Abstract
Six new cembranolides, michaolides L–Q (1–6), and a known cembranolide, lobomichaolide (7) were isolated from the CH2Cl2 extract of the soft coral Lobophytum michaelae. Their structures were established by extensive spectral analysis. The anti-HCMV (human cytomegalovirus) activity of 1–7 and their cytotoxicity against selected cell lines were evaluated.
Keywords: Lobophytum michaelae, cembranolides, cytotoxicity
1. Introduction
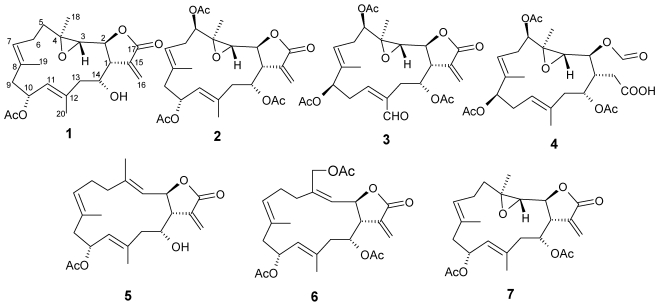
Soft corals of the genus Lobophytum (Alcyoniidae) have been reported as a rich source of secondary metabolites endowed with a range of structural diversity and various biological activities [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Previous bioassay results of some cembranoids and their analogues have demonstrated remarkable pharmacological potential such as cytotoxicity against various cancer cell lines [2,3,4,5,6,7,8,9], anti-inflammatory properties [11,12,13], antimicrobial activities [11], and HIV-inhibitory activity [14]. In previous papers [2,3,4], we reported the isolation of several cytotoxic cembranolides, lobomichaolide, crassolide, and michaolides A–K from samples of the soft coral Lobophytum michaelae Tixier-Durivault (Alcyoniidae) (Figure 1). In this report, a new specimen of the soft coral L. michaelae was studied since the CH2Cl2 solubles exhibited significant cytotoxity against HT-29 (human colon adenocarcinoma) and P-388 (mouse lymphocytic leukemia) cell lines as determined by standard procedures. [25,26] Bioassay-guided fractionation of the extract resulted in the isolation of six new cembranolides, michaolides L–Q (1–6), together with the known cembranolide, lobomichaolide (7) [2,3] (Figure 2).
Figure 1.
Soft coral Lobophytum michaelae.
Figure 2.
Structures of compounds 1–7.
2. Results and Discussion
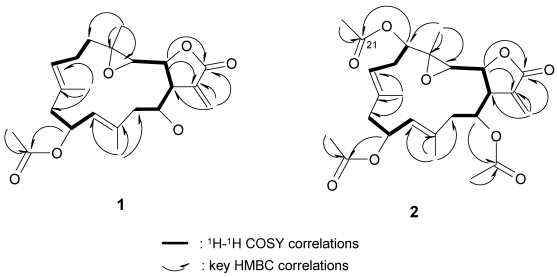
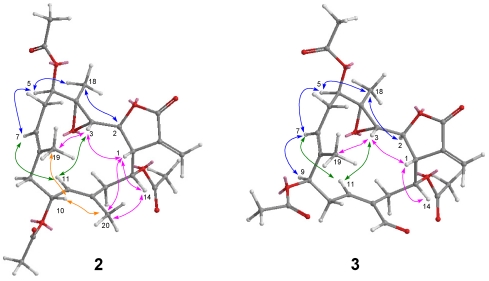
Michaolide L (1) was isolated as a colorless oil, [α]D25 +13.3 (c 0.1, CHCl3). HRESIMS, 13C NMR, and DEPT spectroscopic data established the molecular formula of 1 as C22H30O6. The IR spectrum of 1 indicated the presence of the functionalities of ester group(s) (νmax 1734 cm−1) and an α-methylene-γ-lactone (νmax 1766, 1668 cm−1). The presence of the α-methylene-γ-lactone system in 1 was also demonstrated by UV absorption at 222 (log ε 3.68) nm and signals at δ 5.67 (H-16) and 6.44 (H-16) in the 1H NMR spectrum (Table 1). The 1H NMR spectrum of 1 also showed signals for two olefinic protons at δ 5.65 (H-11), and 5.19 (H-7) ppm; four oxymethine protons at δ 4.46 (t, J = 6.8 Hz, H-2), 2.70 (d, J = 6.8 Hz, H-3), 5.64 (m, H-10), and 4.38 (m, H-14); one methine proton at δ 2.90 (m, H-1), two olefinic methyl groups at δ 1.55 (H3-19) and 1.72 (H3-20); and a methyl group in acetate ester at δ 2.05. HMBC spectrum exhibited a methyl-bearing trisubstituted epoxide [δH 2.70 (d, J = 6.8 Hz, H-3), 1.42(H3-18); δC 59.6 (CH), 64.0 (qC), 20.4 (CH3)] (Table 1). The spectral data of 1 indicated some similarities to those of lobomichaolide (7) [2,3], except for the data due to C-14. The H1–H1 COSY spectrum exhibited correlations from H-13 to H-3, H-5 to H-7, and H-9 to H-11. 1H–1H long-range correlations were also observed between H-1 to H2-16, H-7 to H3-19, and H-11 to H3-20. These spectroscopic findings and the nine degrees of unsaturations indicated that 1 was a 14-membered cembrane-type diterpene skeleton with an α-methylene-γ-lactone.
