SUMMARY
We previously reported that lipopolysaccharide (LPS) related sugars are associated with the glycosylation of the collagen adhesin EmaA, a virulence determinant of Aggregatibacter actinomycetemcomitans. In this study, the role of LPS in the secretion of other virulence factors was investigated. The secretion of the epithelial adhesin Aae, the immunoglobulin Fc receptor Omp34 and leukotoxin were examined in a mutant strain with inactivated TDP-4-keto-6-deoxy-d-glucose 3,5-epimerase (rmlC), which resulted in altered O-antigen polysaccharides (O-PS) of LPS. The secretion of Aae and Omp34 was not affected. However, the leukotoxin secretion, which is mediated by the TolC-dependent Type I secretion system, was altered in the rmlC mutant. The amount of secreted leukotoxin in the bacterial growth medium was reduced 9-fold, with a concurrent 4-fold increase of the membrane-bound toxin in the mutant compared with the wild type strain. The altered leukotoxin secretion pattern was restored to the wild-type by complementation of the rmlC gene in trans. Examination of the ltxA mRNA levels indicated that the leukotoxin secretion was posttranscriptionally regulated in the modified O-PS containing strain. The mutant strain also showed increased resistance to vancomycin, an antibiotic dependent on TolC for internalization, indicating that TolC was affected. Overexpression of TolC in the rmlC mutant resulted in an increased TolC level in the outer membrane but did not restore the leukotoxin secretion profile to the wild-type phenotype. The data suggest that O-PS mediate leukotoxin secretion in A. actinomycetemcomitans.
Keywords: Lipopolysaccharides, Leukotoxin, Type I secretion system, TolC
INTRODUCTION
The Gram-negative, non-motile, microaerophilic bacterium Aggregatibacter actinomycetemcomitans is an endogenous oropharyngeal colonizer of humans and primates. The bacterium can be recovered from the subgingival sulcus, tongue, buccal mucosa, and the saliva from 10–15% of healthy young individuals in the United States (Sirinian et al., 2002) as well as in Brazil (Cortelli et al., 2008). A. actinomycetemcomitans is strongly implicated with localized aggressive periodontitis (LAP) (Haubeck et al., 2008), which is featured with pubertal onset and results in rapid deterioration of the periodontium. LAP affects approximately 0.5% of adolescents in the United States (Löe & Brown, 1991). In addition, A. actinomycetemcomitans causes disseminated infections, including lung and brain abscesses (Stepanović et al., 2005; Hagiwara et al., 2009), and it is also the most common HACEK (Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.) microorganism associated with infective endocarditis (Paturel et al., 2004; Tang et al., 2008). Recent clinical studies (Gaetti-Jardim et al., 2009), as well as in vivo mouse model experiments (Zhang et al., 2010), suggest that A. actinomycetemcomitans accelerates the pathogenesis of atherosclerosis.
The outer membrane of Gram-negative bacteria is an asymmetric bilayer, composed of phospholipids, proteins, and lipopolysaccharides (LPS) (Raetz & Whitfield, 2002). Lipopolysaccharides are comprised of lipid A, core oligosaccharides and O-antigen polysaccharides (O-PS). Lipid A moieties are associated with the hydrophobic compartment of the bilayer. Core oligosaccharides are covalently attached to the lipid A moieties, which are bonded to the O-PS. O-polysaccharides define the serotypes of this organism, and A. actinomycetemcomitans has been assigned seven different serotypes (Takada et al., 2010). Lipopolysaccharides are the predominant molecules on the bacterial surface and important for the viability and membrane stability of Gram-negative bacteria (Raetz & Whitfield, 2002). LPS have also been suggested involved in lateral diffusion of membrane proteins, including large autotransporters and porin protein OmpF (Jain et al., 2006; Straatsma & Soares, 2009), protein secretion (Wandersman & Létoffé, 1993; Bulieris et al., 2003; Bengoechea et al., 2004), and biological activities of proteins (Stanley et al., 1993; Iredell et al., 1998).
Multiple virulence factors contribute to the colonization and pathogenesis of A. actinomycetemcomitans. These factors include adhesins: epithelial cell adhesins ApiA and Aae (Rose et al., 2003; Yue et al., 2007), and the extracellular matrix protein adhesin (EmaA) (Mintz 2004; Tang et al., 2008; Yu et al., 2008). These adhesins are autotransporters secreted using Type V secretion system (T5SS), which comprises an N-terminal passenger domain that typically mediates a virulence function and a C-terminal β-barrel domain for member insertion (Henderson et al., 2004). In addition to these adhesins, other virulence factors are characterized including toxins: leukotoxin (Tsai et al., 1979; Taichman et al., 1980) and cytolethal extending toxin (Shenker et al., 2006); and the immunoglobulin Fc receptor: OmpA-like heat modified protein Omp34(Omp29) (Mintz and Fives-Taylor, 1994) (Komatsuzawa et al., 1999).
The leukotoxin is a 114 kDa protein, which targets human polymorphonuclear leukocytes (PMNs) and monocytes (Tsai et al., 1979; Taichman et al., 1980). The biosynthesis, activation and secretion of leukotoxin is determined by the four-gene operon ltxCABD and tolC (or tdeA, toxin and drug export protein A) (Lally et al., 1989; Crosby & Kachlany, 2007). The ltxCABD operon structure and secretion pathway is homologous to haemolysin (HlyA) found in Escherichia coli (Fath & Kolter, 1993). The pro-toxin of LtxA is synthesized in the cytoplasm and is covalently attached (acylated) with short chain fatty acyl groups to the internal lysine residues by LtxC (Fong et al., 2011). Analogous to the haemolysin secretion, the leukotoxin is translocated across the inner membrane by an integral inner membrane ATPase, LtxB (HlyB in E. coli) and transits the periplasmic space through a channel formed by LtxD (HlyD in E. coli) and TolC (Koronakis et al., 1993; 2004). In E. coli, TolC is a trimeric outer membrane protein, composed of a β-barrel domain and an α-helical domain (Koronakis et al., 2000). The β-barrel domain integrates into the outer membrane to form a pore, whereas the α-helical domain forms a channel extending into the periplasm and interacts with HlyD (Koronakis et al., 2004). In addition to the TolC-dependent Type I secretion system (T1SS) (Fath & Kolter, 1993), leukotoxin is also found secreted with membrane vesicles in A. actinomycetemcomitans (Kato et al., 2002).
The heat-modified outer membrane protein Omp34 is a homologue of the structural outer membrane protein OmpA found in E. coli, and one of the major outer membrane proteins of A. actinomycetemcomitans (Komatsuzawa et al., 1999; 2002). This protein is secreted using the general secretion system, Type II secretion system (T2SS), which utilizes the Sec translocon for transport across the inner membrane (Henderson et al., 2004). This group of proteins plays a pivotal role in maintaining membrane structure (Kleinschmidt, 2006). Omp34 also binds to the Fc portion of IgG, which may inhibit complement activation in the host (Mintz and Five-Taylor, 1994).
