Abstract
Objective To examine the effectiveness of a family-based behavioral group intervention (Positively Fit; PF) for pediatric obesity relative to a brief family intervention (BFI) in a sample of treatment-seeking children and adolescents. Methods Families (n = 93) were randomized to treatment condition. Assessments were conducted at pre- and posttreatment and at 12-month follow-up. Outcome indices included standardized body mass index (BMI) and quality of life (QOL). Results Results indicated a significant reduction in zBMI at posttreatment and follow-up across both conditions. At follow-up, BFI and PF participants evidenced average reductions of .12 and .19 zBMI units, respectively. Children demonstrated better outcomes than adolescents across both conditions. Results indicated clinically significant improvements in parent-reported QOL at postintervention and in self-reported QOL at follow-up for PF participants. Conclusions Results suggest the effectiveness of family-based interventions for pediatric obesity in clinical settings among younger children. Neither intervention was effective in terms of reducing zBMI among adolescents.
Keywords: adolescents, children, obesity, quality of life, RCT, treatment
Introduction
Although there is some evidence that the prevalence of pediatric obesity may no longer be increasing (Ogden, Caroll, & Flegal, 2008; Ogden, Carroll, Curtin, Lamb, & Flegal, 2010), current rates of overweight and obesity in children and adolescents remain unacceptably high. Data provided by the National Health and Nutrition Examination Surveys (NHANES; Ogden et al., 2008, 2010) as well as the Birth Cohort of the Early Childhood Longitudinal Survey (Anderson & Whitaker, 2009) indicate that between 16% and 18% of children and adolescents in the United States are obese (BMI ≥ 95th percentile) and that >30% of youth are overweight (BMI ≥ 85th percentile). Unfortunately, children and adolescents with obesity are at much higher risk for concomitant health and mental health conditions, poorer health-related quality of life (QOL), and continued obesity and its attendant physical and mental health consequences (Jelalian & Hart, 2009).
In response to the prevalence of pediatric obesity, as well as the increasing evidence for associated health problems, the American Medical Association, the Health Resources Services Administration, and the Centers for Disease Control and Prevention convened an Expert Committee to update previous recommendations for the assessment and treatment of overweight children and adolescents (Barlow and the Expert Committee, 2007). Based on their review of the available literature, the Expert Committee identified strategies with “consistent evidence” supporting their efficacy including (a) structured dietary and physical activity changes to yield a negative energy balance, (b) behavior modification techniques to support these changes, (c) involvement of the family in lifestyle changes, (d) parental participation in therapy, and (e) frequent contact with the treatment team.
Consistent with these recommendations, Positively Fit (PF) was developed as a manualized, behaviorally-based group intervention for children with obesity and their families (Steele et al., n.d.). Building upon our earlier work in this area (Herrera, Johnston, & Steele, 2004; Johnston & Steele, 2007), the intervention focuses on the promotion of healthy lifestyle behaviors among the entire family using nutrition and exercise education and behavior modification techniques. The intervention derives from similar behaviorally based interventions (e.g., The Stoplight Diet; Epstein & Squires, 1988), but adopts a multisystemic perspective by attempting to impact individual child behaviors, family interactions, and child and parent management of other micro- and exo-systemic interactions (e.g., with peers or relatives, eating out, etc.). Furthermore, the intervention is delivered in a group format, which was expected to serve as both social support and an analog to naturally occurring peer microsystems.
Similar to our previous evaluations (Herrera et al., 2004; Johnston & Steele, 2007), and consistent with calls for pediatric interventions conducted in “real world” settings (e.g., Klesges, Dzewaltowski, & Glasgow, 2008), our ultimate goal was to examine a promising treatment protocol conducted with a high degree of internal validity in a setting and with a population that would allow a high degree of external validity. Specifically, the present investigation was a practical clinical trial (Tunis et al., 2003) of PF in comparison to a brief family intervention (BFI) in a sample of families seeking outpatient treatment for pediatric overweight and obesity. As described in more detail below, practical clinical trials may confer a number of specific advantages to the literature, including more representative samples of the population, control conditions that mirror actual clinical practice and measurement of variables that easily impact organizational and policy decisions (Glasgow, Magid, Beck, Ritzwoller, & Estabrooks, 2005).
Consistent with the previous investigations of the PF protocol, we used few exclusion criteria to screen the participants, allowing greater generalization of study findings to families seeking treatment for obesity. Writing about clinical trials in general, Glasgow et al. (2005) noted that “… most efficacy studies use stringent selection criteria, which result in study participants who are highly motivated, relatively homogeneous, and do not have comorbid illnesses” (p. 552). This generalization is consistent with much of the treatment literature on pediatric obesity (see Delamater, Jent, Moine, & Rios, 2008; Kitzmann et al., 2010; Raynor 2008, for reviews), with many studies using exclusion criteria that place constrains on the clinical populations that can be served by the literature (e.g., weight above 100% of ideal body weight, concurrent or previous psychiatric contact, parents with previous or concurrent psychiatric contact, and the presence of comorbid medical diagnoses).
Furthermore, and consistent with the concept of a practical clinical trial (Tunis et al., 2003), the current study compared the PF protocol to a “clinically relevant alternative” (p. 1626), specifically, the Trim Kids treatment program (Sothern, von Almen, & Schumacher, 2002) with support from a registered dietician. Thus, the fundamental question for this investigation was “Does this intervention work in clinical practice?” rather than “Can this intervention work under ideal circumstances?” Correspondingly, the results of this study may be particularly well suited to inform clinic and policy decisions, since the “test” intervention is compared to what clients could expect to receive in an actual treatment setting (Drotar, 2011).