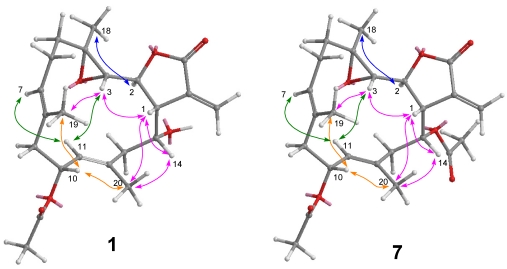
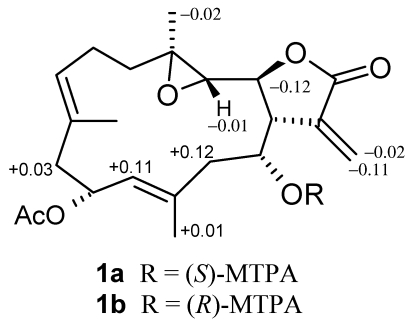
After assignments between all the C–H bondings were made based on an HSQC experiment, the planar structure was determined by HMBC analysis. The correlations according to HMBC are shown in Figure 3. The stereochemistry for the trisubstituted olefins of 1 was determined by NOESY analysis. The NOESY correlations between H-7 and H-9, and H-11 and H-13 disclosed the E configurations for the trisubstituted olefins. The chemical shift values at δC 15.6 and 15.9 (for C-19 and C-20, respectively) also supported the E configurations [2,3]. The NOESY correlations (Figure 4) observed between H-3 and H-1/ H-11/H3-19, H-14 and H-1/H3-20, H-7 and H-9/ H-11, H-10 and H3-20/H3-19, and H3-18 and H-2 indicated the relative configurations for the 14-membered ring carbons, which were identical to those of lobomichaolide (7). Analysis of the ΔδS–R values (Figure 5) according to the Mosher model pointed to an R configuration for C-14 of 1, because H2-13, H-11, and Me-20 of (S)-MTPA ester 1a were less shielded by the phenyl ring of MTPA products. Therefore, the absolute stereochemistry of Michaolide L (1) was established as (1R,2S,3S,4R,10S,14R,7E,11E)-10-acetoxy-14-hydroxy-3,4-epoxycembra-7,11-dien-17,2-olide ambiguously.
Figure 3.
COSY and HMBC correlations of compounds 1 and 2.
Table 1.
1H and 13C NMR data for compounds 1–3.
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) a | δCb | δH (J in Hz) a | δCb | δH (J in Hz) a | δCb | |
| 1 | 2.90 m | 48.1 | 3.07 m | 44.1 | 2.97 m | 45.2 |
| 2 | 4.46 t (6.8) c | 76.7 | 4.68 t (6.8) | 75.2 | 4.52 t (6.8) | 75.3 |
| 3 | 2.70 d (6.8) | 59.6 | 2.83 d (6.8) | 60.3 | 2.76 d (6.8) | 59.9 |
| 4 | 64.0 | 62.7 | 63.4 | |||
| 5 | 1.91 m | 23.8 | 4.83 dd (10.8, 3.2) | 75.5 | 5.05 dd (11.2, 3.6) | 74.3 |
| 6 | 2.03 m, 2.18 m | 33.5 | 2.21 m, 2.42 m | 30.3 | 2.33 m, 2.54 m | 29.1 |
| 7 | 5.19 br d (8.0) | 129.5 | 5.10 t (5.6) | 122.5 | 5.46 t (6.4) | 121.7 |
| 8 | 127.5 | 132.3 | 131.3 | |||
| 9 | 2.18 m, 2.45 m | 44.7 | 2.38 m | 44.5 | 5.25 br s | 75.3 |
| 10 | 5.64 m | 67.8 | 5.67 m | 68.4 | 2.82 m, 3.27 m | 29.4 |
| 11 | 5.65 m | 127.8 | 5.45 m | 128.1 | 6.73 dd (9.6, 4.4) | 147.0 |
| 12 | 137.5 | 135.8 | 137.8 | |||
| 13 | 2.48 m, 2.91 m | 44.2 | 2.37 m, 2.51 m | 41.8 | 2.28 m, 2.89 m | 34.7 |
| 14 | 4.38 m | 68.6 | 5.40 m | 71.1 | 5.77 m | 69.3 |
| 15 | 136.7 | 134.9 | 136.1 | |||