In this study, the impact of LPS in the secretion of virulence determinants was investigated. A mutant strain of A. actinomycetemcomitans with O-PS defect showed an elevated amount of membrane-associated leukotoxin, whereas the secreted leukotoxin in the culture medium was diminished when compared with the wild type strain. Further examination of the mRNA level of ltxA indicated the changes in leukotoxin secretion occurred posttranscriptionally. Additional evidence suggested that the impaired leukotoxin secretion in this mutant was associated with disruption of the function of the TolC-dependent T1SS, but not the relative amount of TolC in the outer membrane. In contrast, no change was associated with either Omp34 or Aae, which are secreted by Sec-dependent pathways.
MATERIALS AND METHODS
Bacterial strains
All strains used in this study are listed in Table 1. VT1169, the wild-type strain, was transformed with the shuttle plasmid, pKM2 containing the 520-bp leukotoxin promoter (ltxP) of A. actinomycetemcomitans (Tang & Mintz, 2010). This strain was used as the positive control and for the effect of the plasmid on gene expression. ltxP was used as the promoter for the expression of rmlC and tolC in the strains used in this study (Table 1). The rmlC mutant, which was transformed with pKM2/ltxP, and the complemented strains were described previously (Tang & Mintz, 2010). All A. actinomycetemcomitans strains were stored at −80 °C and grown in 3% Trypticase Soy Broth and 0.6% Yeast Extract (TSBYE) with/without 1.5% agar (Becton, Dickinson and Company, Franklin Lakes, NJ), and 1 μg/ml chloramphenicol (Sigma, St. Louis, MO) in a 37°C incubator with 10% humidified carbon dioxide.
TABLE 1.
Strains and plasmids
| Strains and plasmids | Genotypes/Remarks | Sources and references |
|---|---|---|
| A. actinomycetemcomitans | ||
| VT1169 | a Rifr, Nalr derivative of SUNY465, serotype b | (Mintz, 2004) |
| VT1169/pKM2/ltxP | VT1169 transformed with plasmid pKM2/ltxP, which is referred as the “wild type” | This study |
| aae mutant (aae::Kan) | An isogenic aae (epithelial adhesin) mutant of SUNY465 | (Rose et al., 2003) |
| rmlC (rmlC::pLOF/Sp) | Rhamnose mutant, transposon pLOF/Sp inserted into rmlC(TDP-4-keto-6-deoxy-D-glucose 3, 5-epimerase) | (Tang & Mintz, 2010) |
| rmlC/pKM2/ltxP | rmlC mutant transformed with pKM2/ltxP | This study |
| rmlC /rmlC+ | rmlC mutant complemented with plasmid pKM2/ltxP/rmlC | (Tang & Mintz, 2010 |
| rmlC /tolC+ | rmlC mutant transformed with plasmid pKM2/ltxP/tolC to over-express TolC | This study |
| Plasmids | ||
| pLOF/Sp | Tn10-based transposon vector; Apr, Spr | (Mintz, 2004) |
| pKM2 | A plasmid containing chloramphenicol acetyltransferase, which can be transformed into serotype b A. actinomycetemcomitans , Cmr | (Gallant et al., 2008) |
| pKM2/ltxP | pKM2 containing ~500-bp ltx_promoter, Cmr | (Tang & Mintz, 2010) |
| pKM2/ltxP/rmlC | pKM2 containing ~500-bp ltx promoter + rmlC sequence, Cmr | (Tang & Mintz, 2010) |
| pKM2/ltxP/tolC | pKM2 containing ~500-bp ltx promoter + tolC sequence, Cmr | This study |
Ap: ampicillin; Cm: chloramphenicol; Nal: nalidixic acid; Rif: rifampicin; Sp: spectinomycin
Analysis of LPS by gas chromatography/mass spectrometry (GC/MS)
The glycosyl composition of the LPS extracted from the wild type A. actinomycetemcomitans strain and the isogenic mutants was analyzed using combined GC/MS, as described before (Tang & Mintz, 2010), at the Complex Carbohydrate Research Center, the University of Georgia.
Cell fractionation of A. actinomycetemcomitans
Membrane and cytoplasmic proteins of A. actinomycetemcomitans were prepared as described previously (Mintz, 2004). Briefly, 200 ml late-logarithmic phase cells were harvested and resuspended in 3.0 ml of 10 mM 4-(2-HydroxyEthyl)-1-PiperazineEthaneSulfonic acid (HEPES, pH 7.4) with 1 mM PhenylMethylSulphonyl Fluoride (PMSF, Roche, Mannheim, Germany) and complete protease inhibitor Cocktail (Roche, Mannheim, Germany). The cells were lyzed using a French press minicell, and intact cells and debris were removed. The cytoplasmic and membrane fractions were separated by ultra-centrifugation (100,000 × g for 40 min at 4 °C). Inner and outer membrane proteins were separated by detergent solubilization using 1% N-lauroylsarcosine sodium salt (L-5125, Sigma, St. Louis, MO) in HEPES, at room temperature for 30 min, without agitation (Filip et al., 1973; Nikaido, 1997), followed by centrifugation at 15,600 ×g for 30 min at 4 °C. The proteins, insoluble in lauroylsarcosine, are defined as outer membrane proteins, and the detergent-soluble proteins are defined as inner membrane proteins.
Leukotoxin isolation from bacterial culture medium
Two hundred ml of A. actinomycetemcomitans were grown to the late-logarithmic growth phase, and the cells were centrifuged at 7,650 ± g for 30 min. The resulting supernatant was filtered through a low protein binding polyethersulfone (PES) membrane (0.22 μm) (Corning, Lowell, MA) to remove any remaining bacteria. Fifteen ml of filtered spent medium was concentrated 150-fold using Amicon Ultra centrifugation filter devices with 50,000 molecular weight cutoff (Millipore, Billerica, MA), by centrifugation at × 5,000 g for 30 min at 4 °C, to a final volume of 100 µl.
