Abstract
The encapsulated fungal pathogens Cryptococcus neoformans and Cryptococcus gattii are significant agents of life-threatening infections, particularly in persons with suppressed cell-mediated immunity. This chapter provides detailed methodology for the purification of two of the major antigen fractions of C. neoformans: glucuronoxylomannan (GXM) and mannoprotein (MP). GXM is the primary component of the polysaccharide capsule, which is the major cryptococcal virulence factor. In contrast, MPs have been identified as key antigens that stimulate T-cell responses. Purification of GXM and MP should assist investigators studying the antigenic, biochemical, and virulence properties of Cryptococcus species.
Keywords: Cryptococcus, glucuronoxylomannan, mannoprotein
1. Introduction
Cryptococcus neoformans is an opportunistic fungal pathogen that has a predilection to cause disease in persons with compromised T-cell function. Major risk factors for cryptococcosis include acquired immunodeficiency syndrome (AIDS), lymphoid malignancies, and immunosuppressive medications to prevent transplant rejection (1-4). Cryptococcus is divided into serotypes A to D, based on the structure of its major capsular component, glucuronoxylomannan (GXM). Serotypes B and C were previously classified as Cryptococcus neoformans var. gattii and have recently been classified as a new species, Cryptococcus gattii (5). C. neoformans can be isolated from environmental sources worldwide, particularly soil contaminated with bird excreta and rotting wood (6). In contrast with C. neoformans, C. gattii is found mostly in tropical and subtropical regions, although Vancouver Island, Canada, has emerged as an endemic focus (7). Environmental niches of C. gattii include eucalyptus trees and Douglas fir trees (8). For both species, infections are thought to be typically acquired after inhalation of aerosolized organisms.
The C. neoformans capsule has been identified as a major virulence factor of the organism. It is composed primarily of the polysaccharide GXM but also contains galactoxylomannan (GalXM). The capsule inhibits phagocytosis, and it can be shed from the cryptococcal cells into blood, cerebrospinal fluid (CSF), or infected tissues (9-11). In experimental models, upon shedding of the capsule, GXM accumulates in marginal zone macrophages in the spleen (10) and in Kupffer cells in the liver (9). In patients suffering from cryptococcosis, GXM circulates in blood and CSF at high concentrations (11) and can be detected for months to years after successful antifungal therapy (12).
GXM has many immunomodulatory properties. It has been shown to downregulate proinflammatory cytokine secretion from human monocytes (13), inhibit leukocyte migration (13), impair neutrophil anticryptococcal activity (14), and diminish T-cell responses (15). GXM is recognized by many receptors on immune cells, including CD14, CD11/CD18, TLR2 and TLR4, and can be internalized by monocytes, neutrophils, and macrophages (16, 17). Accumulation of GXM in human monocyte–derived macrophages (MDMs) results in decreased human neutrophil anticryptococcal activity (14) as well as modulation of MHC II and costimulatory molecule expression on MDMs (18). Recent studies also show that both human and murine dendritic cells (DCs) can internalize GXM in vitro (15, 19). However, soluble GXM does not impair human DC maturation when examined for the markers MHC I, MHC II, CD40, or CD86 (19). GXM from all serotypes of C. neoformans and C. gattii directly inhibits T-cell proliferation, without affecting antigen presentation by DCs or macrophages, and without inhibiting T-cell activation (15).
An adaptive Th1-type immune response is required for protection against cryptococcal infection (20-24). The search for protective antigens began when Bennett and colleagues characterized a cryptococcal skin test antigen that caused delayed-type hypersensitivity (DTH) reactions (25) and continued when Murphy and colleagues isolated a crude C. neoformans culture supernatant (CneF) (26) that stimulated DTH reactions and cytokine production in mice (27, 28). After separation of CneF by concanavalin A (Con A) affinity columns, the adherent mannoprotein (MP) fraction was found to be responsible for the DTH reaction (29). Since that finding, both clinical and experimental studies have identified cryptococcal MPs as critical antigens responsible for stimulating T-cell responses (30-33).
