Abstract
Behçet’s disease is characterized by recurrent oral ulcers, genital ulcers, uveitis and skin lesions. Myelodysplastic syndrome (MDS) is characterized by problems due to ineffective hematopoiesis. Several studies have identified a relationship between MDS and Behçet’s disease, especially intestinal Behçet’s disease. Trisomy 8 seems to play an important role in these disorders as well. The present case was a 24-year-old woman who had a huge tonsil ulcer with initial symptoms of odynophagia and intermittent fever. We also noted folliculitis on her upper back. Five days later, she began to experience diarrhea and abdominal pain. Abdominal computed tomography and subsequent surgery revealed ileum perforation and enterocolitis with multiple ulcers. Later, she was admitted again for a vulvar suppurative ulcer and suspicious Bartholin’s cyst infection. The patient’s clinical presentations met the criteria for Behçet’s disease. Six months after the bowel perforation event, we noted the development of pancytopenia in a routine laboratory examination. All the examinations led to the diagnosis of MDS with trisomy 8. The most unusual finding was that multiple large vessel thrombi developed during follow-up. Previous studies have suggested that trisomy 8 in MDS leads to concurrent intestinal Behçet’s disease. Moreover, the inflammatory and immune genes related to thrombus formation are overexpressed in cases of MDS with trisomy 8. Trisomy 8 must play a role in thrombosis. Further studies are needed to help clarify the pathophysiology and pathogenesis of these disorders.
Keywords: Behçet’s disease, Myelodysplastic syndrome, Trisomy 8, Intestinal ulcers, Thrombosis
INTRODUCTION
Behçet’s disease is a multisystem inflammatory disease characterized by recurrent oral ulcers, genital ulcers, uveitis, and skin lesions. Many other systems can be involved, such as the gastrointestinal tract, central nervous system and cardiovascular system; the disease can also cause arthritic joints.
Myelodysplastic syndrome (MDS) is a blood disease that easily converts to acute leukemia. It is characterized by stem cell disorders, multi-lineage dysplasia, and pancytopenia due to ineffective hematopoiesis.
Behçet’s disease and MDS are two different disease entities. However, an association between the two diseases has been reported in an increasing number of cases. Most of the patients who suffer from the two diseases have intestinal ulcers. Some previous studies have also identified a statistically significant relationship between trisomy 8 and intestinal Behçet’s disease with MDS.
We report the case of a patient with trisomy 8 who was diagnosed with intestinal Behçet’s disease and MDS. We incidentally found multiple thrombi in the major veins.
CASE REPORT
A 24-year-old woman was admitted to our hospital due to odynophagia and intermittent fever for 1 wk. The initial findings were a huge tonsil ulcer with a pus-like coating. We also noted several spots of folliculitis on her upper back. The laboratory examination revealed the following: white cell count 12.5 × 103/mm3, red blood cell count 3.80 × 106/mm3, hemoglobin 14.1 g/dL, platelet count 150 × 103/mm3, alanine aminotransferase 24 IU/L, creatinine 0.9 mg/dL, Na 141 mEq/L and K 3.3 mEq/L. She was initially treated for acute suppurative tonsillitis. However, the symptoms persisted after the administration of antibiotics. Five days later, she began to experience diarrhea, abdominal pain and dyspnea. We arranged for abdominal computed tomography (CT), and the results showed ileus, edematous bowels, right-side colon dilation, ascites and free air. She then underwent an operation, and ileum perforation and enterocolitis with multiple ulcers were found (Figure 1). The pathology report identified multiple ulcers with transmural necrotizing inflammation in the colon and ileum.
Figure 1.
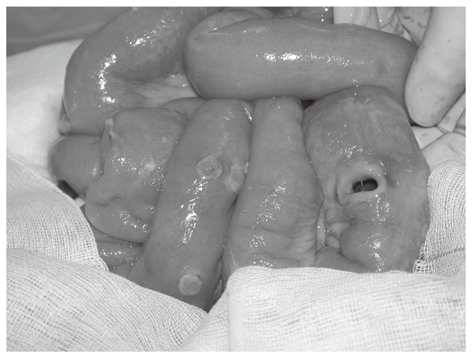
Ileum perforation and multiple transmural ulcers.