Immunoblot analysis for the detection of Aae, Omp34 and Leukotoxin (LtxA)
Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Pierce Thermo Scientific, Rockford, IL). Protein samples were diluted in 10 mM HEPES with 2% SDS before addition to the assay. Equivalent concentration of protein was dissolved in electrophoresis loading buffer containing 10 mM HEPES, 2% SDS, 5% (v/v) β-mercaptoethanol, 2% (v/v) glycerol and 0.05% (w/v) bromophenol blue, at 100 °C for 5 min, and loaded into 4–15% gradient polyacrylamide, Tris-glycine Procast Gels (Bio-Rad, Hercules, CA). The separated proteins were transferred to 0.45 μm Westtran Polyvinylidene fluoride (PVDF) membrane (Whatman Inc., Piscataway, NJ), probed with anti-Aae polyclonal antibody (Rose et al., 2003), anti-Omp34 monoclonal antibody (Komatsuzawa et al., 1999), and anti-leukotoxin polyclonal antibody (Lally et al., 1989; Gallant et al., 2008). The immune complexes were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson Laboratory, Bar Harbor, ME) for the polyclonal antibody, or goat anti-mouse IgG (Sigma, St. Louis, MO) for the monoclonal antibody. Signal was detected using a chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) and visualized by exposure to Kodak X-OMAT LS film (Carestream Health, Rochester, NY).
Protein levels were quantified based on integrated signal densities. Immunoblots were performed 4 times using samples prepared from four independent experiments. Densities were determined using ImageJ 1.43u (http://rsb.info.nih.gov/ij/). The paired t-test was used to compare two groups of data, and one-way ANOVA was used to compare three or more than three groups of data (GraphPad, Version 3.00). The level of the proteins in the wild-type strain was arbitrarily set as 1.0. P <0.05 was considered significant.
Isolation of total RNA from A. actinomycetemcomitans
A. actinomycetemcomitans wild-type and mutant strains were recovered directly from −80 °C. After a two-day growth on TSBYE plates, one colony of the wild-type strain (VT1169) and the rmlC mutant were transferred into 8 ml of freshly prepared TSBYE broth, with or without spectinomycin, and grown overnight. One ml of the bacterial suspension was transferred into 9 ml of fresh TSBYE without antibiotics and grown for an additional 4 hours (one and a half doublings) to reach mid-logarithmic growth phase (OD495 = 0.25–0.3). A total of 5 × 108 bacteria in 1 ml of TSBYE were mixed with 2 ml of RNAprotect Bacteria Reagent (QIAGEN Inc., Valencia, CA) to stabilize the RNA. The cells were centrifuged at 5000 ×g for 10 min and resuspended in 100 µl TE buffer (10 mM Tris-Cl, 1 mM EDTA, pH 8.0) containing 1 mg/ml of lysozyme Type VI (MP Biomedicals, Aurora, OH). The cells were incubated for 5 min at room temperature with agitation. Total RNA was purified from the lysate using an RNeasy Mini Kit (QIAGEN Inc., Valencia, CA).
Quantitative, real-time, reverse transcription PCR (RT-PCR) analysis of leukotoxin expression
The purified total RNA was pretreated with deoxyribonuclease I (DNase), amplification grade (Invitrogen, Carlsbad, CA), to remove contaminating DNA. A total of 200 ng of DNase-treated RNA from each sample was reverse transcribed into complementary DNA (cDNA) in a 20 µl reaction using the SuperScript III First-Strand Synthesis system (Invitrogen, Carlsbad, CA). Random hexamer primers, provided by the manufacturer, were used in the reverse transcription reaction. The quantitative real time PCR was performed using an ABI Prism 7900HT Sequence Detection System in the Vermont Cancer Center at the University of Vermont. The 16S ribosomal RNA gene (16S rRNA) was chosen as the endogenous housekeeping gene. The primers and probes used in this study are listed in Table 2. The probes were labeled with the 5’ reporter dye 6-FAM and the 3’ quencher dye BHQ-1. Three cDNA samples, derived from three individual RNA samples, were prepared for each strain. Each cDNA sample was run in duplicate for each real-time PCR reaction. The data were analyzed using paired t-test, and P < 0.05 was set as a significant difference. The relative expression level was calculated based on the threshold cycle (CT):
Expression level=2−ΔΔCT, and
TABLE 2.
Primers and probes for quantitative real-time, reverse transcriptional PCR
| Targets | Primers and probes | Sequence (5’–3’) | Amplicons (bp) |
|---|---|---|---|
| LktA | Forward primer | 5’-GGACCTGTCGCAGGGTTAATT-3’ | 98 |
| Reverse primer | 5’-AGCATTCTCGCACGATCAAA-3’ | ||
| Dual-labeled probe | 5’-FAM-TCAGCTTGGCAATCAGCCCTTTGTC-BHQ-3’ | ||
| 16S rRNA* | Forward primer | 5'-GAACCTTACCTACTCTTGACATCC-3' | 122 |
| Reverse primer | 5'-GGACTTAACCCAACATTTCACAAC-3' | ||
| Dual-labeled probe | 5'-FAM-CTGACGACAGCCATGCAGCACCTG-BHQ1-3' |
Endogenous control
Reporter dye: 6-FAM; Quencher dye: BHQ-1
The wild type strain VT1169 was chosen as the calibrator.
Antimicrobial susceptibility assay
Three compounds were chosen to determine the antimicrobial susceptibility of this O-PS mutant: bile salt, erythromycin and vancomycin hydrochloride. Bile salt (S719191, Fisher, Pittsburgh, PA), which contains ~50% glycocholic sodium salt and ~45% taurocholic sodium salt, was incorporated into TSBYE agar, initially with 10 mg/ml, and decreased by 0.5 mg/ml intervals to a final concentration of 5 mg/ml. Erythromycin (E6376, Sigma, St. Louis, MO) was initiated with 10 μg/ml, and decreased by 1.25 μg/ml to a final concentration of 2.5 μg/ml. Vancomycin from Streptomyces orientalis (V2002, Sigma, St. Louis, MO) was started with 350 μg/ml and decreased by 12.5 μg/ml to a final concentration of 150 μg/ml. 1.0–2.5 ±103 mid-logarithmic growth phase cells were evenly spread on each plate and grown for 7 days in 10% humidified CO2 at 37°C. The minimal inhibitory concentration (MIC) of each compound was defined as the lowest concentration of antibiotic on plates containing less than five colony forming units (CFUs) (Wandersman & Létoffé, 1993). The assay was performed in triplicate.
Cloning of tolC
The tolC (tdeA) gene was amplified and engineered with XhoI at the 5’ and EcoRI at the 3’ of the gene: sense primer (5’-GCTCGAGATGTTCACAATAAAAA-3’) and antisense primer (5’-GAATTCTTATTTTTTTACGGAATAAT-3’). The tolC gene of VT1169 was found to be 99.9% similar to the sequence of the HK1651 strain (http://www.oralgen.lanl.gov/; Gene ID: AA02077) and the IDH781 strain (Crosby & Kachlany, 2007). The gene was amplified using high-fidelity polymerase (Roche, Mannheim, Germany), cloned into the Topo vector (Invitrogen, Carlsbad, CA), and treated with restriction enzymes XhoI and EcoRI (New England Biolabs, Ipswich, MA). The fragment 5'-XhoI-tolC-EcoRI-3' was gel-purified (QIAGEN Inc., Valencia, CA), ligated (Invitrogen, Carlsbad, CA) with the vector pKM2/ltxP treated with the same enzymes, and dephosphorylated using shrimp alkaline phosphatase (USB, Cleveland, OH). The ligation mix was transformed into Top10 cells, and colonies were selected on LB agar plates with 20 µg/ml chloramphenicol. The new construct pKM2/ltxP/tolC was transformed into the A. actinomycetemcomitans rmlC mutant. The rmlC/tolC+ strain was selected on the TSBYE plate containing 1 µg/ml chloramphenicol and 50 µg/ml spectinomycin.
