Abstract
Polyphenol-rich grape seeds have a beneficial effect on human health. The present study was performed to investigate the effects of grape seeds on antioxidant activities in rats. Male Sprague-Dawley rats were randomly divided into a control diet group (C), a high-fat diet group (HF), a 5% grape seed-supplemented control diet group (G), and a 5% grape seed-supplemented high-fat diet group (HG). Dietary supplementation with grape seeds reduced serum concentrations of lipid peroxides compared with those in the C and HF groups. The hepatic level of lipid peroxides decreased significantly in the grape seed groups compared with that in the C and HF groups. Superoxide dismutase activity in the G group increased significantly compared with that in the C group. Catalase activity tended to be higher by feeding grape seeds. The grape seed diet increased glutathione peroxidase activity in the C group. Glutathione-S-transferase activity increased significantly in the G group compared with that in the C group. Hepatic content of total glutathione increased significantly in the HG group but decreased significantly in the HF group. The ratio of reduced glutathione and oxidized glutathione increased by feeding the grape seed diet. Total vitamin A concentration was significantly higher in HG group than in other groups. Liver tocopherol content of the G and HG groups was significantly higher than that of the control groups. These results suggest that dietary supplementation with grape seeds is beneficial for suppressing lipid peroxidation in high fat-fed rats.
Keywords: Grape seed, oxidative stress, antioxidant, high fat, rat
Introduction
Increasing dietary fat is associated with an increased prevalence of obesity, which can lead to disturbances in metabolism due to body fat accumulation [1]. Obesity may contribute to chronic diseases such as atherosclerosis, diabetes mellitus, and hypertension through increased oxidative stress [2].
Many studies have shown an inverse relationship between consumption of polyphenol rich-fruits or vegetables and the incidence of obesity [3,4]. In particular, grape polyphenols are well-known for their antioxidant and health promoting properties [5,6]. Grapes are a rich source of polyphenols such as phenolic acid, anthocyanins, and flavonoids [7]. Among whole grapes, 60-70% of grape polyphenols are found in grape seeds [8]. Grape seeds contain a number of polyphenols including proanthocyanidins and procyanidins. These grape polyphenols have antioxidant, antibacterial, anticarcinogenic, and antiinflammatory actions [9,10]. Grape seeds also contain α-, β-, and γ-tocopherols as well as α-and β-tocotrienols, which exhibit strong antioxidant activity [11].
The beneficial effects of grape seeds are due to their antioxidant activities, scavenging free radicals, and inhibiting lipid peroxidation [12,13]. Furthermore, grape seeds possess cardioprotective effects by alleviating inflammatory conditions and reducing oxidative stress [14,15]. However, large quantities of grape seeds are discarded as waste from the wine making industry. By-products resulting from the grape industry could be utilized through results of diverse studies on their bioactivities. Therefore, the present study was designed to evaluate the protective effect of grape seeds against oxidative stress in rats fed a high-fat diet.
Materials and Methods
Preparation of grape seed samples
Grape (Vitis labruscana Bailey, Campbell Early) was purchased from Gyeongsan-si Gyeongsangbuk-do, Korea. Grape seeds were manually separated, freeze-dried, and ground in a grinder (Cyclotec 1093 Sample Mill, Foss Tecator, Hoganas, Sweden) into a powdered form. The grape powder was stored at -70℃ until used in the experiment. The grape seed powder yield from grape was 3.1%.
Experimental animals and diets
Male Sprague-Dawley rats (n = 32) were purchased from Orient Bio NHP (Seongnam, Korea). They were housed in stainless steel cages in a room with a 12:12-hour light-dark cycle at 25 ± 5℃. After a 1-week acclimation, the animals were divided randomly into four experimental groups (Table 1). Group I (C): control diet (5% fat); group II (HF): high-fat diet (20% fat); group III (G): control diet with 5% grape seed, group IV (HG): high-fat diet with 5% grape seed. The HF and HG groups were fed a high-fat diet containing an additional 15% lard in addition to the AIN-93 based basal diet, and the G and HG groups were fed experimental diets containing 5% grape seed for 4 weeks. All aspects of the experiment were conducted according to the guidelines provided by the Ethical Committee of Experimental Animal Care.
Table 1.