Clinically, MPs stimulate lymphoproliferative responses and cytokine production from patients recovered from cryptococcosis (30, 31). Experimentally, in human monocytes and murine macrophages, MPs have been reported to induce the production of the cytokines TNF-α (32, 34), IL-12, and IFN-γ (33, 35), which are critical for host defenses in murine models of cryptococcosis (36-39). Additionally, in a mouse model of infection, mice vaccinated with MP and then challenged with C. neoformans had increased survival, increased TNF-α, IFN-γ, and IL-2 in the brain, and a stronger infiltrate of immune cells into the brain, kidney, and liver compared with nonvaccinated mice (40). Whereas most MPs promote proinflammatory cytokine production, one fraction, termed MP-4, has been shown to inhibit neutrophil migration, downregulate neutrophil expression of L-selectin, and desensitize neutrophils toward chemotactic factors (41).
This chapter provides detailed methods for isolating GXM and MP. The protocol described for isolating GXM is applicable to all strains and serotypes of C. neoformans and C. gattii. The protocol described for isolation of MP is applicable to the MPs that are >10 kDa but can be easily modified in order to isolate lower-molecular-weight MPs. Moreover, subfractionization of the MPs can be performed to isolate specific antigenic components.
2. Materials
2.1. GXM Preparation
Cryptococcus neoformans grown on Sabouraud dextrose agar plates (Remel, Lenexa, KS).
10x YNB: Yeast Nitrogen Base with amino acids (Difco, Detroit, MI) 6.7 g, dextrose (d-glucose) 5.0 g, dH2O to 100 mL. Sterile filter using an 0.22-μm filter bottle, and store at 4°C. Before using, dilute to 1x with dH2O from a pure water system and add penicillin (50 U/mL) and streptomycin (50 μg/mL).
Sodium acetate (powder) and acetic acid (to pH the solution).
Hexadecyltrimethyl ammonium bromide (CTAB), make a 0.3% (w/v) solution of CTAB in water at room temperature
Ethanol.
Phenol, glucose, sulfuric acid, glass test tubes, and 96-well U-bottom plates (for polysaccharide concentration assay).
2 M NaCl.
1 M NaCl.
Baked glassware, sterile latex gloves, and nanopure water.
Limulus amoebocyte lysate assay (Associates of Cape Cod, East Falmouth, MA).
2.2. MP Preparation
YNB medium (described in Section 2.1).
Cryptococcus neoformans acapsular strain Cap67 (ATTC no. 52817) grown on Sabouraud dextrose agar plates (Remel).
Filter bottles, 0.45 μm and 0.22 μm (Corning, Lowell, MA).
Tangential filtration cassette, 10,000 MW cutoff (Millipore, catalog no. CDUF001LG, Billerica, MA).
Veristaltic pump (Manostat, division of Barnant Company, Barrington, IL model 72-310-000).
Dulbecco’s phosphate-buffered saline (DPBS) with Ca+2 and Mg+2.
Concanavalin A (Con A) immobilized on 4% cross-linked Sepharose beads (Sigma, St. Louis, MO).
0.2 M methyl α-d manno-pyranoside (Sigma).
Slide-a-lyzer dialysis cassettes (10,000 MW cutoff) (Pierce, Rockford, IL).
BCA protein assay kit (Pierce).
SDS gels (12%), Coomassie blue GelCode Blue Stain reagent (Pierce), Silver stain kit (Biorad, Hercules, CA), and Artisan Periodic Acid–Schiff (PAS) Stain kit (Dako, Carpinteria, CA).
3. Methods
GXM is the major polysaccharide that comprises the capsule of C. neoformans, and, as noted above, it has many immunomodulatory functions. These immunomodulatory properties make GXM a useful tool for examining immune responses. Other methods exist for isolating GXM from C. neoformans, but the method described herein summarizes the protocol that our laboratory routinely uses to obtain purified GXM. GXM can be isolated from all serotypes of C. neoformans and C. gattii (A to D). Differences in GXM structure (Fig. 7.1) affect virulence, inhibition of neutrophil migration, and tissue accumulation of GXM (42-44). Because of these differences, the GXM isolated from different serotypes may have differing degrees of immunomodulation.