Liquid chromatography/mass spectrometry (LC/MS)-based, label-free, relative quantification of TolC
Equivalent amounts of outer membrane fractions from the wild type, the rmlC mutant, and the TolC overexpression strain (rmlC/tolC+) were dissolved in electrophoresis loading buffer, boiled for 5 min and loaded into a 5–15% gradient polyacrylamide-SDS gel, with a 3% stacking gel. Electrophoresis was performed at 60 V, 4 °C for 24 h. The gel was fixed, stained with colloidal blue (Invitrogen, Carlsbad, CA), and destained in deionized water. The bands containing TolC were excised, washed, reduced and alkylated. The hydrated gel slices were treated with trypsin, and analyzed by ion trap LC-MS. The trypsin-generated peptide fingerprints were scanned by the mass spectrometer, with a mass-to-charge ratio between 400 and 1,600 Dalton. The generated peptide spectrum was compared with the TolC protein sequence of an A. actinomycetemcomitans serotype b strain (HK1651) found in the oral pathogen database (http://www.oralgen.lanl.gov). The relative amount of TolC protein in each sample was quantified based on the total spectral counts (Zybailov et al., 2005; Daly et al., 2011), which were matched with the TolC protein sequence in the database. The proteomic relative quantification was duplicated. The LC/MS was performed at the Vermont Genetics Network proteomics facility located at the University of Vermont.
RESULTS
Characterization of sugar residuals of the rhamnose mutant strain (rmlC)
The impact of O-PS on protein secretion was investigated in a strain with a mutation in the gene coding for the TDP-4-keto-6-deoxy-d-glucose 3,5-epimerase (rmlC). Carbohydrate analysis of isolated LPS from the rmlC mutant strain revealed differences in the sugar composition of the O-PS when compared with the wild-type strain (Table 3). The serotype b O-PS of A. actinomycetemcomitans consist of trisaccharide repeating units, and each unit is composed of l-rhamnose (l-Rha), d-fucose (d-Fuc) and d-N-acetyl galactosamine (d-GalNAc) (Perry et al., 1996). Neither Rha nor GalNAc was detected, and the mole percentage of Fuc was reduced 75% when compared with the wild-type LPS. The changes in O-PS sugars could be restored to wild-type levels when the mutant strain was transformed with the wild-type rmlC gene in trans (Tang & Mintz, 2010).
TABLE 3.
Glycosyl composition analysis
| Glycosyl residues | Mole percentage (%) 1 |
|
|---|---|---|
| Wild type | rmlC mutant | |
| Ribose(Rib) | 8.1 | 8.7 |
| Rhamnose (Rha) | 20.5 | n.d. |
| Fucose (Fuc) | 15.7 | 3.9 |
| Galactose (Gal) | 4.2 | 4.8 |
| Glucose (Glc) | 21.9* | 36.2* |
| N Acetyl Galactosamine (GalNAc) | 2.2 | n.d. |
| N Acetyl Glucosamine (GlcNAc) | 12.8 | 16.4 |
| Heptose (Hep) | 14.7 | 30.0 |
| Total amount | 100 | 100 |
The values are mole percentage of total carbohydrate.
The most predominant glycosyl residues
Secretion of Omp34, Aae, and leukotoxin in the rmlC mutant strain
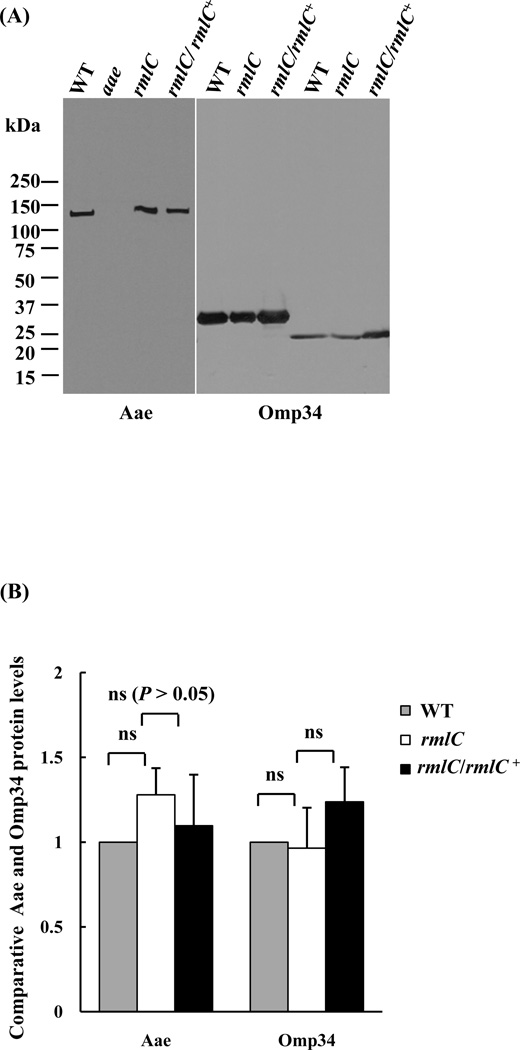
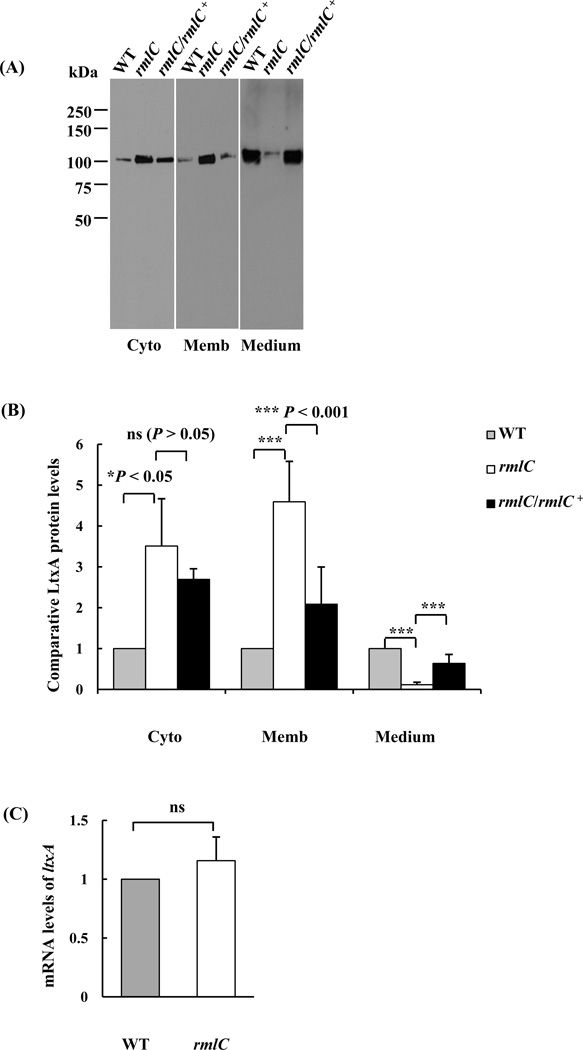
Three proteins representing different secretion pathways in A. actinomycetemcomitans (Omp34, Aae, and Ltx), were examined for the impact of the O-PS defect on secretion. As demonstrated in Figure 1, no difference in the relative immunostaining intensity of Omp34 in the outer membrane was observed in the rmlC mutant strain, as compared with the wild type strain. A similar result was observed for the epithelial cell adhesin Aae (Fig.1). However, the relative amount of leukotoxin in the different bacterial cellular compartments (cytoplasm, membrane and culture medium) was altered, as compared with the wild-type strain (Figs. 2A and B). The leukotoxin detected in the membrane fraction