Experimental diet composition (%)
1)C, control diet; G, control diet with grape seeds; HF, high-fat diet; HG, high-fat diet with grape seeds
2)Mineral mixture (AIN-93; Teklad, Madison, WI, USA)
3)Vitamin mixture (AIN-93; Teklad)
Analytical sample preparation
After 4 weeks, all rats were fasted overnight, anesthetized with ethyl ether, and anatomized. Blood was collected via the abdominal aorta, and serum was obtained by centrifugation at 3,000 rpm for 10 min. Livers were carefully removed and rinsed in physiological saline. The mitochondrial, microsomal, and cytosol fractions were separated using the Hogeboom method [16]. All samples were stored at -70℃ for subsequent analysis.
Measurement of serum and liver lipid peroxide concentrations
Serum and liver lipid peroxidation was measured by evaluating the production of thiobarbituric acid-reactive substances. The levels of serum lipid peroxide were estimated by the method of Yagi [17] and measured fluorometrically at 515 nm excitation and 553 nm emission wavelengths. The hepatic concentration of lipid peroxide was determined by the method of Ohkawa et al. [18] and measured spectrophotometrically at 535 nm. The lipid peroxide level was determined using 1,1,3,3-tetraethoxypropane (Sigma Chemical Co., St. Louis, MO, USA) as the standard.
Antioxidant enzyme assay
Catalase activity was assayed spectrophotometrically using the rate of H2O2 decomposition at 240 nm [19]. Superoxide dismutase (SOD) activity was determined using the pyrogallol autoxidation inhibition assay [20]. Glutathione peroxidase (GSH-Px) activity was measured using a modified method of Paglia and Valentine [21]. Enzyme activity was determined by measuring the disappearance of NADPH at 340 nm and was expressed as nmol/min/mg protein. Glutathione S-transferase (GST) activity was determined spectrophotometrically using dichloro-2,4-dinitrobenzene as the substrate [22].
Measurement of total glutathione and the GSH/GSSG
Total glutathione and oxidized glutathione (GSSG) were determined by the method of Anderson [23] with a slight modification. Total glutathione was determined spectrophotometrically at 412 nm using a solution of 0.1 M potassium phosphate buffer (pH 7.0) containing 6 mM DTNB, 1 mM NADPH, 1 mM GSSG, and 6 unit glutathione reductase. Results are expressed as nmol/mg protein. GSSG was determined using a reaction mixture containing 0.1 M potassium phosphate buffer (pH 7.0), 1 mM EDTA, 1 mM NADPH, and 20 units of glutathione reductase. Reduced glutathione (GSH) content was calculated by subtracting GSSG from total glutathione.
Measurement of hepatic retinol, retinol palmitate, and α-tocopherol
Hepatic concentrations of retinol, retinol palmitate, and α-tocopherol were determined simultaneously by high performance liquid chromatography analysis [24]. The liver was triturated with anhydrous sodium sulfate and was dissolved in dichloromethane containing 500 mg/ml retinyl acetate and 5 mg/ml tocopherol acetate. The mixture was centrifuged at 1,000 × g for 10 min, and the supernatant was transferred to another tube. The supernatant was evaporated under nitrogen gas and was redissolved in 200 µl of ether: methanol (1:3, v/v). The sample was analyzed using a Shimadzu SCL-10A (Kyoto, Japan) instrument equipped with a Waters W12461N column (3.9 × 300 mm) (Milford, MA, USA) and was measured with a UV detector (SPD-10Avp) at 280 nm. The mobile phase was a mixture of methanol and water (95:5, v/v).
Statistical analysis
All data are expressed as mean ± SD. Statistical significance was evaluated with a one-way analysis of variance using SPSS ver. 18.0 (Chicago, IL, USA). Significant differences between groups were obtained by Duncan's multiple range tests. Differences were considered significant at P < 0.05.
Results
Growth performance of rats
The feed intake in the C and G groups increased significantly as compared with that in the high-fat diet groups. In contrast, weight gain and feed efficiency ratio in the C and G diet groups decreased compared with those in the high-fat diet groups (Table 2).
Table 2.
Effect of grape seed supplementation on feed intake, weight gain, and feed efficiency ratio in rats fed a control or high-fat diet
1)Groups are the same as in Table 1.
FER, feed efficiency ratio
Mean ± SD (n = 8).
Values with the same superscript letter within a row are not significantly different at P < 0.05.