Fig. 7.1.

Shown are the different structures for the glucuronoxylomannan (GXM) of Cryptococcus serotypes A to D. Serotypes A and D belong to C. neoformans, and serotypes B and C belong to C. gattii. The mannose backbone of GXM has variable degrees of O-acetylation (not shown). GlcpA, glucopyranosyluronic acid; Manp, mannopyranan; Xylp, xylopyranosyl. (Reproduced and adapted with permission from Cherniak, R., Valafar, H., Morris, L.C., and Valafar, F. 1998. Cryptococcus neoformans Chemotyping by Quantitative Analysis of 1H Nuclear Magnetic Resonance Spectra of Glucuronoxylomannans with a Computer-Simulated Artificial Neural Network. Clin. Diagn. Lab. Immunol. 5, 146–159.)
Mannoproteins (MPs) are major T-cell antigenic determinants isolated from C. neoformans (29, 40). MPs are mannosylated proteins that contain both N- and O-linked glycans (Fig. 7.2) and can be recognized by mannose receptors on antigen-presenting cells, which results in efficient antigen uptake, processing, and presentation to T cells (45). They are readily purified from the Cap67 acapsular mutant of C. neoformans, because this mutant does not have GXM on its surface to interfere with MP purification. However, other laboratories have successfully isolated MP from various other strains, including the encapsulated strains B3501 (41) and 184-A (46, 47), as well as other nonencapsulated strains, such as strain 602 (47). The MP isolation described in this protocol is used to isolate total MP, not individual MPs. Additional purification is necessary to subfractionate MPs or to purify individual MPs. This can be accomplished using standard techniques, including size-exclusion chromatography, ion-exchange chromatography, hydrophobic interaction chromatography, and elution of bands from excised gels (30, 41, 48). MPs have also been subfractionated based on the molar strength of methyl α-d manno-pyranoside required to elute it from Con A beads (41). Once purified, MP (either total MP or specific MPs) can serve as components of candidate vaccines against cryptococcosis. Additionally, because MPs are effectively taken up by mannose receptors on dendritic cells and macrophages, they can be used for in vitro studies to examine cytokine production, antigen presentation, and T cell activation. Finally, the biochemical properties of MPs can be investigated by assaying for functions such as enzymatic activity.
Fig. 7.2.
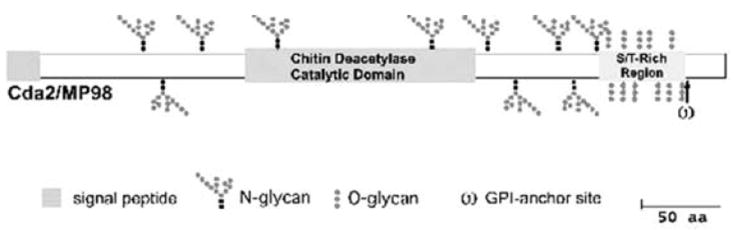
Mannoprotein Cda1/MP-98 Cryptococcus neoformans is shown as a model mannoprotein. The major defining characteristics of MPs are their S/T rich region and their GPI anchor. The putative N-glycans are indicated by the branching structures, and the putative O-glycans are indicated by the straight dotted structures in the S/T rich regions. (Reproduced and adapted with permission from Levitz, S. M., and Specht, C. A. 2006. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Research 6, 513-524.)
3.1. GXM Isolation
Inoculate a colony of C. neoformans grown on a Sabouraud dextrose agar plate into 1 L of 1x YNB culture medium. Shake at 30°C overnight.
Spin down C. neoformans to pellet fungi. Discard fungal pellet and save supernatant.
Precipitate the polysaccharide in the supernatant by slowly adding sodium acetate (add as a powder) to make the solution 10% (w/v). Use a stir bar and adjust the pH to 7.0 with acetic acid as the sodium acetate is being added (see Note 1). Add 2.5 volumes of EtOH. As the EtOH is added, a precipitate will form on top with the appearance of a “glob of cheese.”
