in the OVX NOD.B10.H2b mice compared to the sham operated group.
3.5. Effect of sex hormones treatment on the lacrimal gland of ovariectomized mice
Treatment with physiological doses of E2 or DHT prevented the increase in the numbers of CD4+ T (Fig. 3A), and B220+ B (Fig. 3E) cells in the lacrimal glands of the C57BL/10 mice observed at 3 and 7 days after surgery. In addition, treatment with E2 or DHT also prevented the increase in the numbers of CD4+ T (Fig. 3B), CD8+T (Fig. 3D), and B220+ B (Fig. 3F) cells at all 4 experimental times, as well as the increase in nuclear DNA degradation (Fig. 5B) and the numbers of cells stained positive for cleaved caspase-3 activity (Fig. 5D) in the lacrimal gland of the NOD.B10.H2b mice at 7, 21, and 30 days after surgery.
4. Discussion
Previously, it was shown that the NOD.B10.H2b mouse exhibits spontaneous increase in the levels of proinflammatory cytokines and acinar cell apoptosis around 12–20 weeks of age (Humphreys-Beher et al., 1994; Robinson et al, 1998). In our experiments, we observed changes in the lacrimal glands between 6 and 10 weeks of age, suggesting that removal of the ovarian hormones in these mice can accelerate the development of the disease.
In addition, while the numbers of lymphocytes, cells positive for cleaved caspase-3 and nuclear DNA degradation in the lacrimal glands of NOD.B10.H2b mice increased over time, the OVX C57BL/10 mice showed a significant increase in the numbers of CD4+T and B220+ B cells only at 3 and 7 days after surgery, while no changes in the numbers of CD8+T cells, cleaved caspase -3 positive cells or nuclear DNA degradation were observed compared to the sham operated group. The reason why the numbers of CD8+ T cells did not increase in the lacrimal glands of OVX C57BL/10 mice compared to the sham operated group could be that C57BL/10 mice are not genetically predisposed to develop autoimmune disease and therefore the decrease in sex hormones levels caused only a small and transient increase in the number of CD4+ T cells which was insufficient to activate CD8+ T cells. CD4+ T cells have been shown to modulate CD8+ T cell differentiation by regulating IL-2 homeostasis (Mc Nally et al., 2011), and to maintain sustained CD8+ T cell responses during chronic infection (Wherry and Ahmed, 2004; Fahey et al., 2010). Thus, our results showed that the effects of removing ovarian hormones on the lacrimal gland in a non-predisposed mice is mild and transient. This supports our hypothesis that, in addition to a decrease in the levels of sex hormones, a predisposed genetic background seems to be necessary to produce a more severe and persistent response.
The mechanism(s) by which decreased levels of sex hormones trigger lymphocytic infiltration in the lacrimal gland is still unknown. It has been shown that lacrimal epithelial cells (De Saint et al., 2009) as well as regulatory CD+4 T cells in the lacrimal gland (Barabino and Dana, 2007) secrete TGF-β and other mediators that suppress the proliferation of the immune cells, thereby playing a role in immunoregulation of the gland. In addition, subcutaneous administration of DHT has been shown to increase TGF-β expression by lacrimal epithelial cells and to cause suppression of inflammation in a mouse model of SS (Rocha et al., 1998). Therefore, one of the mechanisms by which decreased levels of sex hormones initiate autoimmune responses could be by impairing lacrimal epithelial cells or CD+4 T regulatory cells from producing immunomodulatory factors such as TGF-β.
Previously, we have shown that ovariectomy causes two surges of plasma cell apoptosis in the lacrimal glands of normal rabbits. The first surge peaked at approximately 4 to 6 hours and the second was observed between 3 and 9 days with a peak of about 6 days after ovariectomy (Azzarolo et al., 1999). In the present experiments we observed apoptosis only in the epithelial cells from day 7 after ovariectomy. However, we do not know if apoptosis of the plasma cells in the lacrimal gland of the NOD.B10.H2b or C57BL/10 mice occurred before day 3 after ovariectomy. If this occurred it could also be responsible of provoking an autoimmune response in these mice by releasing autoantigens.