Serum and liver lipid peroxide concentrations
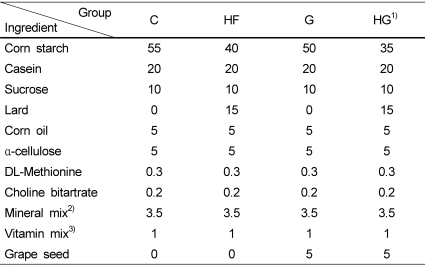
Serum levels of lipid peroxide decreased in the grape seed supplemented groups compared with those in the control groups (Fig. 1A). Liver homogenate level of lipid peroxide showed a similar tendency as the change in serum level. Hepatic microsomal concentration of lipid peroxide tended to decrease with grape seed feeding, but the difference was not significant (Fig. 1B).
Fig. 1.
Effect of grape seed supplementation on the serum concentration (A) and hepatic concentration (B) of lipid peroxides in rats fed a control or high-fat diet. Groups are the same as in Table 1. MC, microsome. Means ± SD (n = 8). Values with the same superscript letters are not significantly different at P < 0.05.
Hepatic activity of antioxidant enzymes
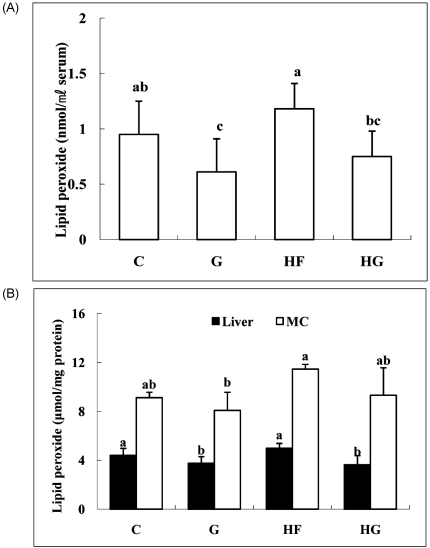
Hepatic antioxidant enzyme activities after 4 weeks of treatment are shown in Table 3. Catalase activity was lowest in the HF group. Catalase activity following grape seed supplementation tended to be higher than that of the control groups, but the difference was not significant. SOD activity in hepatic tissue increased in the G group compared to that in the C group, but high fat supplementation did not change the activity significantly. Grape seed supplementation significantly elevated hepatic GST activity. GSH-Px activity increased in the G and HG groups compared with that in the control groups.
Table 3.
Effect of grape seed supplementation on catalase, superoxide dismutase, glutathione S-transferase and glutathione peroxidase activities in liver fraction of rats fed a control or high-fat diet (unit/min/mg protein)
1)Groups are the same as in Table 1.
Means ± SD (n = 8).
Values with the same superscript letter within a row are not significantly different at P < 0.05.
Concentration of total glutathione and the ratio of GSH and GSSG
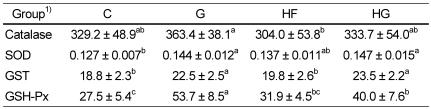
Total glutathione content decreased in the group fed a high fat diet with grape seed as compared to groups without grape seed (Fig. 2A). The ratio of GSH to GSSG increased significantly in the grape seed groups compared to that in the control groups (Fig. 2B).
Fig. 2.
Effect of grape seed supplementation on hepatic level of total glutathione (A) and liver GSH/GSSG (B) in rats fed a control or high-fat diet. Groups are the same as in Table 1. Means ± SD (n = 8). Values with the same superscript letter are not significantly different at P < 0.05.
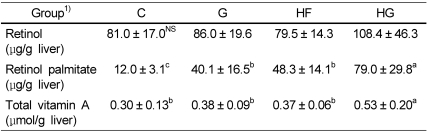
Hepatic concentrations of retinol, retinol palmitate, and tocopherol
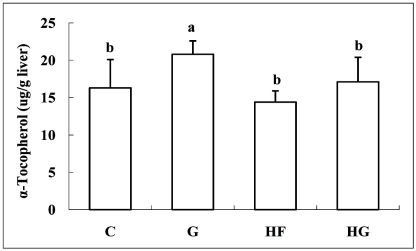
Although retinol concentration was not significantly different among the experimental groups, liver retinol palmitate concentration increased in the G and HG groups compared with that in the C and HF groups (Table 4). Hepatic concentration of total vitamin A was higher in the HS group than that in the other groups. As shown in Fig. 3, hepatic tocopherol concentration increased in the G group compared with that in the C group. No significant difference was observed between the HF and HG groups.