Finally, and consistent with recommendations by Klesges et al. (2008), we also examined self- and parent-reported QOL as a primary outcome variable. Glasgow et al. (2005) include the measurement of QOL as a “priority” (p. 555) for practical clinical trials, with clear implications for policy and organizational decisions in relation to available treatment alternatives. Tsiros et al. (2009) have lamented the paucity of studies examining QOL in treatment outcome studies for obesity treatments. Furthermore, McGovern et al. (2008) suggested that BMI may not completely inform health risk in children with overweight and obesity and advocated for examination of treatment outcomes that are more responsive to immediate change, which may predict long-term health risk.
Based on previous literature, we hypothesized that children assigned to the PF condition would evidence greater decreases in zBMI pre- to posttreatment and at a 1-year follow-up than children assigned to the BFI condition. We also hypothesized that children and adolescents in the PF condition would evidence greater improvements in QOL than children and adolescents in the BFI condition. Beyond these primary aims, we also explored condition (PF vs. BFI) by age group (adolescent vs. preadolescent) and condition by gender effects. Given recent findings suggesting developmental differences in the role of parents’ vis-à-vis interventions for children's weight management (Janicke et al., 2008), we anticipated that the whole-family approach of PF would be more efficacious for children than for adolescents.
Methods
Study Design
The present investigation was designed as a practical clinical trial comparing PF to a BFI condition and was registered as a clinical trial with the National Institutes of Health (NIH; www.clinicaltrials.gov study number NCT00365807). Participants meeting eligibility requirements completed an initial (pretreatment) assessment, after which they were block randomized into either PF or BFI conditions by the sixth author using a random number generator. A total of 17 treatment groups comprised of three to eight families were randomized over a period of 26 months. Participants completed a posttreatment assessment within approximately 2 weeks of the final treatment session. Participants completed a follow-up assessment 1 year subsequent to the final treatment session.
Participants
Families were made aware of the study by physicians at outpatient pediatric medical clinics, school nurses in public primary and secondary schools, flyers posted in community centers, and by advertisements in newspapers or child-related newsletters. Eligibility criteria for participation in the study included: (a) the participating child or adolescent was between 7 and 17 years of age; (b) the participant's body mass index (BMI) percentile was categorized as overweight (i.e., BMI ≥ 85th percentile) or obese (i.e., BMI ≥ 95th percentile); (c) a parent was willing to participate in the intervention; (d) the participant had no reported serious mental illness (i.e., those requiring current inpatient psychiatric care) or developmental delay that would prevent participation in the group intervention; (e) the parent and child spoke English; (f) the parent provided written informed consent; and (g) the child verbally assented to participation. Children with comorbid medical or mental health conditions that did not prevent group participation (e.g., diabetes, learning disorders, mood disorders, ADHD) were not excluded from the study.
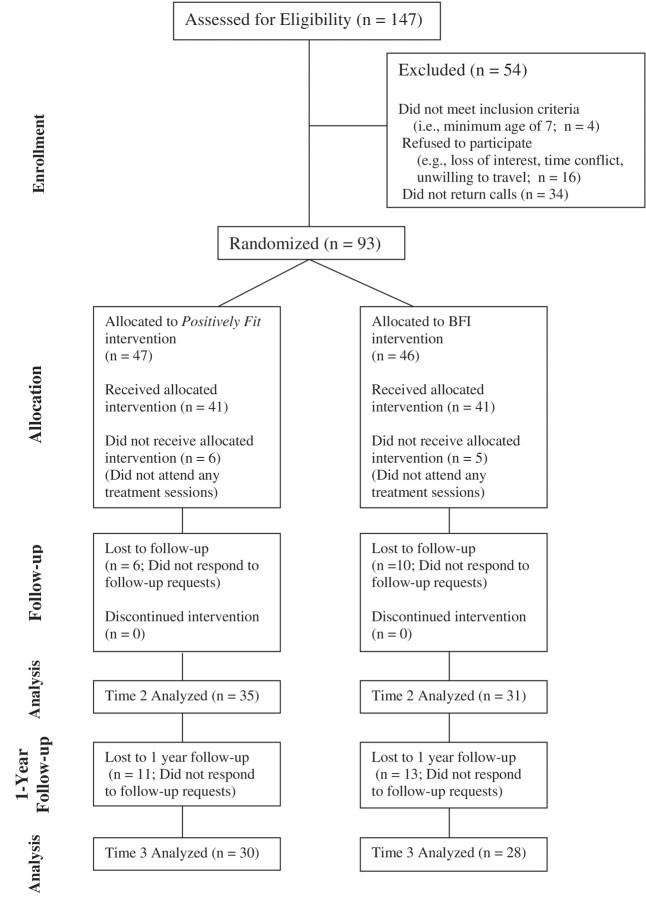
A total of 147 families were screened for eligibility, 93 of which were enrolled in the study (see Figure 1 for the CONSORT flowchart). Families were stratified by child age group (i.e., 7–12 or 13–18) and were randomized into one of two intervention conditions. Of the 93 families that were enrolled in the study, 11 did not complete any treatment sessions, 66 completed the posttreatment follow-up assessment, and 58 were available for the 1-year follow-up. These rates of participation and follow-up are similar to other studies involving children and adolescents with obesity (Jelalian et al., 2008; Zeller et al., 2004).