Leave the solution overnight on the benchtop. By the morning, the polysaccharide will have settled to the bottom and formed a “glaze” (see Note 2). Decant the supernatant and invert the flask so the EtOH is completely removed. Air dry. Dissolve the polysaccharide in 2 to 3 mL dH2O. This is the GXM and GalXM.
Measure the polysaccharide concentration by the phenol sulfuric method of Dubois (49). First, make a standard curve: Add 1 g glucose to 100 mL dH2O. Set up six tubes for the standard curve. To each tube add 2 mL dH2O and 50 μL phenol. Add 0, 5, 10, 20, 40, or 80 μL of glucose solution to the six tubes. For the unknowns, set up tubes with 2 mL dH2O, 50 μL phenol, and 40 μL of unknown. In a fume hood, add 5 mL sulfuric acid directly into the solution in the tubes (see Notes 3 and 4). Take 100-μL samples from the glass test tubes after 2 to 3 inversions to mix, place in a U-bottom 96-well plate, and read at 485 nm using a spectrophotometric plate reader. Compare GXM unknowns to the standard curve to determine carbohydrate content.
To purify the GXM, adjust the polysaccharide solution to 0.2M NaCl. Make 0.3% (w/v) solution of CTAB in water at room temperature. Add CTAB dropwise and slowly (1 mL/min) to a stirring mixture of polysaccharide. The total amount of CTAB added should be 3 times the total amount of polysaccharide (w/w). The amount of polysaccharide is calculated from the phenol sulfuric measurement (described previously). Thus, if you have 100 mg of polysaccharide, you would add 100 mL of 0.3% CTAB solution (=300 mg of CTAB). A milky precipitate will form, which is the CTAB-GXM. The GalXM should remain in solution.
Spin the CTAB-GXM precipitate at 10,000 × g for 10 min. Discard supernatant. Wash the CTAB-GXM precipitate with 10% EtOH in ddH20 (v/v). Centrifuge the sample at 10,000 × g for 10 min and discard the supernatant.
To remove the CTAB: At room temperature, dissolve the CTAB-GXM in 1 M NaCl. The solution should be clear at this point. It cannot be filtered, but it can be centrifuged to clarify if it is not too thick (see Note 5). Add EtOH dropwise with stirring (about 2 volumes EtOH will be needed). The EtOH will precipitate the GXM while the CTAB will remain in solution. Centrifuge the precipitate at 10,000 × g for 10 min at room temperature. Discard the supernatant (see Note 6).
Dissolve precipitate in 2 M NaCl (keep adding NaCl until the pellet dissolves) until a viscous solution forms. Put this viscous solution in a dialysis cassette with a 10,000 MW cutoff and dialyze overnight against 1 M NaCl. Then dialyze against dH2O for 1 week, changing dialysate every day (see Note 7).
Measure the polysaccharide concentration by the method of Dubois (49), as described above. The expected yield varies greatly depending upon the strain of C. neoformans used. Typical yields range from 5 to 200 mg per liter. Lyophilize.
Reconstitute the lyophilized GXM in sterile PBS (or tissue culture media) for use. Perform the Limulus amoebocyte lysate assay to determine levels of endotoxin, if desired (see Note 8).
3.2. MP Preparation
Inoculate a single colony of Cryptococcus neoformans (Cap 67) from a Sabouraud dextrose agar plate into 15 mL 1x YNB culture medium. Shake at 30°C overnight.
Transfer the entire culture into 20 L of 1x YNB medium (see Note 9). Shake at 30°C for 5 to 6 days.
Spin down the yeast cells in 500 mL centrifuge bottles at 5000 × g for 20 min (this will require many spins in order to spin the entire 20 L of culture).
Filter the supernatant through an 0.45-μm filter bottle, followed by filtering through an 0.22-μm filter bottle.
Concentrate the supernatant with a Millipore tangential filtration cassette from 20 L to <100 mL (Fig. 7.3) (see Note 10). Run DPBS (including Ca++ and Mg++) through the filtration cassette several times in order to remove any remaining YNB present in the concentrated material (see Note 11).