About 2 mo later, the patient returned to our hospital due to increased vaginal discharge, itching and pain. Fever and chills followed these symptoms. She was admitted again for a vulvar suppurative ulcer and a suspicion of Bartholin’s cyst infection. After antibiotic treatment, these symptoms improved, and she then received outpatient clinical follow-up care.
The patient’s clinical presentations met the International Study Group’s criteria for Behçet’s disease; these include recurrent oral ulcers (> 3 times in a year), frequent genital ulcers (twice in the past 3 mo), folliculitis on the upper back and multiple bowel ulcers with perforation.
Three months after the second hospitalization, the patient was admitted again due to adhesion ileus. Unexpectedly, abdominal CT found thrombi in the patient’s bilateral internal iliac vein, common iliac vein and inferior vena cava (Figure 2); the multiple thrombi were not noted in the previous CT scan.
Figure 2.
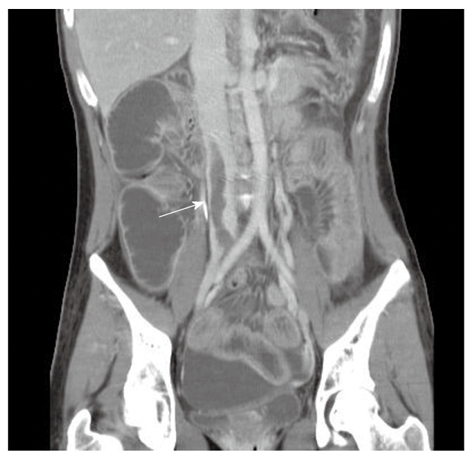
Abdominal computed tomography image shows a dilated bowel loop and a large thrombus (arrow) in the inferior vena cava.
Six months after the bowel perforation event, we noted the development of pancytopenia in a routine laboratory examination. The initial hemogram showed the following: white cell count 1.0 × 103/mm3, red blood cell count 1.45 × 106/mm3, hemoglobin 5.8 g/dL and platelet count 13 × 103/mm3. The differential count of the white blood cells was as follows: neutrophil-seg 18%, neutrophil-band 13%, lymphocytes 62%, monocytes 1%, eosinophils 2%, metamyelocytes 2% and atypical lymphocytes 62%. Bone marrow biopsy was performed for further evaluation and revealed profound hypocellularity of the marrow with a marked decrease of trilinear hematopoietic elements and focal aggregates of lymphoplasmacytic cells. Cytogenetic analysis demonstrated the presence of two cell lines: 48, XX, +8, +9 (11)/46, XX (9). Of the 20 cells examined, 11 showed an abnormal female karyotype with extra 8 and 9 chromosomes, and the remaining nine cells had normal, complete sets of chromosomes. These findings led to the diagnosis of MDS and refractory anemia. The overall picture was compatible intestinal Behçet’s disease with MDS and chromosomal abnormality.
DISCUSSION
Behçet’s disease is characterized by recurrent oral ulcers, genital ulcers, uveitis, and skin lesions. Other systems can be involved, such as the gastrointestinal tract, central nervous system and cardiovascular system, and the disease can lead to arthritic joints. The exact etiology and pathogenesis of Behçet’s disease are still being investigated. From previous studies, we know that genetic factors play key roles in its pathogenesis. MDS, however, is characterized by stem cell disorders, multi-lineage dysplasia and pancytopenia due to ineffective hematopoiesis.
Behçet’s disease is generally considered to be an immunological disease. MDS is related to many immunological abnormalities, and presently, several types of immunomodulatory therapies, such as antithymocyte globulin and cyclosporin A[1], have been used to treat MDS. These two diseases are currently thought to share some immunological characteristics. For example, extensive intramedullary cell death in an MDS patient is proposed to be strongly related to tumor necrosis factor (TNF)-α. In addition, the concentration of TNF and soluble TNF receptors is increased in the serum of patients with active Behçet’s disease[2]. We conclude that some correlation exists in the pathogenesis of the two diseases.