Figure 1.
Overview of study flow.
Power Analysis
Since the study compared the PF protocol to an alternate treatment, an appropriate effect size for an a priori power analysis was difficult to locate. As noted by Kitzmann et al. (2010), effect sizes from alternate treatment studies represent the effects of treatments relative to a wide range of comparison groups, complicating the interpretation of such effect sizes. However, previous evaluations of our protocol resulted in (within-subjects) effect sizes (Cohen's d) of between 0.93 and 1.45 (Herrera et al., 2004; Johnston & Steele, 2007). Based on these previous results, a targeted enrollment of 120 would allow detection of a moderate effect size, Cohen's d = .60, in participant zBMI scores and QOL with power greater than .995. As indicated above, participant recruitment did not achieve this targeted accrual.
Procedures
After completing an initial telephone screening to confirm eligibility, families attended a pretreatment orientation session, and, if interested, parents signed the informed consent and children completed the assent procedure. Pretreatment data were then collected from the participating child or adolescent and the parent or guardian most responsible for preparing meals for the child, after which participants were randomized to treatment. If multiple children from a single family were participating, the oldest overweight or obese child was identified as the “target” child for all study measures. Participants completed all study measures and anthropometric data were obtained by study staff following the 10-week treatment period and approximately 1 year after treatment completion. Participants received $20 for completing pre- and posttreatment assessments and $50 for completing the 1-year follow-up assessment. All procedures were approved by the Human Subjects Committee of the first author's institution.
Description of Interventions
Positively Fit
This manualized intervention (Steele et al., n.d.) is comprised of 10 weekly group treatment sessions lasting approximately 90 min each in duration. Separate sessions were held for children (7–12) and adolescents (13–17), in order to accommodate varied developmental levels. Approximately 40 min of each treatment session consisted of nutrition/physical activity education followed by 40 min of behavioral intervention, with a 10-min summary and goal-setting period at the conclusion of each session. Behavioral sessions were facilitated by two masters-level clinical psychology therapists and nutrition sessions were administered by a registered dietician. Parents and children attended separate meetings for both nutritional/physical activity education and behavioral components of the treatment and reconvened for the concluding goal-setting portion of the session. Nutritional sessions focused on understanding nutritional information and portion control, planning for special occasions, and increasing knowledge of and participation in physical activity. Behavioral treatment sessions addressed topics including stimulus control, rewards for change, modeling, goal setting, social support, and maintenance of lifestyle change.
BFI
Participants randomized to the BFI condition participated in the Trim Kids treatment program (Sothern et al., 2002). Consistent with Sothern et al.'s recommendations, families received three 60-min individual face-to-face visits with one of two registered dietitians involved in the study. These visits were approximately evenly spaced over a 10-week period. Families in this condition received the Trim Kids manual at initial (pretreatment) assessment and were instructed to read the first four chapters prior to their first meeting with the dietitian. Additional chapters were assigned at the first and second sessions. Topics discussed in the BFI condition included meal planning, basic nutritional principles, physical activity, and energy balance principles.
Treatment Fidelity
The PF behavioral treatment protocol was administered by two of four possible Masters’ level clinical psychology therapists under the supervision of a licensed clinical psychologist. Weekly group supervision sessions were conducted to ensure treatment integrity and consistency across group cohorts. All group intervention sessions were taped for supervision purposes. Approximately 40% of treatment tapes were stored and coded as a means of assessing treatment fidelity. Three independent research assistants reviewed the tapes, coding the degree to which specific treatment components were administered in each session. The average fidelity rating across all raters and sessions was 94.7%. Fidelity within each session across cohorts ranged from 83.3% to 100%.
Measures
Anthropometric Variables
Weight and height were collected once at each assessment for both children and participating parents using a calibrated electronic scale (model number SECA 813, SECA Corp., Hanover, MD, USA), and a portable stadiometer (model number SECA 214, SECA Corp.), respectively, with participants in light clothing and no footwear. Conversion of height, weight, sex, and age values to standardized BMI scores (zBMI) was performed using a SAS application provided by the Centers for Disease Control and Prevention (CDC, 2007). All anthropometric data were collected by graduate research assistants who received specific training and periodic retraining from a nurse manager/trainer at one of the participating institutions. Due to clinic staffing limitations, assessors of height and weight were not blind to participants’ treatment condition. The electronic scale and stadiometer were calibrated periodically (i.e., as recommended by the manufacturer) and in conjunction with research assistant retraining.
Quality of Life (PedsQL)
Self- and parent-reported health-related quality of life was measured using the PedsQL 4.0 Generic Core Scales (Varni, Seid, & Kurtin, 2001), which has demonstrated good reliability and validity. Previous estimates of internal consistency for both parent and child report are above 0.70 (Varni et al., 2001). Cronbach's alphas in the current sample were .87, .90, and .89 for the child report at baseline, posttreatment, and follow-up, respectively, and .92, .89, and .91 for the parent report. Minimal clinically important difference (MCID) scores (defined as the smallest difference in PedsQL domain scores that patients perceive as beneficial/detrimental and that require a change in the patient's care and management) are available for both child self-report (MCID = 4.36) and parent proxy report (MCID = 4.50; Varni, Burwinkle, Seid, & Skarr, 2003).