| 16 | 5.67 m, 6.44 d (2.8) | 122.3 | 5.72 s, 6.40 s | 124.0 | 5.75 d (2.8), 6.39 d (2.8) | 124.7 |
| 17 | 169.4 | 168.9 | 168.4 | |||
| 18 | 1.42 s | 20.4 | 1.25 s | 14.7 | 1.49 s | 15.7 |
| 19 | 1.55 s | 15.6 | 1.66 s | 16.5 | 1.67 s | 12.9 |
| 20 | 1.72 s | 15.9 | 1.83 s | 16.0 | 10.14 s | 190.3 |
| 5-OAc | 2.01 s | 170.1 | 2.13 s | 170.1 | ||
| 21.1 | 21.2 | |||||
| 9-OAc | 2.13 s | 169.8 | ||||
| 20.8 | ||||||
| 10-OAc | 2.05 s | 170.5 | 2.05 s | 170.2 | ||
| 21.4 | 21.2 | |||||
| 14-OAc | 2.11 s | 170.3 | 2.02 s | 169.8 | ||
| 21.3 | 20.5 |
a 400 MHz in CDCl3 (assigned by COSY, HSQC, and HMBC experiments); b 100 MHz in CDCl3 (assigned by DEPT, COSY, HSQC, and HMBC experiments); c J values (Hz) in parentheses.
Figure 4.
NOESY correlations of compounds 1 and 7.
Figure 5.
Absolute stereochemistry of 1: ΔδS–R values in ppm for MTPA esters 1a and 1b.
Michaolide M (2) was shown to have the molecular formula of C24H34O9 by HRESIMS and from its 13C NMR data. The 1H and 13C NMR spectral data (Table 1) of 2 closely resembled those of 7 except for the signals at C-5. 1H–1H COSY cross peak (Figure 3) between H-5 and H-6/H-7 as well as HMBC correlations (Figure 3) between H-5 and C-6/C-4/C-21 revealed the presence of an additional acetoxyl [δH 4.83 (dd, J = 10.8, 3.2, H-5), δC 75.5 (CH, C-5), 170.1 (qC), 21.2 (CH3)] at C-5 in 2. NOESY correlations (Figure 6) between H-5 and H-7, H-3 and H-1/ H-11/H3-19, H-14 and H-1/H3-20, H-7 and H-9/H-11, H-10 and H3-20/H3-19, and H3-18 and H-2 indicated the relative configurations for 2 resembled those of 7 except for the additional C-5 (R) acetoxy.
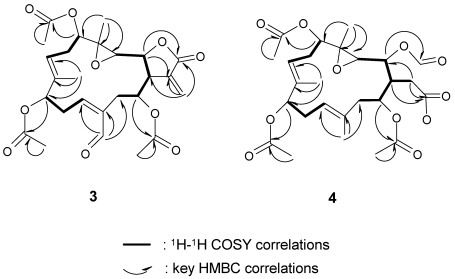
Michaolide N (3) analyzed for C26H32O10 from its HRESIMS and NMR spectroscopic data. The NMR features of compound 3 were analogous to those of 2 with exception that the secondary acetoxyl attached to C-10 was shifted to C-9 and the methyl attached to C-12 was replaced by an aldehyde [δH 10.14, δC 190.3] (Table 1). 1H–1H COSY cross peaks (Figure 6) between H-9 and H-10/H-11 as well as HMBC correlations (Figure 7) between H-20 and C-11/C-12/C-13 as well as between H3-19 and C-7/C-8/C-9 helped to ascertain these assignments. The relative stereochemistry of 3 was determined by NOESY correlations (Figure 6) between H-7 and H-5/H-9/H-11, H-3 and H-1/H-11, H-14 and H-1/H-20, H-10 and H-20, H3-18 and H-2, and H-11 and H2-13.
Figure 6.
NOESY correlations of compounds 2 and 3.
Figure 7.
COSY and HMBC correlations of compounds 3 and 4.