of the rmlC mutant was ~4-fold higher than the wild-type strain (Figs. 2A and B). Concurrent with the increase of leukotoxin in the cytoplasm and membrane, the amount of leukotoxin detected in the culture medium of the rmlC mutant decreased to 12% of the wild-type strain (Figs. 2A and B). The altered leukotoxin secretion pattern in the mutant was restored to the wild type by complementation of the rmlC mutant (Figs. 2A and B). The change in the relative protein level of leukotoxin was not associated with transcriptional changes of the leukotoxin structural gene (ltxA). The transcription of ltxA was found to be similar in the rmlC mutant, when compared to the wild-type strain, as determined by quantitative real-time PCR (Fig. 2C). Taken together, these results suggest that the TolC-dependent T1SS appears to be impacted in the rmlC mutant, and the O-antigen polysaccharide is necessary for leukotoxin secretion in A. actinomycetemcomitans.
Figure 1. Analysis of outer membrane proteins.
(A). Outer membrane proteins were separated by SDS-PAGE, transferred to the PVDF membrane, and probed with specific antibodies for the indicated proteins. Proteins were detected from the wild type (WT), aae mutant (aae), rmlC mutant (rmlC), and the rmlC complemented strain (rmlC/rmlC+). Omp34 migrates at a molecular mass of 34 kDa, when the protein is heated at 100 °C; and migrates at 29 kDa, when the protein is denatured at room temperature (Komatsuzawa et al., 1999). (B). Quantification of Aae and Omp34 proteins. The integrated signal intensity of each band shown in panel A, which represents the relative protein level, was quantified using the ImageJ program and analyzed using One-way ANOVA. The signal intensity of the wild-type strain was arbitrarily set as 1.0. (ns: nonsignificant).
Figure 2. Analysis of leukotoxin in the cytoplasm, membrane, and growth medium.
(A). Immunoblot analysis. Equivalent amounts of proteins from the cytoplasm (Cyto), membrane (Memb) and culture medium (Medium) of the wild type (WT), the rmlC mutant (rmlC) and the complemented strain (rmlC/rmlC+) were separated, transferred to PVDF, and probed with anti-LtxA antibody. (B). Quantification of leukotoxin (LtxA). The integrated intensity of each band in panel A, which represents the relative protein level, was quantified using the ImageJ program and analyzed using One-way ANOVA. The signal intensity of the wild type in each fraction was arbitrarily set as 1.0. (C). Quantitative, real-time, reverse transcriptional-PCR analysis of ltxA mRNA levels. The relative mRNA levels of ltxA were calculated based on the threshold cycle (CT): relative expression level= 2−ΔΔCT with ΔΔCT=CT(ltxA)-CT(16s rRNA)-CT(calibrator). The wild-type strain VT1169 was chosen as the calibrator. (ns: nonsignificant, *P<0.05, ***P< 0.001)
Susceptibility to antimicrobial compounds
The TolC-mediated influx/efflux system actively transports vancomycin and the internalization of vancomycin mediated by TolC is required for its antibiotic activity (Wandersman & Létoffé, 1993). To examine if the TolC-mediated influx/efflux system was affected in the rmlC mutant, vancomycin-induced bacterial cell death was examined. The rmlC mutant strain showed a significantly (P < 0.05) higher resistance to vancomycin (MIC: 262.5 +/− 12.5 µg/ml) when compared with the wild-type strain (MIC: 212.5 +/− 12.5 µg/ml). In contrast, the rmlC mutant showed similar susceptibility to bile salt (MIC: 8.5 +/− 0.5 mg/ml) and erythromycin (5.0 +/− 1.25 μg/ml) when compared with the wild type strain. The increased vancomycin resistance in the rmlC mutant of A. actinomycetemcomitans suggests that the influx/efflux function of TolC is altered in the mutant.