Run a gravity ConA column. For this step, the running buffer is DPBS and the elution buffer is DPBS with 0.2 M methyl α-d manno-pyranoside (see Note 12). Collect the eluate in fractions.
Take aliquots of the eluate fractions and measure protein using the BCA protein assay (see Note 13). To measure carbohydrates, perform the DuBois assay (described in Section 3.1, step 5).
After the protein assay, perform dialysis on the eluate fractions containing protein using a Slide-A-Lyzer dialysis cassette (10,000 MW cutoff), and dialyze against dH2O at 4°C for 2 days. The dH2O should be replaced at least 4 times.
To analyze MP, run three 12% SDS gels with 20 to 30 μg/well of the purified MP. Stain one gel each with Coomassie blue (to visualize protein), silver (to visualize protein) (see Note 14), and periodic acid–Schiff (PAS; to visualize carbohydrates), according to the manufacturer’s instructions (Fig. 7.4).
Filter sterilize the dialyzed sample with an 0.22-μm filter and freeze at −80°C in a dry ice–alcohol bath.
Lyophilize and store the sample at −80°C. Reconstitute the MP in PBS before use. Perform another BCA protein assay on the reconstituted MP to confirm the concentration (see Note 15).
Fig. 7.3.
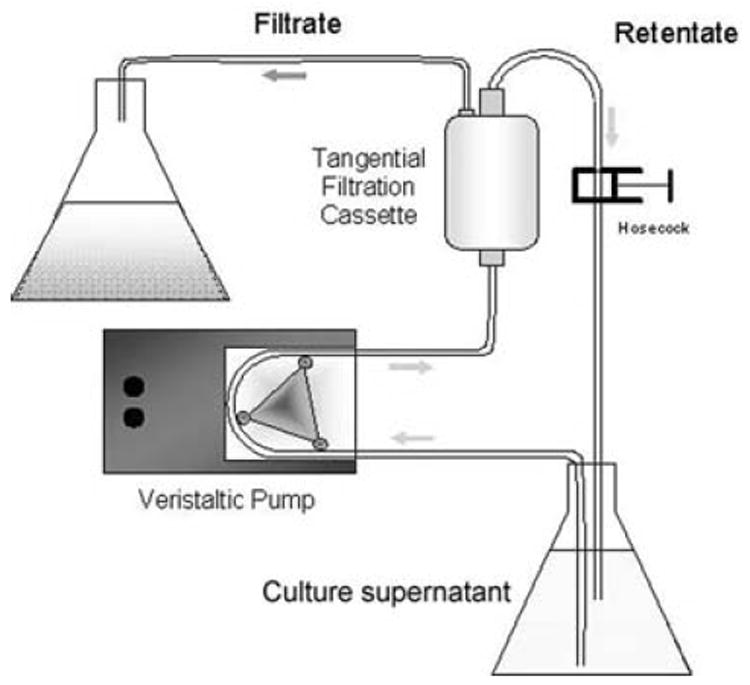
Diagram of the mannoprotein concentration system. Culture supernatant is placed in the lower flask, pumped through a tangential filtration cassette, and concentrated before MP purification. (Figure courtesy of Dr. Michael Mansour.)
Fig. 7.4.
