Abstract
Purpose
Chronic arsenic exposure at levels found in US drinking water has been associated with bladder cancer. While arsenic is a known carcinogen, recent studies suggest that it is useful as a therapeutic agent for leukemia. This study examined the relationship between arsenic exposure and bladder cancer mortality.
Methods
We studied 832 cases of bladder cancer diagnosed in New Hampshire from a population-based case–control study. Individual exposure to arsenic was determined in home drinking water using ICP-MS and in toenail samples by instrumental neutron activation analysis.
Results
Among the high arsenic exposure group, found using toenail arsenic level or arsenic consumption, cases experienced a de-escalated survival hazard ratio (HR) [high (≥ 75 percent) versus low (<25th percentile) toenail arsenic overall survival HR 0.5 (95% CI 0.4–0.8)], controlled for tumor stage, grade, gender, age and treatment regimen. This association was found largely among invasive tumors, in smokers and was not modified by TP53 status. Bladder cancer cause-specific survival showed a similar trend, but did not reach statistical significance [HR 0.5 (95% CI 0.3–1.1)].
Conclusions
Arsenic exposure may be related to the survival of patients with bladder cancer.
Keywords: Arsenic, Bladder cancer, Survival, Drinking water
Introduction
Arsenic is a common trace element present in groundwater in varying amounts across the US. While arsenic is an established bladder carcinogen, the influence of this arsenic exposure on the prognosis for patients with bladder cancer remains to be elucidated. Chronic exposure to arsenic through drinking water occurs in several regions of the US, particularly the northeast and southwest. Approximately 2% of the drinking water serving US households contains 2μg/L arsenic or more [1], primarily the result of geologic contamination. Approximately, 40% of households in New Hampshire are served by un-regulated privately supplied drinking water wells [2, 3].
Although arsenic’s mechanism of action remains under investigation, it induces apoptosis, inhibits cell proliferation, and modulates immune function and angiogenesis [4-6]. Medicinal use of arsenic was common historically; however, in 1992, inorganic trivalent arsenic, in the form of arsenic trioxide (ATO), induced complete acute promyelocytic leukemia (APL) remission in over two-thirds of patients enrolled in a clinical trial [7]. Similar effects were also demonstrated for multiple myeloma and lymphoma [8]. The clinical activity of arsenic in solid tumors, including transitional cell carcinoma of the bladder, is being evaluated [9-11].
Thus, we investigated the relationship between inorganic arsenic exposure from contaminated drinking water and bladder cancer survival using a large, population-based study of 832 cases diagnosed in New Hampshire, USA. Household drinking water arsenic levels were assessed and toenail arsenic was used as an individualized internal biomarker of exposure. We hypothesized that the arsenic exposure may impact survival rates. We also considered whether the TP53 status of the tumors was related to survival and arsenic exposure. TP53 protein levels are positively associated with tumor grade and stage for invasive tumors [12, 13]. Mutations in the TP53 gene result in the production of a non-functional protein with an abnormally long half-life [14].
Methods
Study group
Briefly, we identified all cases of bladder cancer diagnosed among New Hampshire residents, ages 25–74 years, from 1 July, 1994 to 31 December, 2001 from the state cancer registry and interviewed a total of 832 bladder cancer cases, which was 85% of the cases confirmed to be eligible for the study. Tumor tissue samples were re-reviewed by the study pathologist to assess tumor histology, stage and grade. The stage assigned by the pathologist was used for tumors <stage 2A, while cancer registry data were used for higher stage. Treatment and size data were obtained from the state cancer registry. Death of cases was determined as of 15 June, 2009 using the Social Security and the National Death Indices (NDI). The NDI-Plus service was used to assign bladder cancer as the cause of death. Due to the lag in the release of NDI data, cause of death information was available only on cases that died prior to 2006. Of the 298 total deaths among the cases, 87 can currently be specifically attributed to bladder cancer. Death from other causes or death from a cause that is currently unknown were excluded for the analysis of bladder cancer cause-specific mortality. The median duration of follow-up was 9.3 years.
Personal interview
Informed consent was obtained from each participant, and all procedures and study materials were approved by the Committee for the Protection of Human Subjects at Dartmouth College. Consenting participants underwent a detailed in-person interview covering sociodemographic information and lifestyle factors such as the use of tobacco.