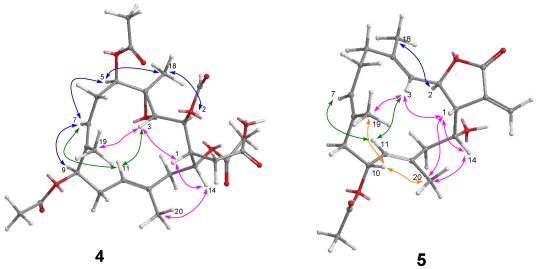
Michaolide O (4) had the molecular formula, C26H36O11. Detailed comparison of the 1H and 13C NMR spectral data (Table 2) of 4 and 3 revealed that 4 differed from 3 at C-20 and the α-exo-methylene-γ-lactone moiety. A COSY correlation (Figure 7) from H-1 to H2-15 and HMBC correlations (Figure 7) from H2-15 to C-16/C-2 and from H-2 toC-17 revealed that the α-exo-methylene-γ-lactone moiety in 3 was oxidized to a formyloxyl (δH 8.27 s, δC 161.6) at C-2 and carboxylmethyl at C-1 in 4. The relative stereochemistry of 4 was determined by NOESY correlations (Figure 8) between H-7 and H-5/H-9/H-11, H-3 and H-1/H-11/H3-19, H-14 and H-1/H3-20, H3-18 and H-2, and H-11 and H2-13.
Table 2.
1H and 13C NMR data for compounds 4–6.
| Position | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) a | δCb | δH (J in Hz) a | δCb | δH (J in Hz) a | δCb | |
| 1 | 2.61 m | 43.2 | 2.68 br s | 48.0 | 2.79 m | 46.1 |
| 2 | 4.52 t (6.8)c | 76.4 | 5.38 dd (8.8, 2.7) | 73.5 | 5.53 dd (8.7, 6.8) | 72.5 |
| 3 | 2.82 d (6.8) | 60.2 | 5.03 d (8.8) | 123.3 | 5.18 m | 127.2 |
| 4 | 63.4 | 140.0 | 140.6 | |||
| 5 | 4.89 dd (10.8, 3.6) | 75.1 | 2.30 m | 24.1 | 2.33 m | 24.1 |
| 6 | 2.82 m, 2.55 m | 29.8 | 2.21 m | 37.9 | 2.36m, 2.40 m | 33.1 |
| 7 | 5.34 m | 120.1 | 4.96 m | 129.7 | 4.93 m | 128.8 |
| 8 | 133.5 | 130.6 | 131.1 | |||
| 9 | 5.16 m | 76.6 | 2.32 m, 2.39 m | 44.5 | 2.29 m, 2.40 m | 44.5 |
| 10 | 2.37 m, 2.48 m | 41.8 | 5.65 m | 68.5 | 5.66 m | 68.0 |
| 11 | 5.30 m | 123.4 | 5.16 d (9.1) | 126.6 | 5.19 m | 127.7 |
| 12 | 131.7 | 134.5 | 136.8 | |||
| 13 | 2.31 m, 2.68 m | 43.8 | 2.21 m, 2.43 m | 45.5 | 2.28 m, 2.53 m | 41.8 |
| 14 | 5.32 m | 69.2 | 4.15 m | 72.9 | 5.28 m | 74.0 |
| 15 | 3.75m, 3.85 m | 35.2 | 136.8 | 136.6 | ||
| 16 | 175.6 | 5.65 s, 6.41 s | 122.7 | 5.70 s, 6.37 s | 124.4 | |
| 17 | 8.26 s | 161.6 | 167.8 | 167.3 | ||
| 18 | 1.48 s | 15.7 | 1.80 s | 17.3 | 4.55 d (12.6), 4.91 d (12.6) | 62.3 |
| 19 | 1.60 s | 12.3 | 1.64 s | 16.7 | 1.61 s | 16.6 |
| 20 | 1.74 s | 15.6 | 1.75 s | 16.3 | 1.81 s | 16.0 |
| 5-OAc | 2.11 s | 170.1 | ||||
| 21.1 | ||||||
| 9-OAc | 2.11 s | 170.3 | ||||
| 21.2 | ||||||
| 10-OAc | 2.04 s | 170.0 | 2.01 s | 170.2 | ||
| 21.4 | 21.0 | |||||
| 14-OAc | 2.12 s | 170.3 | 2.02 s | 170.8 | ||
| 21.3 | 21.3 | |||||
| 18-OAc | 2.03 s | 169.3 | ||||
| 20.9 | ||||||
| 16-OH | 6.18 brs |
a 400 MHz in CDCl3 (assigned by COSY, HSQC, and HMBC experiments); b 100 MHz in CDCl3 (assigned by DEPT, COSY, HSQC, and HMBC experiments); c J values (Hz) in parentheses.