Results
Overview of Analysis
To assess the efficacy of PF on zBMI and child- and parent-reported QOL, a series of longitudinal multilevel models were implemented as mixed linear models. This study employed an intent-to-treat strategy (ITT) to ensure relatively unbiased estimates of treatment effects, adequate Type I error control, and realistic portrayal of clinical practice (Holmbeck, Zebracki, & McGoron, 2009). Participant attrition resulted in missing data that were accounted for statistically using Full Information Maximum Likelihood (FIML; Enders, 2001).
Descriptive Analyses
As indicated in Table I, participants fell primarily within the “obese” category (BMI > 95th percentile for age and sex), with a mean BMI percentile at study entry of 98.2 (SD = 1.79), a mean zBMI of 2.22 (SD = 0.34), and an average percent overweight of 85.15 (SD = 35.15). Additional descriptive data are presented in Table I. Neither zBMI nor mean QOL scores were significantly different across treatment groups at the initial (pretreatment) assessment. Chi-squared analyses did not indicate significant differences in ethnicity across treatment condition (p > .50). To ensure equivalence of study participants, the 11 families that dropped out before receiving any treatment were compared against all other families on zBMI, monthly income, and QOL; independent samples t-tests were not significant for any of the above variables.
Table I.
Means (SDs) and Percentages of Demographic Variables and Sample Characteristics at Study Entry, by Treatment Condition
| Overall (n = 93) | PF (n = 47) | BFI (n = 46) | |
|---|---|---|---|
| Child Age (years) | 11.57 (2.64) | 11.63 (2.48) | 11.52 (2.82) |
| Gender (% Female) | 59.1 | 53.2 | 65.2 |
| Initial zBMI | 2.22 (.34) | 2.20 (.34) | 2.24 (.36) |
| BMI percentile | 98.18 (1.79) | 98.13 (1.92) | 98.24 (1.67) |
| Percent overweight | 85.15 (35.15) | 80.66 (34.49) | 89.73 (37.20) |
| Monthly income | $4 072.54 (2724.70) | $4 295.56 (2717.66) | $3 884.74 (2752.73) |
| Ethnicity (%) | |||
| African American | 14.0 | 10.6 | 17.4 |
| European American | 71.0 | 74.5 | 67.4 |
| Latino/a | 4.3 | 2.1 | 6.5 |
| Biracial | 4.3 | 4.3 | 4.3 |
| Other | 6.5 | 8.5 | 4.3 |
Planned Analyses
zBMI
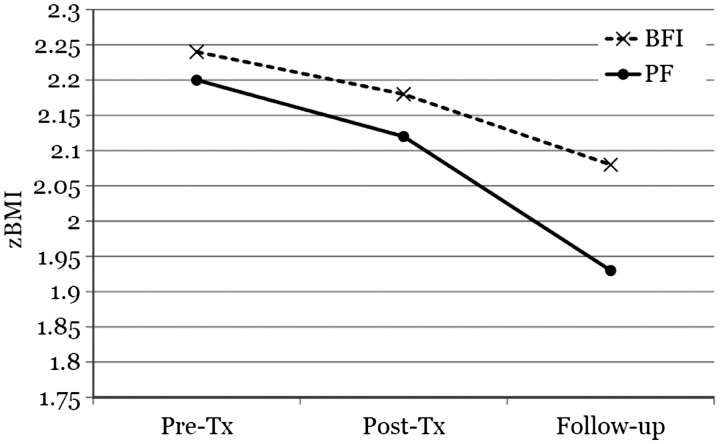
Results indicated a significant average reduction in zBMI at posttreatment relative to baseline levels regardless of condition, t (66.1) = −2.04, p < .05, dRM = 0.941. On average, BFI participants showed a 0.02 standard deviation unit decrease in zBMI by the postintervention assessment, and PF participants showed an average 0.04 standard deviation unit decrease. This difference was not statistically significant, t (65.8) = −1.43, p > .05. At the 1-year follow-up, BFI participants showed an average zBMI reduction of 0.12 standard deviation units, and PF participants showed a 0.19 standard deviation unit reduction. However, this difference did not reach statistical significance, t (134.0) = −0.80, p > .05 (Figure 2). There was no evidence of differential change (PF vs. BFI) over time due to gender at posttreatment, t (64.1) = −0.88, p > .05, or at follow-up, t (134.0) = −1.60, p > .05.
Figure 2.
zBMI over time by treatment condition.
When age group (child vs. adolescent) and its interactions with other variables were added to the model, only the main effect for time, t (130.0) = −4.23, p < .05, dRM = 0.46, and the time by age interaction, t (132.0) = 4.53, p < .05, dRM = 0.43, were significant. Subgroup analyses indicated that children (7–12 years of age) demonstrated declines in zBMI of 0.30–0.32 standard deviation unit, t (96.8) = −3.88, p < .05, dRM = 0.82, whereas adolescents demonstrated a significant increase in zBMI of 0.19–0.25 standard deviation unit, t (33.8) = 4.09, p < .05, dRM = 1.11. There was no evidence of a treatment condition by age interaction, t (129.0) = −0.37, p > .05.