The co-occurrence of Behçet’s disease and MDS has been reported in an increasing number of cases[3]. Most of the patients who suffer from the two diseases have intestinal ulcers. In light of the previous literature, intestinal Behçet’s disease is believed to be partly derived from MDS[4-7], and most of these patients have trisomy 8. Kimura et al[6] have identified a statistically significant relationship between trisomy 8 and intestinal Behçet’s disease with MDS.
Chromosomal abnormalities are observed in about 40% of patients with MDS, but trisomy 8 is found in only about 5% of the MDS population[8]. The high frequency of trisomy 8 in cases of intestinal Behçet’s disease complicated with MDS suggests that trisomy 8 plays an important role in the pathogenesis of intestinal ulcers in the context of Behçet’s disease.
Most cases of trisomy 8 with intestinal Behçet’s disease complicated with MDS have been reported in Japan. A single case or a few cases have been reported in Italy, Korea, the United States, Germany, Spain, Israel and the United Kingdom[3]. We reported the case of a young woman who was diagnosed with intestinal Behçet’s disease with MDS and trisomy 8. There are many compositions of trisomy 8; our patient was 48, XX, +8, +9, which is one of the karyotypes reported previously.
The most unusual finding in our reported case was that multiple large vessel thrombi developed during follow-up. Thrombosis related to Behçet’s disease usually occurs in veins, and involvement of the arteries is less common[9]. Our patient developed thrombi in the bilateral internal iliac veins, common iliac veins and inferior vena cava. Thrombosis related to Behçet’s disease is found more frequently in men[9], although our patient was a young woman.
Kimura et al[6] have found that MDS patients with trisomy 8 tend to develop thrombosis and intestinal ulcers, but no definite cause for the vessel thrombus has been identified. Other researchers have noted neutrophil function abnormality and inflammatory cytokine overproduction in cases of MDS[10,11]. These immunological disorders, particularly cytokine overproduction, may lead to injury and inflammation of the endothelium.
Chen et al[12] have found that inflammatory and immune genes, such as transforming growth factor (TGF)-β, TGF-β receptor, interleukin (IL)-10, IL-7 receptor and vascular cell adhesion molecule (VCAM)-1, are overexpressed in MDS patients with trisomy 8. The exact role of TGF-β is controversial. Some researchers have suggested that it exacerbates neointima formation by inhibiting endothelial regeneration and promoting fibrosis, and some have shown that it protects against lipid lesion formation in atherosclerosis[13]. IL-10 and IL-7 are thought to contribute to the formation of atherosclerosis[14,15]. VCAM-1 has the ability to facilitate thrombus formation.
These previous studies have suggested that the presence of trisomy 8 in MDS leads to the patient having concurrent intestinal Behçet’s disease. Moreover, the inflammatory and immune genes related to thrombus formation are overexpressed in cases of MDS with trisomy 8. Trisomy 8 must play a role in blood vessel thrombosis. We therefore hypothesize that trisomy 8 induces the activation of an abnormal inflammatory process and immune gene expression, eventually leading to or even aggravating blood vessel injury and thrombus formation. This may explain why our patient, with Behçet’s disease complicated with MDS and trisomy 8, developed multiple vessel thrombi. In the near future, trisomy 8 may become a helpful predictor of Behçet’s disease prognosis or outcome, especially in the case of intestinal ulcers or blood vessel thrombosis. Further studies are needed to help clarify the pathophysiology and pathogenesis of these disorders.