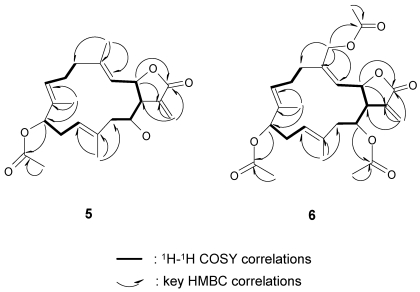
Michaolide P (5) was shown to have the molecular formula of C22H30O5 by HRESIMS and from its 13C NMR data. The 1H and 13C NMR spectral data (Table 2) of 5 closely resembled those of 1 except for the replacement of the trisubstituted epoxy by a trisubstituted olefin at Δ3. HMBC correlations (Figure 9) between H3-18 and C-3/C-4/C-5 confirmed the presence of a trisubstituted olefin at C-3. The relative stereochemistry was determined by NOESY correlations (Figure 8) between H-3 and H-1/H-11/H3-19, H-14 and H-1/H3-20, H-7 and H-9/ H-11, H-10 and H3-20/H3-19, and H3-18 and H-2.
Figure 8.
NOESY correlations of compounds 4 and 5.
Figure 9.
COSY and HMBC correlations of compounds 5 and 6.
Michaolide Q (6) had the molecular formula of C26H34O8 by HRESIMS and from its 13C NMR data. The 1H and 13C NMR spectroscopic data (Table 2) of 6 closely resembled those of 5 except for the signals at C-18 and C-14. The low field chemical shift and HMBC correlations (Figure 9) between H2-18 and C-3/C-4/C-5/C-21 confirmed the presence of an acetoxy group at C-18. HMBC correlations (Figure 8) between H-14 and C-22 revealed the presence of a second acetoxy group at C-14. The relative stereochemistry was determined by NOESY correlations between H-3 and H-1/H-11/H3-19, H-14 and H-1/H3-20, H-7 and H-9/H-11, H-10 and H3-20/H3-19, and H3-18 and H-2.
The cytotoxicity toward P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), A-549 (human lung epithelial carcinoma) tumor cells, and human embryonic lung (HEL) cells of michaolides L–Q (1–6) and lobomichaolide (7) were shown in Table 3. Non-cytotoxic cembranoid, michaolide O (4) was tested for anti-HCMV activity and showed a negative result (IC50 > 200 μM/mL). The α-exo-methylene-γ-lactone moiety is important for cytotoxicity by comparing the cytotoxicity of 4 with those of 1–3, 5, and 6 [4]. The absolute stereochemistry of the known cembranolide, lobomichaolide (7) [2,3] should be drawn as in Figure 2 since cembranolides 1 and 7 both exhibited positive optical rotations.
Table 3.
Cytotoxicityof 1–7.
| Compounds | Cell Lines ED50 (μM/mL) | |||
|---|---|---|---|---|
| A549 | HT-29 | P-388 | HEL | |
| 1 | 1.2 | 0.8 | 0.3 | 1.0 |
| 2 | 2.0 | 4.9 | 1.5 | 3.2 |
| 3 | 2.1 | 1.6 | 0.4 | 2.0 |
| 4 | 61.3 | 61.5 | 39.6 | 60.2 |
| 5 | 3.2 | 2.8 | 2.0 | 2.9 |
| 6 | 2.0 | 1.5 | 1.0 | 1.8 |
| 7 | 1.9 | 1.4 | 0.4 | 1.7 |
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were determined with a JASCO P1020 digital polarimeter. Ultraviolet (UV) and infrared (IR) spectra were obtained on JASCO V-650 and JASCO FT/IR-4100 spectrophotometers, respectively. NMR spectra were recorded on a Varian MR 400 NMR spectrometer at 400 MHz for 1H and 100 MHz for 13C. 1H NMR chemical shifts are expressed in δ (ppm) referred to the solvent peaks δH 7.27 for CDCl3, and coupling constants are expressed in Hz. 13C NMR chemical shifts are expressed in δ (ppm) referred to the solvent peaks δC 77.0 for CDCl3. ESI-MS were recorded by ESI FT-MS on a Bruker APEX II mass spectrometer. Silica gel 60 (Merck, Germany, 230–400 mesh) and LiChroprep RP-18 (Merck, 40–63 μm) were used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254s plates (Merck) were used for thin-layer chromatography (TLC) analysis. High-performance liquid chromatography (HPLC) was carried out using a Hitachi L-7100 pump equipped with a Hitachi L-7400 UV detector at 220 nm together with a semi-preparative reversed-phase column (Merck, Hibar LiChrospher RP-18e, 5 μm, 250 × 25 mm).
3.2. Biological Material
The soft coral L. michaelae Tixier-Durivault (Alcyoniidae) was collected at Ken-Ting, Ping-Tong County, Taiwan, in June 2002, at a depth of 3–4 m and was stored for 2 weeks in a freezer until extraction. Identification was kindly verified by Prof. Keryea Soong, Institute of Marine Biology, National Sun Yat-sen, Taiwan. A voucher specimen, MR-004, was deposited in the Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Taiwan.