Our findings also showed that lymphocytic infiltration caused by decreased circulating levels of sex hormones preceded lacrimal epithelial cell death in the OVX NOD.B10.H2b mice. Thus, we observed a significant increase in CD4+ and CD8+ lymphocyte infiltration in the lacrimal glands of NOD.B10.H2b mice at 3 days after surgery, that continued to rise progressively over time, while nuclear DNA degradation and cleaved caspase-3 activity were found significantly increased at 7 days and remained increased at 21 and 30 days after ovariectomy.
Several possible mechanisms by which lymphocytic infiltration of the lacrimal gland could lead to cell death are conceivable. Activated CD4+ T cells can trigger CD8+T cell differentiation which could lead to lacrimal epithelial cell death through Fas-FasL system or by releasing perforin-granzyme. Expression of FasL and its receptor Fas, have been observed in the lacrimal (Tsubota et al., 2003) and salivary glands of SS patients (Matsumura et al., 1996) and in the salivary glands of the NOD mice (Van Blokland et al., 2003). The interaction of Fas and FasL initiates a chain of biochemical events which leads to cleavage and activation of caspase 3 with subsequent substrate cleavage and apoptosis (Manganelli and Fietta, 2003; Kulkarni et al., 2006; O’Brien and Kirby, 2008). Since, in our experiments, lymphocytic infiltration occurred before apoptosis and cleaved caspase-3 activity follows the same time course as nuclear DNA degradation, it suggests that interaction of Fas-FasL could be a possible mechanism in the initiation of apoptosis. The exact time at which apoptosis occurred after lymphocytic infiltration is not known because measurements of apotosis were not done between days 3 and 7 after ovariectomy. However it requires some time for the autoreactive T cells to get to the tissue. Therefore, it is possible that over time, the increase in the number of infiltrating lymphocytes causes more CD8+ T cell differentiation which leads to a rise in apoptosis at 7, 21 and 30 days after ovariectomy.
In addition, activated CD4+ TH2 cells produce IL-4 that plays an important role in the proliferation of B cells (Gao et al., 2006). B lymphocytes, in turn, participate in lacrimal gland inflammation by producing autoantibodies. Preliminary data in our laboratory showed a significant increase in IL-4 in the lacrimal gland of the OVX NOD.B10.H2b compared to the sham operated groups (data not shown).
Another mechanism could be through Tumor Necrosis Factor alpha (TNF-α). It has been reported that TNF-α and INF-γ directly induce apoptosis in salivary gland cells of SS patients (Kamachi et al., 2002). Preliminary data from our laboratory showed a significant increase in the level of TNF-α in the lacrimal glands of OVX NOD.B10.H2b mice compared to the sham operated group (data not shown). Therefore, it is possible that decreased levels of sex hormones activate immune cells in the lacrimal gland resulting in an increase of the proinflammatory cytokine TNF-α, which then cause lacrimal epithelium cell death.
Sex hormone replacement with physiological doses prevented the increase in lymphocytic infiltration and cell death in the lacrimal glands of the NOD.B10.H2b or C57BL/10 mice. These findings are supported in part by the findings from Ishimaru et al., 1999, which showed that estrogen deficiency after ovariectomy accelerated inflammatory infiltration in the lacrimal and salivary glands of NSF/sld mice and that estrogen administration prevented it. These data indicate that estrogen has a protective role in the lacrimal and salivary glands. These lesions, however, were not recovered by subcutaneous administration of T. In our study we found that physiological doses of E2 or DHT prevented the increase in lymphocytic infiltration and cell death observed in the lacrimal glands of the NOD.B10.H2b. There are several possible reasons that can explain the discrepancy between their findings and ours. One is the type of mouse used in these experiments. Ishimaru et al. used a non-autoimmune prone NFS/sld mouse which develops mainly sialoadenitis that is characterized by inflammatory lesions containing CD3+ and CD4+ cells with few CD8+ and B cells. These mice carry an autosomal recessive gene, sld, with sublingual gland differentiation arrest (Hayashi et al., 1988; Haneji et al., 1994). Our NOD.B10.H2b mouse is genetically predisposed to SS that closely mimic the human disease including lacrimal gland secretory dysfunction. Thus, in addition of the lymphocytic infiltration (CD4+, CD8+ and B220+) we found that ovariectomy caused apoptosis of the lacrimal secretory cells. A second reason could be the dose of the sex hormones used in these experiments. Ishimaru et al. did not mention whether they administered physiological or pharmacological doses of estrogen or T to the NSF/sld mouse. Estrogen can increase the levels of sex hormone binding protein that binds to estrogen and T in the plasma and therefore decrease the action of free or active T. Moreover, T can convert peripherally to estrogen by the enzyme aromatase.