Arsenic analysis
Samples of toenail clipping collected at the time of interview were analyzed for arsenic and other trace elements by instrumental neutron activation analysis (INAA) at the University of Missouri Research Reactor, using a standard comparison approach as described previously [2]. The detection limit for arsenic measured by INAA is approximately 0.001 mg/g.
Water samples from the current household drinking water were drawn into two mineral free bottles (I-Chem) with strict precautions taken to avoid contamination. Samples of drinking water were analyzed for arsenic concentration using an Agilent 7500c Octopole ICPMS in the Dartmouth Trace Element Analysis Core Facility. The intraclass correlation between replicate (masked) samples was 0.98 down to concentrations of 0.010 μg/L or less [15]. Groups were created for high versus low arsenic exposure analyses using the 75th percentile as a cutoff, which is a toenail arsenic level of 0.12 g/g, or 0.74 μg/L in the household drinking water. Low arsenic exposure was used as the reference group (≤25th or 0.057 μg/g toenail, 0.11 μg/L drinking water). We also considered the effects by dose (90th percentile: >0.2 μg/g toenail, 50th: >0.081 μg/g toenail, 25th: >0.057 μg/g toenail). In addition, we analyzed drinking water arsenic using the current US maximum contaminant level (MCL) for drinking water (10 μg/L) as a cutoff. Arsenic consumption was calculated by multiplying the drinking water arsenic concentration at home by the number of glasses the subject reported consuming from that water source in the questionnaire.
Immunohistochemistry and mutational analysis
Immunohistochemical staining of paraffin-embedded slides was performed to assess TP53 protein levels in tumor tissue. Briefly, slides were deparaffinized and hydrated into water followed by antigen retrieval. Staining of p53 was performed using a monoclonal antibody (BioGenex, San Ramon, CA, USA) at a 1:100 dilution. The staining was scored by a trained pathologist (percentage of cells, intensity: negative, 1, 2, 3+). Immunohistochemical data for p53 were available on 627 cases and 163 tumors had high staining intensity, classified as ≥3+ staining. Mutational analysis of TP53 exons 5 through 9 was available in a subset of 389 bladder tumor samples (33 subjects of these subjects had identified mutations) [16].
Statistical analysis
Survival analysis for bladder cancer cases was performed using Kaplan–Meier (KM) plots. Differences between groups were assessed using the log-rank test. To adjust for additional factors related to patient survival, Cox-proportional hazards regression analysis was performed with age, gender, smoking status (never, former, and current), as well as AJCC T/N/M tumor stage (0, Cis, I, II, III, and IV), tumor grade (1, 2, and 3), tumor size and treatment (surgery, chemotherapy/radiation, and immunotherapy) in the model. Statistical significances of interactions were assessed using likelihood ratio tests comparing the models with and without interaction terms. P values represent two-sided statistical tests with statistical significance at P < 0.05.
Results
Demographic characteristics of the study populations are shown in Table 1. Subjects tended to be mainly Caucasian with a mean age of 62 years and a large proportion were males. Our analyses were not appreciably altered by restricting to Caucasians. In our population-based study, 75% of the participants had stage Ta or T1 tumors, and 12% had stage T2 or higher. The median survival time for these groups was 8 and 6 years, respectively. At the time of their diagnosis, 33% of the cases were current smokers.
Table 1.