3.3. Extraction and Isolation
The bodies of the soft coral L. michaelae were freeze dried to give 1.10 kg of a solid, which was extracted with CH2Cl2 (3.0 L × 3). After removal of solvent in vacuo, the residue (20 g) was chromatographed over Si gel 60 using n-hexane and n-hexane/EtOAc mixtures of increasing polarity. Elution with n-hexane/EtOAc (49:1) gave fractions containing 7, with n-hexane/EtOAc (9:2) gave fractions containing 2 and 6, with n-hexane/EtOAc (5:2) gave fractions containing 3, with n-hexane/EtOAc (3:2) gave fractions containing 4, with n-hexane/EtOAc (1:1) gave fractions containing 1 and 5. Compounds 1–7 were further purified by RP-18 HPLC eluting with MeOH/H2O (50:50), MeOH/H2O (76:24), MeOH/H2O (70:30), MeOH/H2O (66:34), MeOH/H2O (50:50), MeOH/H2O (76:24), and MeOH/H2O (78:22), respectively.
Michaolide L (1): White amorphous powder (5 mg); [α]D25 +13.3 (c 0.1, CHCl3); UV λmax (MeOH) nm (log ε): 222 (3.68); IR (neat) νmax 3412, 1766, 1734, 1668 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 413.1916 [M + Na]+ (calcd. for C22H30O6Na, 413.1914).
Preparation of Mosher’s Esters of 1. In separate NMR tubes, duplicate (1.0 mg) samples of 5 were dissolved in 0.6 mL of pyridine-d5 and allowed to react for 3 h at room temperature with (R)- and (S)-MTPA chloride (one drop) to yield (S)-MTPA ester 1a and (R)-MTPA ester 1b, respectively. Selected 1H NMR (pyridine-d5, 300 MHz) of 1a: 1H NMR (CDCl3, 300 MHz): δ 1.35 (3H, s, H3-18), 1.51 (3H, s, H3-19), 1.98 (3H, s, H3-20), 2.08 (3H, s, 10-OAc), 2.32 (1H, dd, J = 12.1, 8.9 Hz, H-9), 2.56 (1H, d, J = 12.1 Hz, H-9), 2.86 (2H, m, H-13), 3.02 (1H, d, J = 6.1 Hz, H-3), 3.54 (3H, s, OMe), 4.57 (1H, t, J = 6.9 Hz, H-2), 5.22 (1H, br d, J = 8.2 Hz, H-7), 5.93 (1H, d, J = 8.4 Hz, H-11), 6.05 (1H, d, J = 2.8 Hz, H-16), 6.12 (1H, ddd, J = 10.8, 6.0, 1.5 Hz, H-14), 6.47 (1H, d, J = 3.2 Hz, H-16), 7.41–7.61 (5H, m, Ph). Selected 1H-NMR (pyridine-d5, 300 MHz) of 1b: 1H NMR (CDCl3, 300 MHz): δ 1.37 (3H, s, H3-18), 1.52 (3H, s, H3-19), 1.97 (3H, s, H3-20), 2.08 (3H, s, 10-OAc), 2.32 (1H, dd, J = 12.3, 9.4 Hz, H-9), 2.53 (1H, d, J = 12.3 Hz, H-9), 2.74 (2H, m, H-13), 3.03 (1H, d, J = 6.8 Hz, H-3), 3.53 (3H, s, OMe), 4.69 (1H, t, J = 6.6 Hz, H-2), 5.20 (1H, br d, J = 6.9 Hz, H-7), 5.82 (1H, d, J = 8.8 Hz, H-11), 6.14 (1H, d, J = 2.2 Hz, H-16), 6.58 (1H, dd, J = 11.4, 3.8 Hz, H-14), 6.58 (1H, d, J = 3.2 Hz, H-16), 7.42–7.62 (5H, m, Ph).
Michaolide M (2): White amorphous powder (3 mg); [α]D25 +11.2 (c 0.1, CHCl3); UV λmax (MeOH) nm (log ε): 221 (3.67); IR (neat) νmax 1765, 1736, 1728, 1669 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 489.2102 [M + Na]+ (calcd. for C24H34O9Na, 489.2101).
Michaolide N (3): White amorphous powder (1 mg); [α]D25 +7.6 (c 0.1, CHCl3); UV λmax (MeOH) nm (log ε): 220 (3.76); IR (neat) νmax 2820, 2730, 1765, 1736, 1726, 1669 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 1; HRESIMS m/z 527.1885 [M + Na]+ (calcd. for C26H32O10Na, 527.1884).
Michaolide O (4): White amorphous powder (2 mg); [α]D25 +3.1 (c 0.1, CHCl3); IR (neat) νmax 3420, 1740, 1731,, 1712, 1675 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 2; HRESIMS m/z 547.2155 [M + Na]+ (calcd. for C26H36O11Na, 547.2156).