Table 4.
Effect of grape seed supplementation on hepatic concentrations of vitamin A in rats fed a control or high-fat diet
1)Groups are the same as in Table 1.
NS, not significant
Means ± SD (n = 8).
Values with the same superscript letter within a row are not significantly different at P < 0.05.
Fig. 3.
Effect of grape seed supplementation on hepatic concentrations of α-tocopherol in rats fed a control or high-fat diet. Groups are the same as in Table 1. Means ± SD (n = 8). Values with the same superscript letter are not significantly different at P < 0.05.
Discussion
The purpose of this study was to investigate the effect of grape seeds on oxidative stress. Oxidative stress, a major cause of chronic disease, is caused by various factors such as aging, inflammation, and environmental exposure [25]. Obesity induced by a high fat diet stimulates overproduction of reactive oxygen radicals, which are toxic molecules that lead to oxidative stress. A high fat diet can aggravate oxidative stress [26,27].
Many studies [28,29] have shown that obesity induced by a high-fat diet enhances lipid peroxide and diminishes antioxidant enzyme activity. Lipid peroxidation represents oxidative decomposition of lipids and is an indicator of oxidative stress status in tissues and cells [30]. In the present study, the hepatic and serum levels of lipid peroxidation showed a tendency toward increasing in rats fed a high-fat diet compared to those fed the control diet. However, lipid peroxide concentration decreased significantly in the group supplemented with grape seeds compared with that in the control groups. Choi et al. [31] reported that serum lipid peroxide content decreased significantly with diet supplementation of grape seed extracts and grape seed powder. The reduced levels of lipid peroxides following supplementation with grape seeds in the present study may have been associated with increased antioxidant enzyme activity and glutathione contents. The hepatic level of total glutathione decreased in the HF group but increased significantly following grape seed supplementation. The GSH to GSSG ratio in the G and HG groups was higher than that in the C and HF groups, respectively. Glutathione is an endogenous antioxidant synthesized from amino acids and acts as a reducing agent [32]. Glutathione protects the cells by modulating cellular redox status and acting as a cofactor for antioxidant enzymes [33]. GST, GSH-Px, catalase, and SOD activities increased comparatively following grape seed supplementation. These antioxidant enzymes are efficient for protecting tissues and cells from oxidative stress. Sehirli et al. [34] showed that glutathione content increases significantly after consumption of grape seed extract in humans. Anh et al. [12] also reported that increased SOD and catalase activities are observed in liver tissue after feeding grape seed extract. In contrast, Alía et al. [35] reported that antioxidant enzymes such as SOD, catalase, and glutathione content did not change, but that glutathione peroxidase activity increased after consumption of grape seeds and grape skins.
These results were supposedly caused by polyphenols in the grape seeds. Grape seeds are a rich source of polyphenols, such as catechin, epicatechin, epicatechin gallate, epigallocatechin gallate, epigallocatechin, procyanidin B1, and procyanidin B2 [36]. Moreover, the level of total polyphenols in grape seeds is significantly higher than that of whole grapes, grape pulp, or skin [37]. It has been generally recognized that polyphenols have numerous important beneficial effects on oxidative stress, including inhibition of inflammation, inhibition of LDL oxidation, and protection of cells and tissues from oxidative damage [38,39]. Additionally, polyphenols may prevent cardiovascular disease through their ability to reduce cholesterol absorption and plasma levels of triglycerides [40]. These effects of polyphenols are due to their strong antioxidant activities of scavenging reactive oxygen [8].
The biological function of grape seed is also based on high levels of dietary fiber. Alía et al. [35] reported that dietary fiber composition in grape seed is 10.5 g/kg soluble fiber, 732.7 g/kg insoluble fiber, and 743.2 g/kg total indigestible fibers. Additionally, Goñi et al. [41] reported that the content of total dietary fiber in the indigestible fraction of dried grape seed ranges from 78.9% to 80.9%. Consumption of dietary fiber is associated with health benefits such as prevention of type 2 diabetes mellitus, cardiovascular disease, obesity and colon cancer by reducing the absorption of carbohydrates and bile acids [42,43].
Vitamin A and tocopherol are important nutrients to prevent and treat many diseases. They prevent lipid peroxidation and improve the antioxidant defense system [44,45]. In this study, total vitamin A concentration in the HG group increased compared with that in the HF group. Hepatic tocopherol concentration was higher in the G group than that in the C group, and it tended to increase in the HG group compared with the HF group. This result agreed with that of a previous study [46], in which hepatic and serum concentrations of a-tocopherol increased following dietary supplementation with quercetin, (-)epicatechin, and (+)-catechin.