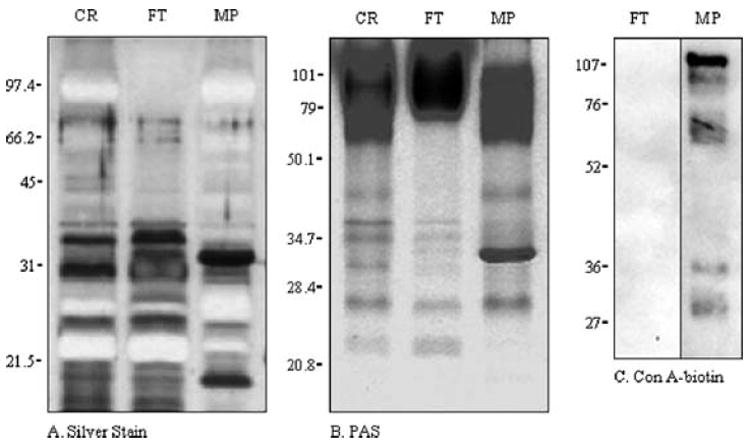
Analysis of C. neoformans strain CAP67 supernatant fractions by SDS-PAGE. Crude (CR), flow-through (FT), and MP fractions were resolved by 12% SDS-PAGE and analyzed by (A) silver stain, (B) PAS, and (C) Con A–biotin blot. Migration of commercial molecular mass standards, expressed in kilodaltons, is indicated to the left of the gels. (Reproduced and adapted with permission from Mansour, M. K., Schlesinger, L.S., and Levitz, S. M. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. Journal of Immunology 168, 2872–2879.)
Acknowledgments
The protocols were developed with the help of Drs. Arturo Casadevall, Robert Cherniak, Michael Mansour, Shuhua Nong, and Lauren Yauch. The research was supported in part by National Institutes of Health grants R01 AI25780 and R01 AI066087.
Footnotes
Make sure to monitor and adjust the pH during the precipitation. GXM has varying degrees of O-acetylation, and if the pH is too high, it will destroy the acetyl groups.
For some C. neoformans strains, the capsular polysaccharide may stay on the top of the solution rather than settle to the bottom.
It is important to add the sulfuric acid directly into the solution and not down the sides of the tubes.
The reaction is strongly exothermic, and the tubes should be cooled for 30 to 60 min in a water bath at 23°C. The colorimetric reaction is directly proportional to the concentration of carbohydrate.
If the NaCl solution is very thick, it is hard to centrifuge, and more NaCl should be added.
Steps 6, 7, and 8 have to be done at room temperature otherwise the CTAB will precipitate. Make sure the centrifuge is room temperature before adding sample!
If the solution becomes cloudy, then the CTAB has not been adequately removed, and the sample needs to be re-dialyzed against NaCl.
If the GXM will be used for immunologic experiments, it is important to minimize endotoxin contamination. For all steps, meticulously avoid the introduction of endotoxin by wearing gloves, baking glassware, using endotoxin-free water, and so forth. Our laboratory is able to routinely purify endotoxin-free GXM (<0.03 endotoxin U/mL).
The reaction can be scaled up or down depending upon the desired amount of MP.
The setup of this device is important for correctly concentrating MP. Incorrect setup can lead to loss of the MP.
This step uses a 10-kDa MW cutoff for concentrating the supernatant. This concentration step will lead to loss of lower MW MPs. In order to retain the smaller MPs, a 3.5-kDa MW cutoff filter may be used (41).
If retention of the MP-4 fraction is desired, elute MP with 0.4 M methyl α-d manno-pyranoside instead of 0.2 M. Additionally, the flow-through (FT) fraction (which contains non-mannoprotein antigens) can be collected.
Perform the BCA protein assay on the eluate fractions prior to dialysis in order to determine which fractions contain MP (these will have measurable protein by the BCA assay). Alternatively, the MP-containing fractions can be determined using an ultraviolet monitor. Then use the MP-containing fractions for the following steps.
Coomassie blue is preferentially used to stain the protein portion of MP instead of the normally more sensitive silver staining. MPs that are extensively glycosylated generally will not stain and often appear as a negative band after silver staining (Fig. 7.4).
A small amount of Con A will inevitably leach off of the affinity column. Because Con A is a potent mitogen, this contamination may have untoward effects in immunologic assays. To biologically inactivate Con A, MP can be boiled for 5 min prior to use. This boiling step will not destroy the antigenic activity of MP.