Michaolide P (5): White amorphous powder (1 mg); [α]D25 +122.0 (c 0.1, CHCl3); UV λmax (MeOH) nm (log ε): 221 (3.96); IR (neat) νmax 3450, 1765, 1735,1666 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 2; HRESIMS m/z 397.1993 [M + Na]+ (calcd. for C22H30O5Na, 397.1992).
Michaolide Q (6): White amorphous powder (1 mg); [α]D25 +81.6 (c 0.1, CHCl3); IR (neat) νmax 1762, 1731, 1675 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data in Table 2; HRESIMS m/z 497.2154 [M + Na]+ (calcd. for C26H34O8Na, 497.2152).
Lobomichaolide (7): Colorless prism (25 mg); m.p. 180–181; [α]D25 +55.6 (c 0.1, CHCl3).
3.4. Cytotoxicity Assay
Cytotoxicity was determined on P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), and A-549 (human lung epithelial carcinoma) tumor cells using a modification of the MTT colorimetric method according to a previously described procedure [25,26]. The provision of the P-388 cell line was supported by J.M. Pezzuto, formerly of the Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago. HT-29 and A-549 cell lines were purchased from the American Type Culture Collection.
3.5. Anti-HCMV Assay
To determine the effects of natural products upon HCMV cytopathic effect (CPE), confluent human embryonic lung (HEL) cells grown in 24-well plates were incubated for 1 h in the presence or absence of various concentrations of tested natural products. Then, cells were infected with HCMV at an input of 1000 pfu (plaque forming units) per well of 24-well dish. Antiviral activity was expressed as IC50 (50% inhibitory concentration), or compound concentration required to reduce virus induced CPE by 50% after 7 days as compared with the untreated control. To monitor the cell growth upon treating with natural products, an MTT-colorimetric assay was employed [27].
4. Conclusion
The α-exo-methylene-γ-lactone moiety is important for cytotoxicity by comparing the cytotoxicity of 4 with those of 1–3, 5, and 6 [4]. The absolute stereochemistry of the known cembranolide, lobomichaolide (7) [2,3] should be drawn as in Figure 2 since cembranolides 1 and 7 both exhibited positive optical rotations.
Acknowledgments
This research was financially supported by grants from the National Science Council (NSC99-2628-B-110-002-MY3) of Taiwan awarded to C.-Y.D.
Footnotes
Samples Availability: Not Available.
References
- 1.Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2010;27:165–237. doi: 10.1039/b906091j. [DOI] [PubMed] [Google Scholar]
- 2.Wang S.-K., Duh C.-Y., Wu Y.-C., Wang Y., Cheng M.-C., Soong K., Fang L.-S. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992;55:1430–1435. doi: 10.1021/np50088a007. [DOI] [PubMed] [Google Scholar]
- 3.Wang S.-K., Duh C.-Y., Wu Y.-C., Wang Y., Cheng M.-C., Soong K., Fang L.-S. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 2009;72:324. [Google Scholar]
- 4.Wang L.-T., Wang S.-K., Soong K., Duh C.-Y. New Cytotoxic Cembranolides from the Soft Coral Lobophytum michaelae. Chem. Pharm. Bull. 2007;55:766–770. doi: 10.1248/cpb.55.766. [DOI] [PubMed] [Google Scholar]
- 5.Coval S.J., Patton R.W., Petrin J.M., James L., Rothofsky M.L., Lin S.L., Patel M., Reed J.K., McPhil A.T., Bishop W.R. A Cembranolide Diterpene Farnesyl Protein Transferase Inhibitor from the Marine Soft Coral Lobophytum cristagalli. Bioorg. Med. Chem. Lett. 1996;6:909–912. [Google Scholar]
- 6.Higuchi R., Miyamoto T., Yamada K., Komori T. Cytotoxic and ichthyotoxic compounds from marine opisthobranchia and soft coral. Toxicon. 1998;36:1703–1705. doi: 10.1016/s0041-0101(98)00163-9. [DOI] [PubMed] [Google Scholar]
- 7.Matthee G.F., Konig G.M., Wright A.D. Three new diterpenes from the marine soft coral Lobophytum crassum. J. Nat. Prod. 1998;61:237–240. doi: 10.1021/np970458n. [DOI] [PubMed] [Google Scholar]
- 8.Duh C.-Y., Wang S.-K., Huang B.-T., Dai C.-F. Cytotoxic cembrenolide diterpenes from the Formosan soft coral Lobophytum crassum. J. Nat. Prod. 2000;63:884–885. doi: 10.1021/np990620h. [DOI] [PubMed] [Google Scholar]