Child-Reported QOL
Overall, there were no significant differences in child-reported QOL between BFI and PF participants at baseline, F (1, 121) = 0.34, p > .05, or at the immediate postintervention assessment F (1, 73.7) = 0.26, p > .05. However, consistent with the study hypotheses, results suggested a trend toward a significant time by condition interaction at follow-up, t (126.0) = 1.93, p = .06 (1-tailed: p = 0.028), dRM = 0.68; BFI participants evidenced virtually no change in self-reported QOL, while PF participants self-reported a 10.3 point increase over the same period (i.e., greater than the MCID of 4.36).
Parent-Reported QOL
There was not a significant difference in parent-reported child QOL between BFI and PF participants at baseline, t (105.0) = −1.13, p > .05, and there was no overall average change in parent-reported child QOL at posttreatment relative to baseline levels, t (65.2) = 1.19, p > .05. However, parents of children in the PF condition reported a significantly greater increase in their children's QOL at postintervention relative children in the BFI condition, t (64.1) = 3.06, p < .05, dRM = 0.59; parents of BFI participants reported virtually no change in their child's QOL (+1.6 points) while parents of PF participants reported an 8.1 point increase on average over the same period (i.e., greater than the MCID of 4.50). The time by condition interaction became nonsignificant by the 1-year follow-up, t (111.0) = 1.19, p > .05.
Clinically Meaningful Change
Another way of examining the data at 1-year follow-up is to determine the number of participants who experienced clinically meaningful changes in zBMI. Changes as small as –0.18 in zBMI have produced clinically meaningful changes in the prevalence and indicators of metabolic syndrome in children at 1-year follow-up (e.g., Reinehr, Kleber, & Toschke, 2009). In the current study, 41% of PF participants with complete available data at the 1-year follow-up demonstrated zBMI reductions greater than 0.18; 38% of BFI participants met this criterion.
Discussion
The current study represents a practical clinical trial (Glasgow et al., 2005; Tunis et al., 2003) of the PF program (relative to a BFI for pediatric obesity. With regard to zBMI, our results are suggestive—but not conclusive—of the effectiveness of both the PF and the BFI interventions in applied clinical settings, primarily among younger children. Despite significant differences in treatment content, the two interventions produced similar outcomes in terms of zBMI change as well as in the percent of participants that achieved clinically significant zBMI reductions (i.e., >0.18; Reinehr et al., 2009).
On the one hand, these results speak to the potential efficacy of a range of family-based interventions for pediatric obesity; both interventions resulted in sustained decreases in zBMI for children. On the other hand, these results beg the question of why such different treatment conditions yielded similar outcomes in terms of zBMI. The most parsimonious reason for this is low power. As noted above, participant recruitment fell short of target accrual, limiting our ability to detect between-groups differences. Nevertheless, it is interesting to consider possible alternative explanations for the similarities in outcome. Relatively few studies have examined the effectiveness of behavioral interventions for children with “severe” obesity (Kitzmann et al., 2010; Levine, Ringham, Kalarchain, Wisniewski, & Marcus, 2001). As noted by Kitzmann et al., in their meta-analysis of interventions for pediatric obesity, the most commonly reported criterion for study entry was the child being 20% overweight—far short of the current sample's mean percent overweight. Thus, it is possible that in a population so overweight (≈85% over ideal body weight), any change in either diet or physical activity has some salutary impact on weight. Alternatively, it is possible that differential adherence to treatment program recommendations may have modulated the possible gains from the two interventions. Anecdotally, we note that many families struggled with implementation of treatment recommendations. Unfortunately, we were not able to examine adherence to treatment as a moderator in the current study. So, ultimately, our data cannot speak to the mechanisms by which each intervention had its effect. Further investigations will be necessary to explore which intervention might be expected to be more effective under particular circumstances, and how individual treatment components influence treatment outcomes.
In general, neither intervention appeared to be particularly effective in terms of reducing zBMI among adolescents. Early adolescence is a high-risk period for the development of obesity, and health care professionals have reported increased challenges and complexity of treating adolescents with chronic illness compared to other age groups (Steinbeck, Baur, Cowell, & Pietrobelli, 2009). Due to the intertwined social contexts of adolescence, prevention and intervention efforts targeting social networks present in families, schools, neighborhoods, and communities hold potential for promoting sustainable and effective weight control behaviors (Koehly & Loscalzo, 2009). Although PF was delivered in a group context (i.e., with other adolescents), the intervention did not specifically target adolescents’ social networks as have other interventions designed specifically for adolescents (e.g., Jelalian, Mehlenbeck, Lloyd-Richardson, Birmaher & Wing, 2006).
The examination of QOL as a primary outcome variable enhanced the practical value of our study. Participants randomized to the PF condition evidenced statistically and clinically significant improvements in parent-reported QOL at the posttreatment assessment (+8.1 points), and clinically significant improvements in self-reported QOL at the 1-year follow-up (+10.3 points). Even with the non-significant changes in zBMI across treatment conditions, our QOL results are not necessarily surprising. PF sessions were specifically designed to increase healthy lifestyle behaviors that are likely to improve QOL (e.g., increasing physical activity; Shoup, Gattshall, Dandamudi, & Estabrooks, 2008), and to address QOL-related issues known to be associated with obesity (e.g., gaining social support from family and friends, dealing with teasing/weight-related criticism). The alternative treatment was more narrowly focused on energy balance, with less attention given to “ancillary” QOL issues. As suggested by McGovern et al. (2008), QOL-related variables (particularly obesity-specific QOL measures; e.g., Impact of Quality of Life–Kids, Kolotkin et al., 2006; Sizing me Up, Zeller & Modi, 2009) may be more responsive to immediate change than traditional weight-related outcomes (such as BMI), over which an individual has only indirect control.