Beveridge et al. [11] reported that grape seed oil contains high levels of α-tocopherol (1.6-3.8 mg/100 g) and γ-tocotrienol (15.23-28.48 mg/100 g). Wie et al. [47] also showed that the total concentration of vitamin E in grapes seed from 14 cultivars ranges from 4.8-9.9 mg/100 g grape seed. Tocopherol, a lipid-soluble antioxidant vitamin, may act as a hydroxyl radical scavenger and lipid peroxidation inhibitor; thus, protecting cell membranes from oxidative stress [48]. Therefore, enhanced tocopherol levels following grape seed supplementation can be an effective deterrent against oxidative stress.
The present results suggest that grape seeds may have a protective effect against oxidative stress by decreasing plasma and hepatic lipid peroxide concentrations and by increasing the antioxidant system. Therefore, grape seeds are expected to be effective for developing functional foods to prevent chronic diseases such as cardiovascular disease.
Footnotes
This research was supported by 2005 grant from Agricultural R & D Promotion Center (ARPC).
References
- 1.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83:549–555. doi: 10.1016/j.physbeh.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, Yilmaz MI, Sayal A, Isimer A, Ozdemir IC. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35:627–631. doi: 10.1016/s0009-9120(02)00363-6. [DOI] [PubMed] [Google Scholar]
- 3.Slattery ML, Curtin KP, Edwards SL, Schaffer DM. Plant foods, fiber, and rectal cancer. Am J Clin Nutr. 2004;79:274–281. doi: 10.1093/ajcn/79.2.274. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Kushi LH, Miller AB, Pietinen P, Rohan TE, Speizer FE, Willett WC, Hunter DJ. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer. 2003;107:1001–1011. doi: 10.1002/ijc.11490. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Juhasz B, Tosaki A, Maulik N, Das DK. Cardioprotection with grapes. J Cardiovasc Pharmacol. 2002;40:762–769. doi: 10.1097/00005344-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Torres JL, Varela B, García MT, Carilla J, Matito C, Centelles JJ, Cascante M, Sort X, Bobet R. Valorization of grape (Vitis vinifera) byproducts. Antioxidant and biological properties of polyphenolic fractions differing in procyanidin composition and flavonol content. J Agric Food Chem. 2002;50:7548–7555. doi: 10.1021/jf025868i. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 9.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 11.Beveridge TH, Girard B, Kopp T, Drover JC. Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: varietal effects. J Agric Food Chem. 2005;53:1799–1804. doi: 10.1021/jf040295q. [DOI] [PubMed] [Google Scholar]
- 12.Ahn HS, Jeon TI, Lee JY, Hwang SG, Lim Y, Park DK. Antioxidative activity of persimmon and grape seed extract: in vitro and in vivo. Nutr Res. 2002;22:1265–1273. [Google Scholar]
- 13.Spranger I, Sun B, Mateus AM, de Freitas V, Ricardo-da-Silva JM. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008;108:519–532. doi: 10.1016/j.foodchem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J Mol Cell Cardiol. 1999;31:1289–1297. doi: 10.1006/jmcc.1999.0961. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–737. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 16.Hogeboom GH. [3] Fractionation of cell components of animal tissues. Methods Enzymol. 1955;1:16–19. [Google Scholar]
- 17.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 11. New York: Academic Press; 1974. pp. 673–684. [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 22.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 23.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 24.Furr HC, Amédée-Manesme O, Olson JA. Gradient reversed-phase high-performance liquid chromatographic separation of naturally occurring retinoids. J Chromatogr. 1984;309:299–307. doi: 10.1016/0378-4347(84)80037-7. [DOI] [PubMed] [Google Scholar]
- 25.Scandalios JG. Oxidative stress responses--what have genome-scale studies taught us? Genome Biol. 2002;3:REVIEWS1019. doi: 10.1186/gb-2002-3-7-reviews1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milagro FI, Campión J, Martínez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity (Silver Spring) 2006;14:1118–1123. doi: 10.1038/oby.2006.128. [DOI] [PubMed] [Google Scholar]