Population characteristics
| Surviving cases n (%) | Deceased cases n (%) | |
|---|---|---|
| Sex | ||
| Males | 381 (71) | 250 (84) |
| Females | 153 (29) | 48 (16) |
| Reference age | ||
| <40 | 21 (4) | 2 (1) |
| 40–55 | 124 (23) | 23 (8) |
| 55–70 | 295 (55) | 156 (52) |
| >70 | 94 (18) | 117 (39) |
| Race | ||
| White | 517 (97) | 277 (93) |
| Non-white | 17 (3) | 21 (7) |
| Smoking status | ||
| Never | 102 (19) | 39 (13) |
| Former | 258 (49) | 149 (52) |
| Current | 169 (32) | 101 (35) |
| AJCC stage | ||
| 0a | 321 (61) | 131(45) |
| 0is | 22 (4) | 9 (3) |
| I | 142 (27) | 84 (29) |
| II–IV | 40 (8) | 64 (22) |
| Grade | ||
| 1 | 227 (50) | 72 (29) |
| 2 | 121 (27) | 66 (27) |
| 3 | 105 (23) | 111 (44) |
| Tumor size (mm) | ||
| <30 | 467 (87) | 255 (86) |
| >30 | 67 (13) | 43 (14) |
| Treatment | ||
| Surgery | 393 | 194 |
| Surgery and immunotherapy | 47 | 38 |
| Surgery and radiation/chemotherapy | 12 | 20 |
| Radiation/chemotherapy | 5 | 6 |
Data were missing on smoking n = 14, stage n = 19, grade n = 130
As shown in Table 2, the Cox-regression analysis comparing high arsenic exposure (>75th percentile) to the low exposure, grouped by toenail arsenic level (≤25th), exhibited a significant hazard ratio (HR) of 0.5 (95% CI 0.4–0.8) indicating a prolonged overall survival associated with arsenic exposure after adjustment for age, sex, smoking status, stage, grade and therapy. High toenail arsenic levels were related to better overall survival in a dose-responsive manner (p-trend = 0.004) (Log-rank p value < 0.001) (Fig. 1a; Table 2). Cox-regression analysis using high versus low arsenic consumption showed a similar trend of HR 0.7 (95% CI 0.5–1.1). There was also a suggestion of slightly improved survival with household drinking water arsenic level above the current US MCL (>10 μg/L), compared to lower levels, but with wide confidence intervals [HR 0.7 (95% CI 0.3–1.5)]. Similar trends were observed for bladder cancer cause-specific survival, but the results were not statistically significant. Toenail arsenic levels >75th were associated with longer survival HR 0.5 (95% CI 0.3–1.1) compared to low arsenic level (<25th).
Table 2.
Bladder cancer case survival based on arsenic exposure
| Surviving n (%) | Deaths n (%) | Overall survival HRa 95% CI | Bladder cancer HRa 95% CI | Invasive bladder cancer HRa 95% CI | |
|---|---|---|---|---|---|
| Toenail arsenic | |||||
| (≤25th) | 149 (28) | 121 (41) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| (>25th) | 385 (72) | 177 (59) | 0.7 (0.5–0.9) | 0.8 (0.5–1.3) | 0.8 (0.5–1.4) |
| (>50th) | 261 | 109 | 0.6 (0.5–0.9) | 0.8 (0.5–1.4) | 0.7 (0.4–1.3) |
| (>75th) | 137 | 49 | 0.5 (0.4–0.8) | 0.5 (0.3–1.1) | 0.5 (0.3–1.1) |
| (>90th) | 60 | 15 | 0.4 (0.2–0.8) | 0.3 (0.1–1.1) | 0.3 (0.1–1.3) |
| Arsenic consumed from water | |||||
| Low (≤25th) | 171 (62) | 111 (70) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| High (>75th) | 107 (38) | 48 (30) | 0.7 (0.5–1.1) | 0.6 (0.3–1.2) | 0.6 (0.3–1.2) |
| Water arsenic level | |||||
| Low (≤25th) | 170 (58) | 101 (62) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| High (>75th) | 125 (42 | 63 (38) | 1.0 (0.7–1.5) | 1.1 (0.6–2.3) | 1.2 (0.6–2.4) |
| Low (≤10 μg/L) | 501 (94) | 291 (98) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| High (>10 μg/L) | 33 (6) | 7 (2) | 0.7 (0.3–1.5) | 1.3 (0.5–3.7) | 1.5 (0.5–4.6) |
Adjusted for gender, age, smoking status, tumor stage, grade and treatment
Fig. 1.