In our experiments, although the serum levels of the androgens androstenedione and T also decreased after ovariectomy, treatment with DHT or E2 alone prevented the lymphocytic infiltration, nuclear DNA degradation and caspase-3 activity observed in the lacrimal gland after ovariectomy. One explanation for this is that androstenedione and T are weaker androgens compared to DHT. In addition, the lacrimal glands could be a DHT dependent organ (Azzarolo et al., 1995, 1997), since lacrimal glands have been shown to express 5α-reductase enzyme that converts T into DHT (Rocha et al., 2000). Therefore, it is possible that, for androstenedione and T to act on the lacrimal gland, they need to be converted into DHT. On the other hand, androstenedione and T can also be converted into E2 in adipose tissue by the enzyme aromatase. Therefore, treatment with DHT or E2 can compensate for the decrease in the levels of androstenedione and testosterone. Since either DHT or E2 can prevent all the effects seen after ovariectomy in the lacrimal gland, it is possible that both hormones are crucial for the maintenance and protection of the lacrimal gland.
The mechanism(s) by which estrogen or androgen prevent the characteristic symptoms of SS in lacrimal glands is still unclear. Estrogen or androgen could exert their effects through their classical intracellular receptors, via the regulation of gene transcription. Estrogen (ER-α and ER-β) and androgen receptor (AR) mRNA have been found in lacrimal epithelial cells of NOD mice (Moore et al., 1996; Richards and Sullivan, 2009) and androgen receptor protein in the MRL mice models of SS (Ono et al., 1995). On the other hand, both estrogen and androgen could exert their actions through their so called “non genomic” effects (Fitts et al., 2011; Cheng et al., 2011; Maiti et al., 2011).
In summary, our results showed that removal of sex hormones in the genetically predisposed mouse model of SS accelerated the symptoms of this disease and support our hypothesis that genetic predisposition is necessary to produce a more severe and persistent response. Moreover, lymphocytic infiltration of the lacrimal gland preceded glandular epithelial cell death in NOD.B10.H2b mice. We also found that treatment with physiological doses of sex hormones prevented the symptoms in the lacrimal glands of these mice observed after ovariectomy. Further research needs to be done to elucidate the relationship between decreased levels of sex hormones, lymphocytic infiltration and cell death in the lacrimal gland. In addition, the interaction between sex hormones and the genetic elements needs to be investigated.
Ovariectomy accelerated the symptoms of Sjögren’s syndrome in the lacrimal gland.
Lymphocytic infiltration preceded lacrimal gland apoptosis after ovariectomy.
These effects were more severe and persistent in the genetically predisposed mice.
Sex hormones replacement at physiological levels prevented these effects.
Acknowledgments
The authors would like to thank Dr. Keith Brew for his comments and helpful discussion. This work has been supported by NIH Grants EY 017995.