Although formal cost-benefit analyses were not included as part of this trial, our results are interesting to consider in light of potential cost-benefit variability across treatment modalities. Yates (1994) notes that cost-benefit must be considered in terms of financial cost to the client/patient, real and opportunity costs to the therapist or institution, and nonspecific costs to the client related to difficulty (“hassle”). Each of these must be considered in relation to the value of the benefit derived by the client/patient (i.e., change in zBMI, QOL) and/or the institution (billable hours). One metric for evaluating cost benefit might reflect incremental loss of zBMI per therapist hour:
Assuming a mean group membership of eight families in PF, the above equation results in a mean zBMI reduction per adjusted therapist hour of 0.10 for PF and .04 for BFI, suggesting somewhat greater cost efficacy for the group intervention. However, this metric does not necessarily reflect client out-of-pocket expenses or perceived benefit to the client in terms of zBMI change and QOL change. More challenging still will be calculations of longer term health care cost savings associated with changes in zBMI or QOL across interventions. Given the economic impact of treatments to clients, institutions, and third party payors, more sophisticated cost-benefit research will be necessary to guide the further development of interventions for pediatric obesity.
Although promising, the current study presents with a number of limitations that must be addressed in future work. First, the study was ultimately underpowered to fully examine moderators of treatment outcome, which limits the conclusions that can be drawn about relative effectiveness across treatment conditions. Although both treatment conditions demonstrated significant reductions in zBMI, our results fail to confirm one treatment's superiority over the other. The substantial differences in zBMI group means across treatment conditions and age groups underscore the importance of understanding differential treatment effects across developmental periods (Janicke et al., 2008). Related to this concern, we observed a trend toward more variability at each assessment (as evidenced by increasing standard deviations at each time point; Table II), which may indicate varied treatment responses within groups. This finding suggests that multiple levels of treatment intensity (i.e., with potentially varying numbers of sessions, formats, and/or settings) may be necessary to maximize clinical improvements for all participants.
Table II.
Overall and Comparison Group Means (SDs) by Outcome Variable and Measurement Occasion
| zBMI |
Child QOL |
Parent QOL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | 1a | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Overall | |||||||||
| BFIb | 2.24 (.36) | 2.18 (.34) | 2.08 (.61) | 73.34 (13.86) | 78.46 (16.07) | 75.14 (13.40) | 71.56 (17.76) | 76.90 (12.37) | 72.11 (15.10) |
| PF | 2.20 (.34) | 2.12 (.41) | 1.93 (.54) | 76.33 (14.03) | 79.13 (12.06) | 87.41 (9.03) | 68.10 (16.53) | 79.32 (14.21) | 75.54 (16.59) |
| Children | |||||||||
| BFI | 2.26 (.34) | 2.10 (.36) | 1.87 (.60) | 74.72 (14.73) | 81.13 (16.12) | 77.90 (15.08) | 70.49 (19.78) | 78.00 (11.96) | 73.11 (15.69) |
| PF | 2.20 (.33) | 2.10 (.41) | 1.79 (.54) | 77.03 (14.15) | 80.21 (11.32) | 86.85 (7.81) | 70.69 (16.07) | 82.84 (12.93) | 78.42 (14.99) |
| Adolescents | |||||||||
| BFI | 2.19 (.39) | 2.37 (.21) | 2.58 (.21) | 70.59 (11.92) | 71.96 (14.80) | 70.99 (9.89) | 73.77 (12.91) | 74.46 (13.55) | 70.48 (14.97) |
| PF | 2.21 (.38) | 2.18 (.42) | 2.29 (.38) | 74.03 (14.04) | 76.30 (14.07) | 88.45 (11.50) | 59.61 (15.80) | 70.87 (14.18) | 70.52 (19.06) |
| Males | |||||||||
| BFI | 2.48 (.24) | 2.36 (.19) | 2.17 (.39) | 77.93 (13.51) | 79.02 (16.90) | 74.32 (15.81) | 67.65 (22.22) | 75.30 (13.24) | 72.67 (15.59) |
| PF | 2.24 (.28) | 2.19 (.33) | 2.05 (.72) | 77.09 (12.92) | 76.43 (11.80) | 88.97 (8.79) | 67.81 (14.99) | 77.39 (14.30) | 73.70 (16.47) |
| Females | |||||||||
| BFI | 2.11 (.34) | 2.09 (.37) | 2.03 (.70) | 71.05 (13.67) | 78.20 (16.08) | 75.68 (12.26) | 73.65 (14.86) | 77.73 (12.14) | 71.76 (15.42) |
| PF | 2.18 (.39) | 2.06 (.46) | 1.85 (.39) | 75.66 (15.17) | 81.29 (12.13) | 86.20 (9.38) | 68.36 (18.09) | 80.84 (14.34) | 77.08 (17.26) |
a 1, baseline; 2, postintervention; 3, 1-year follow-up.
b BFI, control group; PF, Positively Fit intervention group.