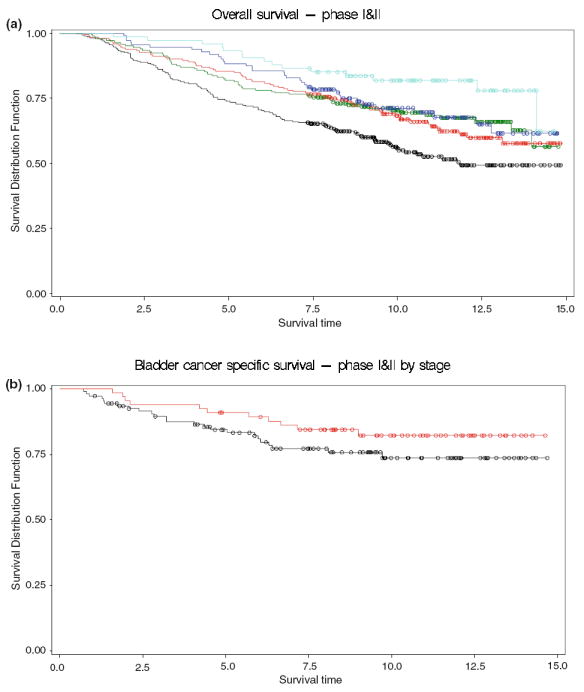
a Overall survival by toenail arsenic level. Kaplan–Meier plots show survival of bladder cancer cases (y-axis) plotted over time in years (x-axis). The colored lines depict cases based on toenail arsenic level (light blue 90th percentile and greater, dark blue 75th–90th, green 50th –75th, red 25th–50th, while black shows all cases below the 25th percentile cutoff (log-rank test: p values < 0.001). b Bladder cancer cause-specific survival for invasive tumors by toenail arsenic level. Kaplan–Meier plots show survival of bladder cancer cases (y-axis) plotted over time in years (x-axis). The red line depicts cases with a toenail arsenic level in the 75th percentile and greater, while black shows all cases below the 25th percentile cutoff (Log-rank test: p values = 0.19)
The effect of high (>75th) versus low (<25th) toenail arsenic level on overall survival did not differ strongly between non-invasive [HR 0.5 (95% CI 0.3–0.9)] and invasive tumors (stage I–IV) [HR 0.5 (95% CI 0.3–0.9)]. Bladder cancer cause-specific mortality was less precise among non-invasive tumors [HR 1.5 (95% CI 0.2–8.7)], but the hazard ratio remained low for invasive tumors [HR 0.5 (95% CI 0.2–1.1)] (Table 2; Fig. 1b). Logistic regression analysis with adjustment for age, gender and smoking showed that high toenail arsenic levels (>75th) decreased the odds of being diagnosed with a tumor of stage I or higher [OR 0.7 (95% CI 0.5–0.9)] compared to having low (<25th) arsenic exposure.
We have previously observed that less TP53 immunohistochemical staining is associated with better overall survival [17] and that arsenic exposure was related to less TP53 staining and a lower prevalence of p53 mutations. The difference in survival was consistent within both the high and low TP53 intensity groups, and remained when we included adjustment for TP53 intensity [overall survival HR 0.5 (95% CI 0.3–0.7); bladder cancer-specific HR 0.5 (0.2–1.0)] or TP53 mutations [overall survival HR 0.4 (95% CI 0.3–0.7); bladder cancer-specific HR 0.5 (0.2–1.2)] in the model. Among subjects without p53 mutations, the overall survival was HR 0.4 (95% CI 0.2–0.7); bladder cancer-specific survival was HR 0.4 (95% CI 0.1–1.0).
The reduced hazard ratio for survival associated with high toenail arsenic exposure was observed among the cases who had a history of smoking [overall survival HR 0.5 (95% CI 0.3–0.7); bladder cancer survival HR 0.4 (95% CI 0.2–0.9)], in contrast to the non-smokers [overall survival HR 1.2 (95% CI 0.4–3.9); bladder cancer survival 1.4 (95% CI 0.3–6.7)]. The interaction between high toenail arsenic and smoking was not statistically significant (interaction p = 0.06). Drinking water arsenic levels were associated with similar differences based on smoking status.
Discussion
Arsenic is an old remedy that has been resurrected due to recently discovered efficacy in acute promyelocytic leukemia, multiple myelomas, lymphomas, pancreatic cancer cell lines and prostate cancer tissue [18], and has been proposed as a treatment for bladder and other epithelial cancers [9-11]. Our study addressed the hypothesis that environmental arsenic exposure might be related to the survival of patients with bladder cancer.