Footnotes
Proprietary Interest: N
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azzarolo AM, Bjerrum K, Maves CA, Becker L, Wood RL, Mircheff AK, Warren DW. Hypophysectomy-induced regression of female rat lacrimal glands: partial restoration and maintenance by dihydrotestosterone and prolactin. Invest Ophthalmol Vis Sci. 1995;36:216–26. [PubMed] [Google Scholar]
- Azzarolo AM, Mircheff AK, Kaswan RL, Stanczyk FZ, Gentschein E, Becker L, Nassir B, Warren DW. Androgen support of lacrimal gland function. Endocrine. 1997;6:39–45. doi: 10.1007/BF02738800. [DOI] [PubMed] [Google Scholar]
- Azzarolo AM, Wood RL, Mircheff AK, Richters A, Oslen E, Berkowitz M, Bachmann M, Huang ZM, Zolfagari R, Warren DW. Androgen influence on lacrimal gland apoptosis, necrosis, and lymphocytic infiltration. Invest Ophthalmol Vis Sci. 1999;40:592–602. [PubMed] [Google Scholar]
- Barabino S, Dana MR. Dry eye syndromes. Chem Immunol Allergy. 2007;92:176–84. doi: 10.1159/000099268. [DOI] [PubMed] [Google Scholar]
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol of Reproduction. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Quinn JA, Graeber CT, Filardo EJ. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-golgi-proteasome pathway. J Biol Chem. 2011;24:22441–22455. doi: 10.1074/jbc.M111.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon DD, Zelinski-Wooten MB, Barkley MS. Hormonal basis of variation in oestrous cyclicity in selected strains of mice. J Reprod Fertil. 1990;89:117–126. doi: 10.1530/jrf.0.0890117. [DOI] [PubMed] [Google Scholar]
- De Saint, Jean M, Nakamura T, Wang Y, Trousdale MD, Schechter JE, Mircheff AK. Suppression of lymphocyte proliferation and regulation of dendritic cell phenotype by soluble mediators from rat lacrimal epithelial cells. Scand J Immunol. 2009;70:53–62. doi: 10.1111/j.1365-3083.2009.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2010;208:987–99. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts JM, Klein RM, Powers CA. Tamoxifen regulation of bone growth and endocrine function in the ovariectomized rat: discrimination of responses involving estrogen receptor {alpha}/estrogen receptor {beta}, g protein-coupled estrogen receptor, or estrogen-related eeceptor {gamma} using fulvestrant (ICI 182780) J Pharmacol Exp Ther. 2011;1:246–254. doi: 10.1124/jpet.110.173955. [DOI] [PubMed] [Google Scholar]
- Gao J, Killedar S, Cornelius JG. Sjögren's syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmune. 2006;26:90–100. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kojima A, Hata M. A new mutation involving the sublingual gland in NFS/N mice. Partially arrested mucous cell differentiation. Am J Pathol. 1988;132:187–91. [PMC free article] [PubMed] [Google Scholar]
- Haneji N, Hamano H, Yanagi K. A new animal model for primary Sjögren’s syndrome in NFS/sld mutant mice. J Immunol. 1994;153:2769–77. [PubMed] [Google Scholar]
- Humphreys-Beher MG, Hu Y, Nakagawa Y, Wang PL, Purushotham KR. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjögren’s syndrome. Adv Exp Med Biol. 1994;350:631–636. doi: 10.1007/978-1-4615-2417-5_105. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Saegusa K, Yanagi K, Haneji N, Ichiro S, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren’s syndrome through fas-mediated apoptosis. Am J Pathol. 1999;155:173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi M, Kawakami A, Yamasaki S, Hida A, Nakashima T, Nakamura H, Ida H, Furuyama M, Nakashima K, Shibatomi K, Miyashita T, Migita K, Eguchi K. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. J Lab Clin Med. 2002;139:13–9. doi: 10.1067/mlc.2002.120648. [DOI] [PubMed] [Google Scholar]
- Kulkarni K, Selesniemi K, Brown TL. Interferon-gamma sensitizes the human salivary gland cell line, HSG, to tumor necrosis factor-alpha induced activation of dual apoptotic pathways. Apoptosis. 2006;11:2205–2215. doi: 10.1007/s10495-006-0281-8. [DOI] [PubMed] [Google Scholar]
- Maiti K, Paul JW, Read M, Chan EC, Riley SC, Nahar P, Smith R. G-1-activated membrane estrogen receptors mediate increased contractility of the human myometrium. Endocrinology. 2011;1:J2448–2455. doi: 10.1210/en.2010-0979. [DOI] [PubMed] [Google Scholar]
- Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. Arthritis Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- Masago R, Aiba-Masago S, Talal N, Zuluaga FJ, Al-Hashimi I, Moody M, Lau CA, Peck AB, Brayer J, Humphreys-Beher MG, Dang H. Elevated proapoptotic Bax and caspase 3 activation in the NOD. scid model of Sjögren's syndrome. Arthritis Rheum. 2001;44:693–702. doi: 10.1002/1529-0131(200103)44:3<693::AID-ANR119>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Matsumura R, Kagami M, Tomioka H, Tanabe E, Sugiyama T, Sueishi M, Nakajima A, Azuma M, Okumura K. Expression of ductal Fas antigen in sialoadenitis of Sjögren's syndrome. Clin Exp Rheumatol. 1996;14:309–11. [PubMed] [Google Scholar]
- McNally A, Hill GR, Sparwasser T, Ranjeny Thomas R, Raymond J, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. PNAS. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progression of the immunopathological lesion of Sjögren’s syndrome. Clin Exp Immunol. 2002;128:562–569. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA, Bounous DI, Kaswan RL, Humphreys-Beher MG. Histologic examination of the NOD-mouse lacrimal glands, a potential model for idiopathic autoimmune dacryoadenitis in Sjögren's syndrome. Lab Anim Sci. 1996;46:125–8. [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Nyugen CQ, Cha SR, Peck AB. Sjögren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Frontiers in Bioscience. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjögren syndrome-associated dry eye disease. Ocular Surf. 2009;7:11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien MA, Kirby R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J Vet Emerg Crit Care. 2008;18:572–585. [Google Scholar]
- Ono M, Rocha FJ, Sullivan DA. Immunocytochemical location and hormonal control of androgen receptors in lacrimal tissues of the female MRL/Mp-lpr/lpr mouse model of Sjögren’s syndrome. Exp Eye Res. 1995;61:659–66. doi: 10.1016/s0014-4835(05)80016-8. [DOI] [PubMed] [Google Scholar]
- Richards SM, Sullivan DA. Do genetic alterations in sex steroid receptors contribute to lacrimal gland disease in Sjögren's syndrome? Open Endocrinol J. 2009;3:5–11. doi: 10.2174/1874216500903010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CP, Cornelius J, Bounous DE, Yamamoto H, Humphreys-Beher MG, Peck AB. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity. 1998;27:29–44. doi: 10.3109/08916939809008035. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Wickham LA, Huang Z, Toda I, Gao J, da Silveira LA, Sullivan DA. Presence and testosterone influence on the levels of anti- and pro- inflammatory cytokines in lacrimal tissues of a mouse model of Sjögren's syndrome. Adv Exp Med Biol. 1998;438:485–491. doi: 10.1007/978-1-4615-5359-5_67. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Wickham LA, da Silveira LA, Krenzer KL, Yu FS, Toda I, Sullivan BD, Sullivan DA. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84:76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Hardin JA, Frank MM, Green I. Identification of cells infiltrating minor salivary glands in patients with Sjögren’s syndrome. J Immunol. 1974;112:641–648. [PubMed] [Google Scholar]
- Tobon GJ, Pers JO, Youinou P, Saraux A. B cell-targeted therapies in Sjögren’s Syndrome. Autoimmunity Reviews. 2010;9:224–28. doi: 10.1016/j.autrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Fujita H, Tsuzaka K, Takeuchi T. Quantitative analysis of lacrimal gland function, apoptotic figures, Fas and Fas ligands expression of lacrimal glands in dry eye patients. Exp Eye Res. 2003;76:233–240. doi: 10.1016/s0014-4835(02)00279-8. [DOI] [PubMed] [Google Scholar]
- Van Blokland SCA, van Helden-Meeuwsen CG, Wierenga-Wolf AF, Tielemans D, Drexhage HA, van de Merwe JP, Homo-Delarche F, Versnal MA. Apoptosis and apoptosis- related molecules in the submandibular gland of the nonobese diabetic mouse model for Sjögren’s syndrome: Limited role for apoptosis in the development of sialoadenitis. Lab Invest. 2003;83:3–11. doi: 10.1097/01.lab.0000048721.21475.d1. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Rafi Ahmed R. Memory CD8 T-cell differentiation during viral infection. J of Virology. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]