References
- 1.Levitz SM. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Reviews of Infectious Diseases. 1991;13:1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS - 100 years after the discovery of Cryptococcus neoformans. Clinical Microbiology Reviews. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoham S, Levitz SM. The immune response to fungal infections. British Journal of Haematology. 2005;129:569–582. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Gayowski T, Wagener MM, Marino IR. Clinical spectrum of invasive cryptococcosis in liver transplant recipients receiving tacrolimus. Clinical Transplantation. 1997;11:66–70. [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Research. 2006;6:574–587. doi: 10.1111/j.1567-1364.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 6.Ellis DH, Pfeiffer T. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. The Lancet. 1990;336:923–925. doi: 10.1016/0140-6736(90)92283-n. [DOI] [PubMed] [Google Scholar]
- 7.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, MacDougall L, Boekhout T, Kwon-Chung KJ, Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan C, Schwantje H, Stephen C, Campbell J, Bartlett K. Cryptococcus gattii in Wildlife of Vancouver Island, British Columbia, Canada. Journal of Wildlife Diseases. 2006;42:175–178. doi: 10.7589/0090-3558-42.1.175. [DOI] [PubMed] [Google Scholar]
- 9.Grinsell M, Weinhold LC, Cutler JE, Han YM, Kozel TR. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: A critical role for tissue macrophages. Journal of Infectious Diseases. 2001;184:479–487. doi: 10.1086/322787. [DOI] [PubMed] [Google Scholar]
- 10.Goldman DL, Lee SC, Casadevall A. Tissue localization of Cryptococcus-neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infection and Immunity. 1995;63:3448–3453. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuck SL, Sande MA. Infections with Cryptococcus-neoformans in the acquired immunodeficiency syndrome. New England Journal of Medicine. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Zhou Y, Yin Y, Pan X, Weng X. Cryptococcal antigen test revisited: significance for cryptococcal meningitis therapy monitoring in a tertiary Chinese hospital. Journal of Clinical Microbiology. 2005;43:2989–2990. doi: 10.1128/JCM.43.6.2989-2990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellerbroek PM, Walenkamp AM, Hoepelman AI, Coenjaerts FE. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Current Medical Chemistry. 2004;11:253–266. doi: 10.2174/0929867043456188. [DOI] [PubMed] [Google Scholar]
- 14.Monari C, Retini C, Casadevall A, Netski D, Bistoni F, Kozel TR, Vecchiarelli A. Differences in outcome of the interaction between Cryptococcus neoformans glucuronoxylomannan and human monocytes and neutrophils. European Journal of Immunology. 2003;33:1041–1051. doi: 10.1002/eji.200323388. [DOI] [PubMed] [Google Scholar]
- 15.Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathogens. 2006;2:e120. doi: 10.1371/journal.ppat.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong ZM, Murphy JW. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infection and Immunity. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. Journal of Immunology. 2001;166:4620–4626. doi: 10.4049/jimmunol.166.7.4620. [DOI] [PubMed] [Google Scholar]
- 18.Monari C, Pericolini E, Bistoni G, Casadevall A, Kozel TR, Vecchiarelli A. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. Journal of Immunology. 2005;174:3461–3468. doi: 10.4049/jimmunol.174.6.3461. [DOI] [PubMed] [Google Scholar]
- 19.Vecchiarelli A, Pietrella D, Lupo P, Bistoni F, McFadden DC, Casadevall A. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. Journal of Leukocyte Biology. 2003;74:370–378. doi: 10.1189/jlb.1002476. [DOI] [PubMed] [Google Scholar]
- 20.Hill JO, Harmsen AG. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T-cells. Journal of Experimental Medicine. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4(+) and CD8(+) T-cells in the protective inflammatory response to a pulmonary cryptococcal infection. Journal of Leukocyte Biology. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T-cells. Journal of Experimental Medicine. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huffnagle GB, Yates JL, Lipscomb MF. T-cell-mediated immunity in the lung - a Cryptococcus neoformans pulmonary infection model using Scid and athymic nude-mice. Infection and Immunity. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody CH, Lipscomb MF, Street NE, Toews GB. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. Journal of Immunology. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 25.Bennett JE. Cryptococcal skin test antigen: preparation variables and characterization. Infection and Immunity. 1981;32:373–380. doi: 10.1128/iai.32.1.373-380.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy JW, Cozad GC. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by hemolytic plaque technique. Infection and Immunity. 1972;5:896. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JW. Protective cell-mediated immunity against Cryptococcus neoformans. Research in Immunology. 1998;149:373–386. doi: 10.1016/s0923-2494(98)80761-x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JW, Moorhead JW. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. Journal of Immunology. 1982;128:276–283. [PubMed] [Google Scholar]