The results suggest that there is a decreased risk of overall death for patients exposed to higher amounts of arsenic, with a non-significant similar trend for cause-specific mortality. Explanations for this observation are: (a) the biology of arsenic-related tumors may differ, (b) arsenic may directly affect tumor growth or (c) arsenic may interact with cancer treatments [6]. Our data suggest that arsenic exposure may modify the biology of the tumor development as high arsenic exposure is associated with less aggressive tumors.
We previously reported that there was a lower incidence of TP53 mutations and over-expression in patients exposed to arsenic [16]. These factors are associated with poor prognosis and could explain our findings [17]. As an alternative explanation, arsenic induces epidermal growth factor receptor (EGFR) activation [19]. EGFR-driven tumors show better response to treatment than other types of tumors (reviewed in [20]).
Recent ecologic studies have explored the relationship between arsenic and bladder cancer survival. Hospital-based Taiwanese studies of transitional cell carcinoma of the urinary bladder found a higher proportion of females, higher grade/stage tumors and a lower 5-year survival rate for subjects living in the area with endemic blackfoot’s disease (≥350 μg/L), compared with other areas (<350 μg/L), but there was no association between arsenic exposure zone and survival time [21, 22]. Potentially confounding differences include gender, stage, delayed diagnosis, emigration, occupation and socioeconomic status between the exposure zones. Our population-based observational US study assessed a lower range of arsenic exposure using individualized exposure biomarker measurements within a single geographic area.
Use of arsenic as an anticancer agent in epithelial cells, including bladder, has been shown to be effective in combination with glutathione synthesis inhibition [10, 23]. Although the exact mechanism of action is still unclear, a majority of arsenic-induced acute promyelocytic leukemia responses occur by activating apoptosis [24]. Arsenic can destabilize lysosomes, degrading the PML/RARα protein [25] and inhibiting proliferation via p21 [26].
While these studies suggest a therapeutic role for arsenic in cancer, others highlight the serious deleterious toxicities and side effects of arsenic, including the possibility of treatment-related secondary malignancies [27], cardiovascular diseases, enhanced angiogenesis and vascular remodeling [28]. Arsenic also initiates defenestration and capillarization of the liver sinusoidal endothelial cells and may impair the cellular immune response [29, 30].
Limitations of our study include a small number of highly exposed individuals. Arsenic measurements from household drinking water do not include arsenic from workplace or other sources of water consumption. Toenail arsenic serves as a biologic indicator of arsenic exposure integrating all sources of exposure and metabolic differences between individuals. We have included overall and cause-specific mortality for bladder cancer cases. Cause of death is known to be problematic, as inconsistent methods are used in assigning cause of death on death certificates.
In summary, we found that bladder cancer patients exposed to higher levels of arsenic had a better survival rate than those with low exposure. Further research is needed to determine whether our observation is due to the etiologic effect of arsenic on the underlying biology of the tumors, a direct effect of continued arsenic exposure on tumor growth or a synergistic effect of continued arsenic exposure combined with treatment. Our results are consistent with the beneficial outcome in patients with an epithelial malignancy and highlight the need for further studies of arsenic exposure in bladder and other epithelial cancers to elucidate the mechanism by which arsenic is associated with bladder cancer survival.
Acknowledgments
Funding for the study was provided by the National Institutes of Health (NIH): K07 CA102327, R03 CA121382, R03 CA99500, R01 CA57494 (NCI) and P20 RR018787 [National Center for Research Resources (NCRR) IDeA program], P42 ES07373 [National Institute for Environmental Health Sciences (NIEHS) and the Dartmouth Superfund Basic Research Program]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement There is no conflict of interest.