Quantification of outer membrane-associated TolC
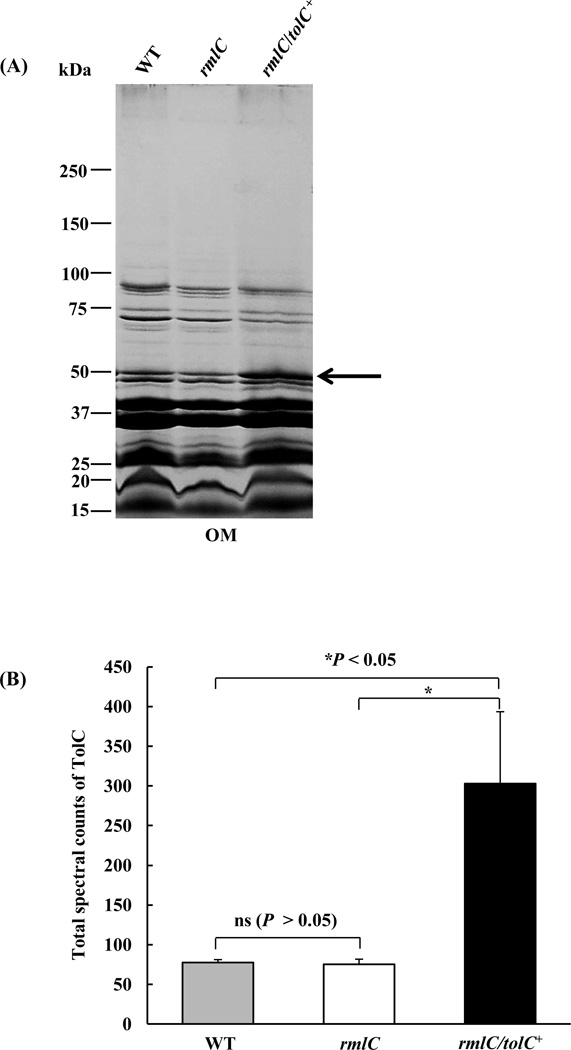
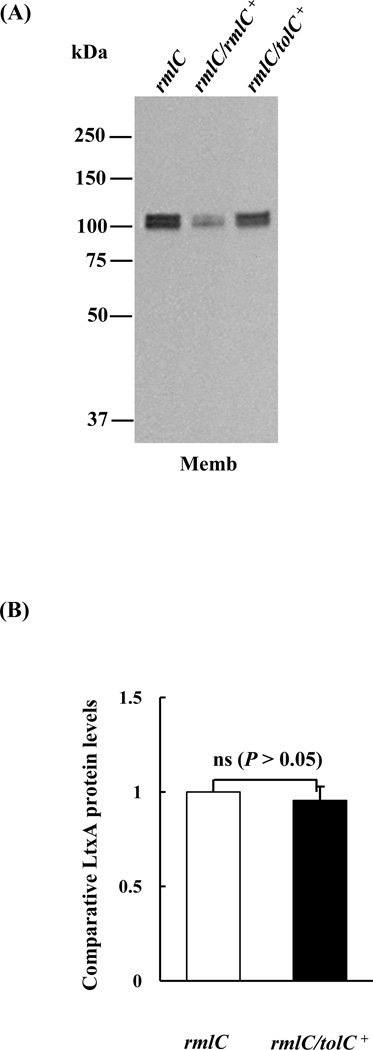
The reduction in leukotoxin secretion and the increase in resistance to vancomycin in the rmlC mutant indicated that the transportation of leukotoxin via the channel-forming protein TolC may be impaired in the O-PS mutant. To address the question whether the impact on secretion is due to altered biological activities or the decreased amount of TolC, the relative amount of TolC in the membrane of the mutant and wild-type strains was quantified using a LC/MS-based, label-free, proteomic approach, due to the lack of anti-A. actinomycetemcomitans TolC antibodies. Outer membrane proteins from these two strains were separated by SDS-PAGE and the bands corresponding to TolC (~51 kDa) were excised and analyzed by mass spectroscopy (Fig. 3A). The amount of TolC in the outer membrane of the mutant and wild type strain was equivalent (Fig. 3B). A two-fold increase in the amount of TolC in the outer membrane was observed in the rmlC mutant transformed with tolC on a replicating plasmid (Fig. 3B). The overexpression of TolC in this background did not rescue the leukotoxin secretion phenotype of the rmlC mutant strain (Figs. 4A and B). The data indicates that the decrease in leukotoxin secretion in the rmlC mutant strain was not dependent on the amount of TolC in the membrane.
Figure 3. Liquid chromatography/mass spectrometry (LC/MS)-based, label-free quantification of TolC.
(A). Colloidal blue-stained SDS-polyacrylamide gel and LC/MS analysis. Equivalent amounts of outer membrane (OM) proteins of the wild type (WT), the rmlC mutant, and the rmlC mutant with overexpression of TolC (rmlC/tolC+) were separated by SDS-polyacrylamide gels, stained with colloidal blue, and the bands corresponding to TolC (shown by arrow) were excised, reduced, alkylated, digested and analyzed using ion trap LC/MS. (B). Relative quantification of TolC proteins using total spectral counts. The amount of TolC protein in each sample was quantified based on the total number of peptides generated by LC/MS and matched with the TolC protein sequence. (ns: nonsignificant, *P<0.05)
Figure 4. Detection of leukotoxin in the rmlC mutant strain overexpressing TolC.
(A). Immunoblot analysis. Equivalent amounts of outer membrane (OM) protein of the rmlC mutant (rmlC), the rmlC complemented strain (rmlC/rmlC+), and the TolC overexpression strain (rmlC/tolC+) were separated, transferred to PVDF, and probed with anti-LtxA antibody. (B). Quantification of leukotoxin (LtxA). The integrated intensity of each band in panel A, which represents the relative protein level, was quantified using the ImageJ program. The membrane-bound leukotoxin found in the rmlC/tolC+ strain remained the same as the rmlC mutant. The signal intensity of the rmlC mutant was arbitrarily set as 1.0. (ns: nonsignificant)
DISCUSSION
Lipopolysaccharide, the predominant molecule on the bacterial surface, is composed of a hydrophobic domain (lipid A), non-repeating core oligosaccharides, and distal O-PS. While lipid A is critical for the onset of immune responses to Gram-negative bacteria via activation of TLR4 (Raetz & Whitfield, 2002), O-PS is reported to be involved in protein secretion, including the alpha-haemolysin of E. coli, the proteases of Erwinia chrysanthemi (Wandersman & Létoffé, 1993) and virulence factors of Yersinia spp. (Bengoechea et al., 2004). However, the role of O-PS in the secretion of virulent determinants in A. actinomycetemcomitans has not been investigated. This study suggests the composition or the structure of the O-polysaccharide impacts selective protein secretion in A. actinomycetemcomitans.
The dTDP-4-keto-6-deoxy-d-glucose 3,5-epimerase (rmlC) converts the precursor dTDP-4-keto-6-deoxy-d-glucose to dTDP-4-keto-l-rhamnose, which is further reduced to dTDP-l-rhamnose. The dTDP-4-keto-6-deoxy-d-glucose is also the substrate for dTDP-4-keto-6-deoxy-d-glucose reductase (fcd), which is metabolized to dTDP-d-fucose (Yoshida et al., 1999). The absence of Rha in the LPS isolated from the rmlC mutant confirmed the inactivation of dTDP-4-keto-6-deoxy-d-glucose 3,5-epimerase. The d-GalNAc moiety of the O-PS is linked to the O-3 position of l-Rha (Perry et al., 1996). Therefore, the absence of GalNAc in the LPS of the rmlC mutant might be attributed to the inability of this sugar to covalently bond to the O-PS backbone in the absence of Rha. The sugar components of the core oligosaccharides were found to be similar to the wild-type LPS. Together, the data indicated that the composition and structure of the O-PS in the rmlC mutant were altered, as compared to the wild type O-PS.
The change in the O-PS had little effect on the amount of selected outer membrane proteins, including Omp34, Aae or TolC. We did, however, observe an increase in the amount of leukotoxin associated with the membrane and a concurrent decrease in the amount of toxin in the bacterial growth medium. The reversion to a wild-type secretion profile by complementation of the rmlC mutant strain with a plasmid expressing dTDP-4-keto-6-deoxy-d-glucose 3,5-epimerase supports the thesis that leukotoxin secretion is indirectly dependent on this enzyme.