- 27.Hirao K, Maruyama T, Ohno Y, Hirose H, Shimada A, Takei I, Murata M, Morii T, Eguchi T, Hayashi M, Saruta T, Itoh H. Association of increased reactive oxygen species production with abdominal obesity in type 2 diabetes. Obes Res Clin Pract. 2010;4:e83–e90. doi: 10.1016/j.orcp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Folmer V, Soares JC, Gabriel D, Rocha JB. A high fat diet inhibits delta-aminolevulinate dehydratase and increases lipid peroxidation in mice (Mus musculus) J Nutr. 2003;133:2165–2170. doi: 10.1093/jn/133.7.2165. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Choi SK, Seo JS. Grape skin improves antioxidant capacity in rats fed a high fat diet. Nutr Res Pract. 2009;3:279–285. doi: 10.4162/nrp.2009.3.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34:171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 31.Choi CS, Chung HK, Choi MK, Kang MH. Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Nutr Res Pract. 2010;4:114–120. doi: 10.4162/nrp.2010.4.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen CK. Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 34.Sehirli O, Ozel Y, Dulundu E, Topaloglu U, Ercan F, Sener G. Grape seed extract treatment reduces hepatic ischemia-reperfusion injury in rats. Phytother Res. 2008;22:43–48. doi: 10.1002/ptr.2256. [DOI] [PubMed] [Google Scholar]
- 35.Alía M, Horcajo C, Bravo L, Goya L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res. 2003;23:1251–1267. [Google Scholar]
- 36.Bozan B, Tosun G, Özcan D. Study of polyphenol content in the seeds of red grape (Vitis vinifera L.) varieties cultivated in Turkey and their antiradical activity. Food Chem. 2008;109:426–430. doi: 10.1016/j.foodchem.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 37.Ramchandani AG, Chettiyar RS, Pakhale SS. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extracts from seedless and seeded Indian grapes. Food Chem. 2010;119:298–305. [Google Scholar]
- 38.Quettier-Deleu C, Voiselle G, Fruchart JC, Duriez P, Teissier E, Bailleul F, Vasseur J, Trotin F. Hawthorn extracts inhibit LDL oxidation. Pharmazie. 2003;58:577–581. [PubMed] [Google Scholar]
- 39.Schreckinger ME, Wang J, Yousef G, Lila MA, de Mejia EG. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J Agric Food Chem. 2010;58:8966–8976. doi: 10.1021/jf100975m. [DOI] [PubMed] [Google Scholar]
- 40.Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr. 2005;135:2291–2294. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
- 41.Goñi I, Martìn N, Saura-Calixto F. In vitro digestibility and intestinal fermentation of grape seed and peel. Food Chem. 2005;90:281–286. [Google Scholar]
- 42.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Cabezas ME, Gálvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, Osuna A, Redondo L, Zarzuelo A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr. 2002;132:3263–3271. doi: 10.1093/jn/132.11.3263. [DOI] [PubMed] [Google Scholar]
- 44.Ciaccio M, Valenza M, Tesoriere L, Bongiorno A, Albiero R, Livrea MA. Vitamin A inhibits doxorubicin-induced membrane lipid peroxidation in rat tissues in vivo. Arch Biochem Biophys. 1993;302:103–108. doi: 10.1006/abbi.1993.1186. [DOI] [PubMed] [Google Scholar]
- 45.van Dam B, van Hinsbergh VW, Stehouwer CD, Versteilen A, Dekker H, Buytenhek R, Princen HM, Schalkwijk CG. Vitamin E inhibits lipid peroxidation-induced adhesion molecule expression in endothelial cells and decreases soluble cell adhesion molecules in healthy subjects. Cardiovasc Res. 2003;57:563–571. doi: 10.1016/s0008-6363(02)00699-5. [DOI] [PubMed] [Google Scholar]
- 46.Frank J, Budek A, Lundh T, Parker RS, Swanson JE, Lourenco CF, Gago B, Laranjinha J, Vessby B, Kamal-Eldin A. Dietary flavonoids with a catechol structure increase alpha-tocopherol in rats and protect the vitamin from oxidation in vitro. J Lipid Res. 2006;47:2718–2725. doi: 10.1194/jlr.M600291-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Wie M, Sung J, Choi Y, Kim Y, Jeong HS, Lee J. Tocopherols and tocotrienols in grape seeds from 14 cultivars grown in Korea. Eur J Lipid Sci Technol. 2009;111:1255–1258. [Google Scholar]
- 48.Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]