Footnotes
Peer reviewer: Francis Seow-Choen, MBBS, FRCSEd, FAMS, Professor, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, 3 Mt Elizabeth Medical Centre #09-10, 228510 Singapore, Singapore
S- Editor Shi ZF L- Editor Kerr C E- Editor Zheng XM
References
- 1.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 2.Turan B, Gallati H, Erdi H, Gürler A, Michel BA, Villiger PM. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Bechçet’s disease; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–132. [PubMed] [Google Scholar]
- 3.Kawabata H, Sawaki T, Kawanami T, Shimoyama K, Karasawa H, Fukushima T, Masaki Y, Ogawa N, Hirose Y, Ozaki K, et al. Myelodysplastic syndrome complicated with inflammatory intestinal ulcers: significance of trisomy 8. Intern Med. 2006;45:1309–1314. doi: 10.2169/internalmedicine.45.1718. [DOI] [PubMed] [Google Scholar]
- 4.Yano K, Eguchi K, Migita K, Takashima H, Tamura M, Izumino K, Sasagawa I, Sadamori N, Nagataki S. Behcet’s disease complicated with myelodysplastic syndrome: a report of two cases and review of the literature. Clin Rheumatol. 1996;15:91–93. doi: 10.1007/BF02231696. [DOI] [PubMed] [Google Scholar]
- 5.Ohno E, Ohtsuka E, Watanabe K, Kohno T, Takeoka K, Saburi Y, Kikuchi H, Nasu M. Behçet’s disease associated with myelodysplastic syndromes. A case report and a review of the literature. Cancer. 1997;79:262–268. doi: 10.1002/(sici)1097-0142(19970115)79:2<262::aid-cncr9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Kimura S, Kuroda J, Akaogi T, Hayashi H, Kobayashi Y, Kondo M. Trisomy 8 involved in myelodysplastic syndromes as a risk factor for intestinal ulcers and thrombosis--Behçet’s syndrome. Leuk Lymphoma. 2001;42:115–121. doi: 10.3109/10428190109097683. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, Kuroda T, Inada M, Yamamoto M, Enomoto H, Kishima Y, Yoshida K, Ito H, Ogawa H, Nakamura H. Intestinal Behçet’s disease associated with myelodysplastic syndrome with chromosomal trisomy 8--a report of two cases and a review of the literature. Hepatogastroenterology. 2001;48:416–420. [PubMed] [Google Scholar]
- 8.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 9.Düzgün N, Ateş A, Aydintuğ OT, Demir O, Olmez U. Characteristics of vascular involvement in Behçet's disease. Scand J Rheumatol. 2006;35:65–68. doi: 10.1080/03009740500255761. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HC, Lee YM, Tsai WH, Jiang ML, Ho CH, Ho CK, Wang SY. Circulating levels of thrombopoietic and inflammatory cytokines in patients with acute myeloblastic leukemia and myelodysplastic syndrome. Oncology. 2002;63:64–69. doi: 10.1159/000065722. [DOI] [PubMed] [Google Scholar]
- 11.Martin S, Baldock SC, Ghoneim AT, Child JA. Defective neutrophil function and microbicidal mechanisms in the myelodysplastic disorders. J Clin Pathol. 1983;36:1120–1128. doi: 10.1136/jcp.36.10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Zeng W, Miyazato A, Billings E, Maciejewski JP, Kajigaya S, Sloand EM, Young NS. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood. 2004;104:4210–4218. doi: 10.1182/blood-2004-01-0103. [DOI] [PubMed] [Google Scholar]
- 13.Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- 14.Cagnin S, Biscuola M, Patuzzo C, Trabetti E, Pasquali A, Laveder P, Faggian G, Iafrancesco M, Mazzucco A, Pignatti PF, et al. Reconstruction and functional analysis of altered molecular pathways in human atherosclerotic arteries. BMC Genomics. 2009;10:13. doi: 10.1186/1471-2164-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishihira K, Imamura T, Yamashita A, Hatakeyama K, Shibata Y, Nagatomo Y, Date H, Kita T, Eto T, Asada Y. Increased expression of interleukin-10 in unstable plaque obtained by directional coronary atherectomy. Eur Heart J. 2006;27:1685–1689. doi: 10.1093/eurheartj/ehl058. [DOI] [PubMed] [Google Scholar]