The change in the leukotoxin secretion profile was not attributed to alteration in ltxA transcription, which suggested a posttranscriptional mechanism to describe this defect. In E. coli, the secretion of α-haemolysin is reduced in a rfaH mutant, a strain producing LPS with the core oligosaccharides lacking hexose (Wandersman & Létoffé, 1993). Although the mutations in these two bacteria targeted different moieties of LPS, the impact of the LPS defect on toxin secretion appeared to be similar. In E. coli, however, the change in α-haemolysin secretion is attributed to the reduction in the amount of TolC present in the outer membrane (Wandersman & Létoffé, 1993). This is in contrast to our observation that the amount of membrane-associated TolC in the rmlC mutant did not differ from the wild-type strain (Fig. 4). Furthermore, overexpression of TolC in the membrane of the rmlC mutant did not revert to the wild-type leukotoxin secretion profile (Fig. 4). However, different membrane isolation and solubilization methods were employed in this study, as compared with Wandersman et al. (Wandersman & Létoffé, 1993), which may have resulted in different interpretations. In addition, the difference in the membrane morphology of these bacteria, convoluted in A. actinomycetemcomitans (Gallant et al., 2008) versus smooth in E. coli, may also be a contributing factor. Nonetheless, our data indicate that the leukotoxin secretion defect in this LPS mutant of A. actinomycetemcomitans was not dependent on the amount of TolC in the membrane.
Multidrug influx/efflux machineries are associated with TolC (Wandersman & Létoffé, 1993; Koronakis et al., 2004; Crosby & Kachlany, 2007). In the E. coli system, the reduction in hemolysin secretion was associated with an increase in the resistance to vancomycin (Wandersman & Létoffé, 1993), a glycopeptide that inhibits peptidoglycan biosynthesis of Gram-positive bacteria (Reynolds, 1989). Vancomycin, due to its large molecular size (1,486 Da), cannot passively diffuse across the outer membrane of Gram-negative bacteria; instead, it is actively transported via the influx/efflux system mediated by TolC (Wandersman & Létoffé, 1993). The increase in vancomycin resistance of the rmlC mutant of A. actinomycetemcomitans suggests a defect in the influx/efflux activity of TolC, since the amount of TolC in the membrane is unaffected by the mutation.
TolC or TdeA in A. actinomycetemcomitans facilitates the influx/efflux of antimicrobials (Crosby & Kachlany, 2007). The MIC of two selected agents used in this study, bile salt and erythromycin, were equally effective in inhibiting the growth of the wild-type and the rmlC mutant strains. Bile salt and erythromycin are the most sensitive antimicrobials in differentiating tolC mutations in A. actinomycetemcomitans (Crosby & Kachlany, 2007) and Vibrio cholera (Bina & Mekalanos, 2001) from the wild-type strains. The apparent activity of TolC for these antibiotics may be attributed to the smaller size of these antimicrobials, as compared with vancomycin. The crystal structure of TolC suggests that the protein acts as a gating mechanism, which has an “open” or “closed” state (Eswaran et al., 2003; Koronakis et al., 2004). The change in O-PS may influence the structure of the protein to limit the pore size of the “open” state and allow only for the passage of low molecular weight compounds and exclude molecules such as leukotoxin and vancomycin. However, we cannot exclude the possibility that other membrane protein(s) may be altered in the rmlC mutant, which lead to changes in the microenvironment of the membrane resulting in a structural perturbation of the transport system.
In summary, this study suggests that the O-PS components of LPS are necessary for secretion of leukotoxin in A. actinomycetemcomitans. Intact O-PS appear to be important for the maintenance of an environment for the membrane integration of a competent TolC-dependent leukotoxin secretion apparatus.
ACKNOWLEDGMENTS
We thank Edward Lally, University of Pennsylvania, for providing the anti-LtxA antibodies and Teresa Ruiz and Silvana Fabiola Parussini, for critical review of the manuscript. We also acknowledge Bin Deng for the Mass spectrometric analysis (Vermont Genetics Network, P20 RR16462 from the INBRE program of the National Center for Research Resources). This research was supported by NIH-NIDCR grant RO1-DE13824 awarded to K.P.M.
REFERENCES
- Bengoechea JA, Najdenski H, Skurnik M. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol Microbiol. 2004;52:451–469. doi: 10.1111/j.1365-2958.2004.03987.x. [DOI] [PubMed] [Google Scholar]
- Bina JE, Mekalanos JJ. Vibrio cholerae tolC is required for bile resistance and colonization. Infect Immun. 2001;69:4681–4685. doi: 10.1128/IAI.69.7.4681-4685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulieris PV, Behrens S, Holst O, Kleinschmidt JH. Folding and insertion of the outer membrane protein OmpA is assisted by the chaperone Skp and by lipopolysaccharide. J Biol Chem. 2003;278:9092–9099. doi: 10.1074/jbc.M211177200. [DOI] [PubMed] [Google Scholar]
- Cortelli JR, Aquino DR, Cortelli SC, Fernandes CB, de Carvalho-Filho J, Franco GC, Costa FO, Kawai T. An etiological study analyzing initial colonization of periodontal pathogens in oral cavity. J. Clin. Microbiol. 2008;46:1322–1329. doi: 10.1128/JCM.02051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JA, Kachlany SC. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene. 2007;388:83–92. doi: 10.1016/j.gene.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Baliff BA, Jaworski DM, Allen GB, Weiss D. Initial Binding and Re-Cellularization of De-Cellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2011.0301. ahead of print. doi:10.1089/ten. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran J, Hughes C, Koronakis V. Locking TolC entrance helices to prevent protein translocation by the bacterial type I export apparatus. J Mol Biol. 2003;327:309–315. doi: 10.1016/s0022-2836(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Fath MJ, Kolter R. ABC transporter: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C, Fletcher G, Wulff JL, Earhart CF. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-laurayl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KP, Tang HY, Brown AC, Kieba IR, Speicher DW, Boesze-Battaglia K, Lally ET. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Mol Oral Microbiol. 2011;26:262–276. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetti-Jardim E, Jr, Marcelino SL, Feitosa AC, Romito GA, Avila-Campos MJ. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 2009;58:1568–1575. doi: 10.1099/jmm.0.013383-0. [DOI] [PubMed] [Google Scholar]