The efficacy and advisability of different “tiers” or intensities of care within a single treatment program remains an open and potentially fruitful question. For example, Johnston et al. (2007) utilized a “tiered” approach to a classroom-based obesity intervention. However, these tiers of treatments were not implemented until after initial participant response became apparent. It may be that pretreatment variables can serve as indicators of likely participant response, and would suggest different levels of care within a treatment package. The Pediatric Psychosocial Preventive Health Model (PPPHM; Kazak, 2006) and its attendant Psychosocial Assessment Tool (i.e., PAT 2.0; Pai et al., 2008)—used to triage families and children into risk groups for pediatric hospital-based services—provides one example of how pretreatment screeners might be used to tailor the treatment experience of children with obesity and their families. Future studies might examine the impact of pretreatment psychosocial functioning, physical activity, dietary habits, parenting-style, and family barriers to treatment adherence (e.g., low-income, distance from treatment site; see also Zeller et al., 2004).
Although a number of previous randomized clinical trials have demonstrated the efficacy of behaviorally based group interventions for children with obesity and their families (Kitzmann et al., 2010), fewer studies have been conducted that allow easy translation into clinical practice (Klesges et al., 2008). Consistent with the concept of the practical clinical trial (Glasgow et al., 2005; Tunis et al., 2003), the current evaluation of the PF treatment program featured fewer constraints on participants’ eligibility, included more broadly defined outcomes (e.g., QOL), and provided an examination of effectiveness in comparison to an active treatment for pediatric obesity. Results suggest that both interventions resulted in beneficial changes to children's (but not adolescents’) zBMI, and that PF outperformed the BFI in term of participants’ health-related quality of life. Future work should address limitations imposed by study attrition, sample size, and instrumentation, and should include cost-benefit analyses capable of guiding future development of interventions for pediatric obesity.
Funding
This study was supported by grant R40 MC 06631 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, U.S. Department of Health and Human Services, awarded to R. G. S.
Conflicts of interest: None declared.
Footnotes
1 The standardized effect size dRM is calculated as the average change score divided by the standard deviation (or pooled standard deviation) of the change scores (Feingold, 2009; Gibbons, Hedeker, & Davis, 1993; Morris & DeShon, 2002).
References
- Anderson S E, Whitaker R C. Prevalence of obesity among US preschool children in different racial and ethnic minorities. Archives of Pediatrics and Adolescent Medicine. 2009;163(4):344–348. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- Barlow S E, the Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescents overweight and obesity: Summary report. Pediatrics. 2007;120(s4):s164–s192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. A SAS program for the CDC growth charts [Computer Software Macro] 2007. Retrieved from http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm.
- Delamater A M, Jent J F, Moine C T, Rios J. Empirically supported treatment of overweight adolescents. In: Jelalian E, Steele R G, editors. Handbook of childhood and adolescent obesity. New York: Springer; 2008. pp. 221–239. [Google Scholar]
- Drotar D. 2011, April. Enhancing the clinical significance of research on treatment adherence: Strategies and future directions. Paper presented at the National Conference in Pediatric Psychology, San Antonio, TX. [Google Scholar]
- Enders C K. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement. 2001;61:713–740. [Google Scholar]
- Epstein L H, Squires S. The stoplight diet for children: An eight-week program for parents and children. Boston, MA: Little, Brown, & Co; 1988. [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analyses. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R D, Hedeker D R, Davis J M. Estimation of effect sizes from a series of experiments involving paired comparisons. Journal of Educational Statistics. 1993;18:271–279. [Google Scholar]
- Glasgow R E, Magid D J, Beck A, Ritzwoller D, Estabrooks P A. Practical clinical trials for translating research to practice: Design and measurement recommendations. Medical Care. 2005;43(6):551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- Herrera E, Johnston C A, Steele R G. Comparison of cognitive and behavioral treatments for pediatric obesity. Children's Health Care. 2004;33:151–167. [Google Scholar]
- Holmbeck G N, Zebracki K, McGoron K. Research design and statistical applications. In: Roberts M C, Steele R G, editors. Handbook of pediatric psychology. 4th. New York: The Guilford Press; 2009. pp. 52–71. [Google Scholar]
- Janicke D M, Sallinen B J, Perri M G, Lutes L D, Silverstein J H, Huerta M, Brumback B. Comparison of parent-only versus family-based interventions for overweight children in underserved rural settings: Outcomes from Project STORY. Archives of Pediatrics and Adolescent Medicine. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelalian E, Hart C. Pediatric obesity. In: Roberts M C, Steele R G, editors. Handbook of pediatric psychology. 4th. New York: Guilford; 2009. pp. 446–463. [Google Scholar]
- Jelalian E, Hart C, Mehlenbeck R, Lloyd-Richardson E, Kaplan J, Flynn-O'Brien K, Wing R R. Predictors of attrition and weight loss in an adolescent weight control program. Obesity. 2008;16:1318–1323. doi: 10.1038/oby.2008.51. [DOI] [PubMed] [Google Scholar]