- 29.Murphy JW. Influence of cryptococcal antigens on cell-mediated-immunity. Reviews of Infectious Diseases. 1988;10:S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- 30.Levitz SM, Nong SH, Mansour MK, Huang C, Specht CA. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10422–10427. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoy JF, Murphy JW, Miller GG. T-cell response to soluble cryptococcal antigens after recovery from cryptococcal infection. Journal of Infectious Diseases. 1989;159:116–119. doi: 10.1093/infdis/159.1.116. [DOI] [PubMed] [Google Scholar]
- 32.Chaka W, Verheul AF, Vaishnav VV, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman AI. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. Journal of Immunology. 1997;159:2979–2985. [PubMed] [Google Scholar]
- 33.Pietrella D, Cherniak R, Strappini C, Perito S, Mosci P, Bistoni F, Vecchiarelli A. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infection and Immunity. 2001;69:2808–2814. doi: 10.1128/IAI.69.5.2808-2814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaka W, Verheul AF, Vaishnav VV, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman IM. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infection and Immunity. 1997;65:272–278. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitzurra L, Cherniak R, Giammarioli M, Perito S, Bistoni F, Vecchiarelli A. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infection and Immunity. 2000;68:558–563. doi: 10.1128/iai.68.2.558-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. American Journal of Respiratory Cell and Molecular Biology. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami K, Qifeng X, Tohyama M, Qureshi MH, Saito A. Contribution of tumour necrosis factor-alpha (TNF-alpha) in host defence mechanism against Cryptococcus neoformans. Clinical and Experimental Immunology. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie QF, Saito A. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunology and Medical Microbiology. 1996;13:123–130. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clinical and Experimental Immunology. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansour MK, Yauch LE, Rottman JB, Levitz SM. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infection and Immunity. 2004;72:1746–1754. doi: 10.1128/IAI.72.3.1746-1754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coenjaerts FEJ, Walenkamp AME, Mwinzi PN, Scharringa J, Dekker HAT, van Strijp JAG, Cherniak R, Hoepelman AIM. Potent inhibition of neutrophil migration by cryptococcal mannoprotein-4-induced desensitization. Journal of Immunology. 2001;167:3988–3995. doi: 10.4049/jimmunol.167.7.3988. [DOI] [PubMed] [Google Scholar]
- 42.Kozel TR, Levitz SM, Dromer F, Gates MA, Thorkildson P, Janbon G. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infection and Immunity. 2003;71:2868–2875. doi: 10.1128/IAI.71.5.2868-2875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellerbroek PM, Lefeber DJ, van Veghel R, Scharringa J, Brouwer E, Gerwig GJ, Janbon G, Hoepelman AIM, Coenjaerts FEJ. O-Acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. Journal of Immunology. 2004;173:7513–7520. doi: 10.4049/jimmunol.173.12.7513. [DOI] [PubMed] [Google Scholar]
- 44.Janbon G, Himmelreich U, Moyrand F, Improvisi L, Dromer F. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Molecular Microbiology. 2001;42:453–467. doi: 10.1046/j.1365-2958.2001.02651.x. [DOI] [PubMed] [Google Scholar]
- 45.Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. Journal of Immunology. 2006;176:3053–3061. doi: 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan KL, Murphy JW. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infection and Immunity. 1993;61:2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong ZM, Murphy JW. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infection and Immunity. 1993;61:5067–5077. doi: 10.1128/iai.61.12.5067-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C, Nong SH, Mansour MK, Specht CA, Levitz SM. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-cell responses. Infection and Immunity. 2002;70:5485–5493. doi: 10.1128/IAI.70.10.5485-5493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]