- Gallant CV, Sedic M, Chicoine EA, Ruiz T, Mintz KP. Membrane morphology and leukotoxin secretion are associated with a novel membrane protein of Aggregatibacter actinomycetemcomitans. J Bacteriol. 2008;190:5972–5980. doi: 10.1128/JB.00548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Fujimaru T, Ogino A, Takano T, Sekijima T, Kagimoto S, Eto Y. Lung abscess caused by infection of Actinobacillus actinomycetemcomitans. Pediatr Int. 2009;51:748–751. doi: 10.1111/j.1442-200X.2009.02899.x. [DOI] [PubMed] [Google Scholar]
- Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredell JR, Stroeher UH, Ward HM, Manning PA. Lipopolysaccharide O-antigen expression and the effect of its absence on virulence in rfb mutants of Vibrio cholerae O1. FEMS Immunol Med Microbiol. 1998;20:45–54. doi: 10.1111/j.1574-695X.1998.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Jain S, van Ulsen P, Benz I, Schmidt MA, Fernandez R, Tommassen J, Goldberg MB. Polar localization of the autotransporter family of large bacterial virulence proteins. J Bacteriol. 2006;188:4841–4850. doi: 10.1128/JB.00326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt JH. Folding kinetics of the outer membrane proteins OmpA and FomA into phospholipid bilayers. Chem Phys Lipids. 2006;141:30–47. doi: 10.1016/j.chemphyslip.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Komatsuzawa H, Asakawa R, Kawai T, Ochiai K, Fujiwara T, Taubman MA, Ohara M, Kurihara H, Sugai M. Identification of six major outer membrane proteins from Actinobacillus actinomycetemcomitans. Gene. 2002;288:195–201. doi: 10.1016/s0378-1119(02)00500-0. [DOI] [PubMed] [Google Scholar]
- Komatsuzawa H, Kawai T, Wilson ME, Taubman MA, Sugai M, Suginaka H. Cloning of the gene encoding the Actinobacillus actinomycetemcomitans serotype b OmpA-like outer membrane protein. Infect Immun. 1999;67:942–945. doi: 10.1128/iai.67.2.942-945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu Rev Biochem. 2004;73:467–489. doi: 10.1146/annurev.biochem.73.011303.074104. [DOI] [PubMed] [Google Scholar]
- Koronakis V, Hughes C, Koronakis E. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR, Taichman NS, Rosenbloom J, Rosenbloom JC, Gibson CW, Demuth DR. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J Biol Chem. 1989;264:15451–15456. [PubMed] [Google Scholar]
- Lally E, Kieba I, Demuth D, Rosenbloom J, Golub E, Taichman N, Gibson C. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989;159:256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- Löe H, Brown LJ. Early onset periodontitis in the United States of America. J Periodontol. 1991;62:608–616. doi: 10.1902/jop.1991.62.10.608. [DOI] [PubMed] [Google Scholar]
- Mintz KP. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology. 2004;150:2677–2688. doi: 10.1099/mic.0.27110-0. [DOI] [PubMed] [Google Scholar]
- Mintz KP, Fives-Taylor PM. Identification of an immunoglobulin Fc receptor of Actinobacillus actinomycetemcomitans. Infect Immun. 1994:4500–4505. doi: 10.1128/iai.62.10.4500-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. In: Isolation of outer membranes. Bacterial Pathogenesis: Selected Methods in Enzymology. Clark V, Bavoil P, editors. San Diego: Academic Press; 1997. pp. 113–122. [Google Scholar]
- Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. Actinobacillus actinomycetemcomitans endocarditis. Clin Microbiol Infect. 2004;10:98–118. doi: 10.1111/j.1469-0691.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- Perry M, MacLean LL, Gmur R, Wilson ME. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect Immun. 1996;64:1215–1219. doi: 10.1128/iai.64.4.1215-1219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- Rose JE, Meyer DH, Fives-Taylor PM. Aae, an autotransporter involved in adhesion of Actinobacillus actinomycetemcomitans to epithelial cells. Infect Immun. 2003;71:5284–5293. doi: 10.1128/IAI.71.5.2384-2393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Demuth DR, Zekavat A. Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect Immun. 2006;74:2080–2092. doi: 10.1128/IAI.74.4.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinian G, Shimizu T, Sugar C, Slots J, Chen C. Periodontopathic bacteria in young healthy subjects of different ethnic backgrounds in Los Angeles. J Periodontol. 2002;73:283–288. doi: 10.1902/jop.2002.73.3.283. [DOI] [PubMed] [Google Scholar]
- Stanley PL, Diaz P, Bailey MJ, Gygi D, Juarez A, Hughes C. Loss of activity in the secreted form of Escherichia coli haemolysin caused by an rfaP lesion in core lipopolysaccharide assembly. Mol Microbiol. 1993;10:781–787. doi: 10.1111/j.1365-2958.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Stepanović S, Tosić T, Savić B, Jovanović M, K'ouas G, Carlier JP. Brain abscess due to Actinobacillus actinomycetemcomitans. APMIS. 2005;113:225–228. doi: 10.1111/j.1600-0463.2005.apm1130312.x. [DOI] [PubMed] [Google Scholar]
- Straatsma TP, Soares TA. Characterization of the outer membrane protein OprF of Pseudomonas aeruginosa in a lipopolysaccharide membrane by computer simulation. Proteins. 2009;74:475–488. doi: 10.1002/prot.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Saito M, Tsuzukibashi M, Kawashima Y, Ishida S, Hirasawa M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol. 2010;25:200–206. doi: 10.1111/j.2041-1014.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- Taichman NS, Dean RT, Sanderson CJ. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Kitten T, Munro CL, Wellman GC, Mintz KP. EmaA, a Potential Virulence Determinant of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Infective Endocarditis. Infect Immun. 2008;76:2316–2324. doi: 10.1128/IAI.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Mintz KP. Glycosylation of the collagen adhesin EmaA of Aggregatibacter actinomycetemcomitans is dependent upon the lipopolysaccharide biosynthetic pathway. J Bacteriol. 2010;192:1395–1404. doi: 10.1128/JB.01453-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979;25:427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C, Létoffé S. Involvement of lipopolysaccharide in the secretion of Escherichia coli alpha-haemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993;7:141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Nakano Y, Nezu T, Yamashita Y, Koga T. A novel NDP-6-deoxyhexosyl-4-ulose reductase in the pathway for the synthesis of thymidine diphosphate-D-fucose. J Biol Chem. 1999;274:16933–16939. doi: 10.1074/jbc.274.24.16933. [DOI] [PubMed] [Google Scholar]
- Yu C, Ruiz T, Lenox C, Mintz KP. Functional mapping of an oligomeric autotransporter adhesin of Aggregatibacter actinomycetemcomitans. J Bacteriol. 2008;190:3098–3109. doi: 10.1128/JB.01709-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G, Kaplan JB, Furgang D, Mansfield KG, Fine DH. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and old world primates. Infect Immun. 2007;75:4440–4448. doi: 10.1128/IAI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol. 2010;59:143–151. doi: 10.1111/j.1574-695X.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]