References
- 1.Council NR. Arsenic in drinking water. National Academy Press; Washington: 1999. [Google Scholar]
- 2.Karagas MR, Tosteson TD, Morris JS, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004;15:465–472. doi: 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- 3.Andrew AS, Burgess JL, Meza MM, et al. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect. 2006;114:1193–1198. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapaj S, Peterson H, Liber K, et al. Human health effects from chronic arsenic poisoning–a review. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:2399–2428. doi: 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- 5.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Fang J, Dong Y, et al. Arsenic in cancer therapy. Anticancer Drugs. 2005;16:119–127. doi: 10.1097/00001813-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Ma L, Hu X. Ai-lin 1 treated 32 cases of acute promyelocytic leukemia. Chi J Integrat Chin West Med. 1992;12:170–172. [Google Scholar]
- 8.Zhu XH, Shen YL, Jing YK, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 9.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6(Suppl 2):22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Hori S, Ohizumi H, et al. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and l-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 11.Pu YS, Hour TC, Chen J, et al. Arsenic trioxide as a novel anticancer agent against human transitional carcinoma-characterizing its apoptotic pathway. Anticancer drugs. 2002;13:293–300. doi: 10.1097/00001813-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Tsai YS, Tzai TS, Chow NH, et al. Prognostic values of p53 and HER-2/neu coexpression in invasive bladder cancer in Taiwan. Urol Int. 2003;71:262–270. doi: 10.1159/000072676. [DOI] [PubMed] [Google Scholar]
- 13.Kelsey KT, Hirao T, Schned A, et al. A population-based study of immunohistochemical detection of p53 alteration in bladder cancer. Br J Cancer. 2004;90:1572–1576. doi: 10.1038/sj.bjc.6601748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung I, Messing E. Molecular mechanisms and pathways in bladder cancer development and progression. Cancer Control. 2000;7:325–334. doi: 10.1177/107327480000700401. [DOI] [PubMed] [Google Scholar]
- 15.Karagas MR, Tosteson TD, Blum J, et al. Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am J Epidemiol. 2000;152:84–90. doi: 10.1093/aje/152.1.84. [DOI] [PubMed] [Google Scholar]
- 16.Kelsey KT, Hirao T, Hirao S, et al. TP53 alterations and patterns of carcinogen exposure in a US population-based study of bladder cancer. Int J Cancer. 2005;117:370–375. doi: 10.1002/ijc.21195. [DOI] [PubMed] [Google Scholar]
- 17.Marsit CJ, Karagas MR, Andrew A, et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 2005;65:7081–7085. doi: 10.1158/0008-5472.CAN-05-0267. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Chen Z, Lallemand-Breitenbach V, et al. How acute promyelocytic leukemia revived arsenic. Nat Rev Cancer. 2002;2:705–713. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]
- 19.Andrew AS, Mason RA, Memoli V, et al. Arsenic activates EGFR pathway signaling in the lung. Toxicol Sci. 2009;109(2):169–171. doi: 10.1093/toxsci/kfp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Chiou HY, Hsueh YM, et al. Clinicopathological characteristics and survival outcome of arsenic-related bladder cancer in Taiwan. J Urol. 2009;181:547–552. doi: 10.1016/j.juro.2008.10.003. discussion 553. [DOI] [PubMed] [Google Scholar]
- 22.Tan LB, Chen KT, Guo HR. Clinical and epidemiological features of patients with genitourinary tract tumour in a Blackfoot disease endemic area of Taiwan. BJU Int. 2008;102:48–54. doi: 10.1111/j.1464-410X.2008.07565.x. [DOI] [PubMed] [Google Scholar]
- 23.Pu YS, Hour TC, Chen J, et al. Cytotoxicity of arsenic trioxide to transitional carcinoma cells. Urology. 2002;60:346–350. doi: 10.1016/s0090-4295(02)01699-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 25.Kitareewan S, Roebuck BD, Demidenko E, et al. Lysosomes and trivalent arsenic treatment in acute promyelocytic leukemia. J Natl Cancer Inst. 2007;99:41–52. doi: 10.1093/jnci/djk004. [DOI] [PubMed] [Google Scholar]
- 26.Park WH, Seol JG, Kim ES, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 27.Liu J, Lu Y, Wu Q, et al. Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J Pharmacol Exp Ther. 2008;326:363–368. doi: 10.1124/jpet.108.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucy NV, Ihnat MA, Kamat CD, et al. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol Sci. 2003;76:271–279. doi: 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- 29.Straub AC, Stolz DB, Ross MA, et al. Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver. Hepatology. 2007;45:205–212. doi: 10.1002/hep.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrosky-Wegman P, Gonsebatt ME, Montero R, et al. Lymphocyte proliferation kinetics and genotoxic findings in a pilot study on individuals chronically exposed to arsenic in Mexico. Mutat Res. 1991;250:477–482. doi: 10.1016/0027-5107(91)90204-2. [DOI] [PubMed] [Google Scholar]