- Jelalian E, Mehlenbeck R, Lloyd-Richardson E, Birmaher V, Wing R. Adventure therapy combined with cognitive-behavioral treatment for overweight adolescents. International Journal of Obesity. 2006;30:31–39. doi: 10.1038/sj.ijo.0803069. [DOI] [PubMed] [Google Scholar]
- Johnston C A, Steele R G. Treatment of pediatric obesity: An examination of effectiveness in an applied clinical setting. Journal of Pediatric Psychology. 2007;32:106–110. doi: 10.1093/jpepsy/jsl010. [DOI] [PubMed] [Google Scholar]
- Johnston C A, Tyler C, Poston W S C, Haddock C K, McFarlin B, Reeves R, Foreyt J P. Obesity prevention for Mexican American children in a school setting. Pediatrics. 2007;120:1450–1457. doi: 10.1542/peds.2006-3321. [DOI] [PubMed] [Google Scholar]
- Kazak A E. Pediatric Psychosocial Preventative Health Model (PPPHM): Research, practice, and collaboration in pediatric family systems medicine. Families, Systems, & Health. 2006;24:381–395. [Google Scholar]
- Kitzmann K M, Dalton W T, Stanley C M, Beech B M, Reeves T P, Buscemi J, Egli C J, Gamble H L, Midgett E L. Lifestyle interventions for youth who are overweight: A meta-analytic review. Health Psychology. 2010;29(1):91–101. doi: 10.1037/a0017437. [DOI] [PubMed] [Google Scholar]
- Klesges L, Dzewaltowski D A, Glasgow R E. Review of external validity reporting in childhood obesity prevention research. American Journal of Preventive Medicine. 2008;34:216–223. doi: 10.1016/j.amepre.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Koehly L M, Loscalzo A. Adolescent obesity and social networks. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 2009;6(3) A99. Published Online 15 June 2009. Retrieved from http://www.cdc.gov/pcd/issues/2009/jul/08_0265.htm. [PMC free article] [PubMed] [Google Scholar]
- Kolotkin R L, Zeller M, Modi A, Samsa G P, Polanichka N, Yanovski J, Bell SK, Maahs D, de Serna DG, Roehrig H R. Assessing weight related quality of life in adolescents. Obesity. 2006;13:448–457. doi: 10.1038/oby.2006.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M D, Ringham R M, Kalarchian M A, Wisniewski L, Marcus M D. Is family-based behavioral weight control appropriate for severe pediatric obesity? International Journal of Eating Disorders. 2001;30:318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- McGovern L, Johnson J N, Paulo R, Hettinger A, Singhal V, Kamath C, Erwin P J, Montori V M. Treatment of pediatric obesity: A systematic review and meta-analysis of randomized trials. Journal of Clinical Endocrinology and Metabolism. 2008;93(12):4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- Morris S B, DeShon R P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups design. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Curtin L R, Lamb M M, Flegal K M. Prevalence of high body mass index in US children and adolescents 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Flegal K M. High body mass index for age among us children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Pai A L H, Patino-Fernandez A M, McSherry M, Beele D, Alderfer M A, Reilly A T, Hwang W, – T, Kazak A E. The Psychosocial Assessment Tool (PAT2.0): psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. Journal of Pediatric Psychology. 2008;33:50–62. doi: 10.1093/jpepsy/jsm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor H A. Evidence-based treatments for childhood obesity. In: Jelalian E, Steele R G, editors. Handbook of childhood and adolescent obesity. New York: Springer; 2008. pp. 201–220. [Google Scholar]
- Reinehr T, Kleber M, Toschke A M. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207:174–180. doi: 10.1016/j.atherosclerosis.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Schwimmer J B, Burwinkle T M, Varni J W. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- Shoup J A, Gattshall M, Dandamudi P, Estabrooks P. Physical activity, quality of life, and weight status in overweight children. Quality of Life Research. 2008;17:407–412. doi: 10.1007/s11136-008-9312-y. [DOI] [PubMed] [Google Scholar]
- Sothern MS, von Almen K, Schumacher H. Trim Kids: The proven 12-week plan that has helped thousands of children achieve a healthier weight. New York: HarperResource; 2002. [Google Scholar]
- Steele R G, the Pediatric Health Promotion and Maintenance Lab . Therapist manual for Positively Fit: A family-based group intervention for child and adolescent weight management. n.d.. Unpublished manuscript. [Google Scholar]
- Steinbeck K, Baur L, Cowell C, Pietrobelli A. Clinical research in adolescents: challenges and opportunities using obesity as a model. International Journal of Obesity. 2009;33:2–7. doi: 10.1038/ijo.2008.263. [DOI] [PubMed] [Google Scholar]
- Tsiros M, Olds T, Buckley J, Grimshaw P, Brennan L, Walkley J, Hills A P, Howe P R C, Coates A M. Health-related quality of life in obese children and adolescents. International Journal of Obesity. 2009;33(4):1–14. doi: 10.1038/ijo.2009.42. [DOI] [PubMed] [Google Scholar]
- Tunis S R, Stryer D B, Clancy C M. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Varni J W, Burwinkle T M, Seid M, Skarr D. The PedsQL™ 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Yates B T. Toward the incorporation of costs, cost-effectiveness analysis, and cost-benefit analysis into clinical research. Journal of Consulting and Clinical Psychology. 1994;62:729–736. doi: 10.1037//0022-006x.62.4.729. [DOI] [PubMed] [Google Scholar]
- Zeller M, Kirk S, Claytor R, Khoury P, Grieme J, Santangelo M, Daniels S. Predictors of attrition from a pediatric weight management program. Journal of Pediatrics. 2004;144:466–470. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Zeller M, Modi A C. Development and initial validation of an obesity-specific quality of life measure for children: Sizing me up. Obesity. 2009;17:1171–1177. doi: 10.1038/oby.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]