- 9.Chao C.-H., Hang H.-C., Wu Y.-C., Lu C.-K., Dai C.-F., Sheu J.-H. Glycolipids from the Formosan Soft Coral Lobophytum crassum. Chem. Pharm. Bull. 2007;55:1720–1723. doi: 10.1248/cpb.55.1720. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W., Krohn K., Ding J., Miao Z.-H., Zhou X.-H., Chen S.-H., Pescitelli G., Salvadori P., Kurtan T., Guo Y.-W. Structural and stereochemical studies of α-methylene-γ-lactone-bearing cembrane diterpenoids from a south China sea soft coral Lobophytum crassum. J. Nat. Prod. 2008;71:961–966. doi: 10.1021/np800081p. [DOI] [PubMed] [Google Scholar]
- 11.Lin S.-T., Wang S.-K., Cheng S.-Y., Duh C.-Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009;14:3012–3014. doi: 10.1021/ol901070e. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S.-Y., Wen Z.-H., Chiou S.-F., Wang S.-K., Hsu C.-H., Dai C.-F., Chiang M.Y., Duh C.-Y. Durumolides A–E, Anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. [Google Scholar]
- 13.Cheng S.-Y., Wen Z.-H., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Duh C.-Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009;17:3763–3769. doi: 10.1016/j.bmc.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S.-Y., Wen Z.-H., Chiou S.-F., Wang S.-K., Hsu C.-H., Dai C.-F., Chiang M.Y., Duh C.-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009;72:152–155. doi: 10.1021/np800686k. [DOI] [PubMed] [Google Scholar]
- 15.Rashid M.A., Gustafson K.R., Boyd M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000;63:531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- 16.Kashman Y., Groweiss A. Lobolide: A new epoxy cembranolide from marine origin. Tetrahedron Lett. 1977;13:1159–1160. [Google Scholar]
- 17.Kashman Y., Carmely S., Groweiss A. Further cembranoid derivatives from the Red Sea soft corals Alcyonium flaccidum and Lobophytum crassum. J. Org. Chem. 1981;46:3592–3596. [Google Scholar]
- 18.Kinamoni Z., Groweiss A., Carmely S., Kashman Y. Several new cembranoid diterpenes from three soft corals of the Red Sea. Tetrahedron. 1983;39:1643–1648. [Google Scholar]
- 19.Bowden B.F., Coll J.C., Tapiolas D.M. Studies of Australian Soft Corals. ХХХШ. New cembranoid diterpenes from a Lobophytum species. Aust. J. Chem. 1983;36:2289–2295. doi: 10.1071/CH9832289. [DOI] [Google Scholar]
- 20.Bowden B.F., Coll J.C., Decosta M.S.L., Desilva E.D., Mackay M.F., Mahendran M., Willis R.H. The structure determination of a new cembranolide diterpene from the soft coral Lobophytum cristigalli (Coelenterata, Octocorallia, Alcyonacea). Aust. J. Chem. 1984;34:545–552. [Google Scholar]
- 21.Bowdon B.F., Coll J.C., Heaton A., Konig G., Bruck M.A., Cramer R.E., Klein D.M., Scheuer P.J. The structures of four isomeric dihydrofuran-containing cembranoid diterpenes from several species of soft coral. J. Nat. Prod. 1987;50:650–659. doi: 10.1021/np50052a013. [DOI] [Google Scholar]
- 22.Subrahmanyam C., Rao C.V., Anjaneyulu V., Satyanarayana P., Rao P.V.S. New diterpenes from a new species of Lobophytum soft coral of the South Andaman coast. Tetrahedron. 1992;48:3111–3120. [Google Scholar]
- 23.Yamada K., Ryu K., Miyamoto T., Higuchi R. Bioactive terpenoids from octocorallia. 4. Three new cembrane-type diterpenoids from the soft coral Lobophytum schoedei. J. Nat. Prod. 1997;60:798–801. doi: 10.1021/np960728m. [DOI] [Google Scholar]
- 24.Cheng S.-Y., Chen P.-W., Chen H.-P., Wang S.-K., Duh C.-Y. New Cembranolides from the Dongsha Atoll Soft Coral Lobophytum durum. Mar. Drugs. 2011;9:1307–1318. doi: 10.3390/md9081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geran R.I., Greenberg N.H., MacDonald M.M., Schumacher A.M., Abbott B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972;3:1–91. [Google Scholar]
- 26.Hou R.-S., Duh C.-Y., Chiang M.Y., Lin C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995;58:1126–1130. doi: 10.1021/np50121a026. [DOI] [PubMed] [Google Scholar]
- 27.Stevens M., Balzarini J., Tabarrini O., Andrei G., Snoeck R., Cecchetti V., Fravolini A., De Clercq E., Pannecouque C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005;56:847–855. doi: 10.1093/jac/dki328. [DOI] [PubMed] [Google Scholar]