Abstract
AIM: To investigate a potential role of S100A4 in esophagus squamous cell carcinoma metastasis (ESCCs).
METHODS: Expression of S100A4 and E-cadherin were analyzed in frozen sections from ESCCs (metastasis, n = 28; non-metastasis, n = 20) by reverse transcription-polymerase chain reaction, quantitative polymerase chain reaction and immunohistochemistry. To explore the influence of S100A4 on esophageal cancer invasion and metastasis, S100A4 was overexpressed or silenced by S100A4 siRNA in TE-13 or Eca-109 cells in vitro and in vivo.
RESULTS: We found the mRNA and protein levels of S100A4 expression in ESCCs was significantly upregulated, and more importantly, that expression of S100A4 and E cadherin are strongly negatively correlated in patients who had metastasis. It was indicated that overexpression of S100A4 in TE-13 and Eca-109 cells downregulates the expression of E-cadherin, leading to increased cell migration in vitro, whereas knockdown of S100A4 inhibited cell migration and upregulation of E-cadherin expression. Moreover, the loss of cell metastatic potential was rescued by overexpression of E-cadherin completely. In addition, nude mice inoculated with S100A4 siRNA-transfected cells exhibited a significantly decreased invasion ability in vivo.
CONCLUSION: S100A4 may be involved in ESCC progression by regulate E-cadherin expression, vector-based RNA interference targeting S100A4 is a potential therapeutic method for human ESCC.
Keywords: Esophagus squamous cell carcinoma, Metastasis, Gene treatment, S100A4, E-cadherin
INTRODUCTION
Despite improvements in detection, surgical resection, and (neo-) adjuvant therapy, the overall survival for esophageal squamous cell carcinoma (ESCC), one of the most aggressive carcinomas of the gastrointestinal tract, remains lower than that of other solid tumors due to distant and lymph node metastasis[1]. Therefore, efforts are ongoing regarding exploration of novel targets and strategies for the management of ESCC, and gene targeting therapies in particular are promising. Multiple studies focusing on the effects of various biological factors on the malignant potential of ESCC have been conducted[2-4]. One of those factors is E-cadherin as the loss of E-cadherin is an important step in the process of epithelial-to-mesenchymal transition (EMT) in cancer[5-8]. In ESCC, loss of E-cadherin expression is associated with tumor invasiveness, metastasis and prognosis[2,9,10]. Mechanisms involved in regulation of E-cadherin in ESCC are likely complex and poorly understood.
The S100 family of calcium binding proteins has been shown to be involved in a variety of physiological functions, such as cell proliferation, extracellular signal transduction, intercellular adhesion, and motility as well as cancer metastasis[11-13]. Of these, S100A4 (mts1, p9Ka, calvasculin) has been identified as a cytoplasmic protein in normal cells, which is associated with the actin/myosin cytoskeleton in fixed cells[14]. Interestingly, elevated levels of S100A4 are closely associated with the process of metastasis in several human solid cancers including gastric cancer[15,16], colorectal adenocarcinoma[17,18], and breast cancer[19]. Patients with S100A4 high expression often appear with advanced stage or lymph node metastasis suggesting correlation of the S100A4 expression and the invasion or metastasis of ESCC[20]. In this study, we investigated the expression of S100A4 and E-cadherin in ESCC patients and the potential functional relationship in tumor metastasis and proliferation.
MATERIALS AND METHODS
Cell lines
EC109 and TE13 were kindly provided by Dr. Zhang (Surgery, the affiliated Hospital of Medical College, Qingdao University, QingDao, China)[21]. All the cells were maintained in 50 mL/L CO2 atmosphere at 37 °C in RPMI 1640/Ham’s F-12 mixed (1:1) medium containing 100 g/L fetal bovine serum.
Tissue sample collection
A total of 48 cryostat sections of frozen ESCC tissue were enrolled in this study: 28 with lymph node (n = 25) or distant metastasis (n = 3) and 20 without metastasis. These patients did not receive any preoperative adjuvant radiation or chemotherapy. All research involving human participants was approved in written form by the patients studied and the ethics committee at the affiliated hospital of Tianjin medical university.
Silencing of S100A4
SiRNAs were commercially purchased from Qiagen (Valencia, CA). The sequence of selected regions to be targeted by siRNAs was 5’-AACGAGGTGGACTTCCAAGAG-3’ for S100A4 and 5’-AATTCTCCGAACGTGTCTCG T-3’ for a nonsilencing siRNA (control). siRNA cloning vector (pGB) was purchased from ABCAM (Shanghai). pGB-S100A4 siRNA and controls were constructed according to the manufacture’s instruction. EC109 cells were transfected with the siRNA plasmids in the presence of Lipofectamine. Stable transfectants were selected with 300 μg/mL G418.
S100A4 cDNA and E-cadherin cDNA plasmid construction and transfection
The commercial pMD vector (produced by TAKARA) and Homo sapiens S100A4 transcript variant 1 DNA ORF (S100A4 cDNA) and Homo sapiens E-cadherin DNA ORF Clone (E-cadherin cDNA) were purchased from Sino Biological Inc. Beijing; The gene sequence is identical with the Gene Bank Ref. ID sequence: S100A4 cDNA: NM_002961.2; E-cadherin DNA: NM_004360.3. The S100A4 cDNA product was then cloned into pMD vector as the manufacture’s instruction. The constructs were confirmed by DNA sequencing and restriction enzyme digestion. For transfection studies, EC109 and TE13 cells were plated at a density of 1 × 106 cells per well in 6-well plates and incubated for 24 h in complete medium. The cells were then transfected with S100A4 cDNA (E-cadherin) construct by using an lipofectamine transfection kit for 48 h. For controls, the same amount of empty vector was also transfected. Stable transfected TE13 cells (pMD-S100A4 cDNA) were selected with 200 μg/mL G418.
Real-time quantitative reverse transcription-polymerase chain reaction analysis of archival material or TE-13 and Eca-109 cell lines
Total RNA was extracted from cryostat sections of frozen tissue or TE-13 and Eca-109 cell lines using Trizol Reagent (Life Technologies, Inc.) according to the manufacturer’s instructions. Real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR) was performed using the ABI Prism 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems). Q-PCR assays were performed in triplicate, and the mean values were used for calculations of mRNA expression. Relative levels were determined using the 2 (-ΔΔCt) method[22].
Reverse transcription-polymerase chain reaction analysis of archival material or TE-13 and Eca-109 cell lines
PCRs were carried out by using forward and reverse primer combinations for S100A4 (forward 5’-TCAGAACTAAAGGAGCTGCTGACC-3’, reverse 5’-TTTCTTCCTGGGCTGCTTATCTGG-3’), E-cadherin (forward 5’-GGAAGTCAGTTCAGACTCCAGCC-3’, reverse 5’-AGGCCTTTTGACTGTAATCACACC-3’), GAPDH (forward 5’-AATCCCATCACCATCTTCCAGGAG-3’, reverse 5’-GCATTGCTGATGATCTTGAGGCTG-3’). The cDNA was amplified with an initial denaturation at 94 °C for 3 min followed by the sequential cycles of denaturation at 94 °C for 50 s, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min for 30 cycles, with final extension at 72 °C for 5 min.
Western blotting
Whole-cell proteins were isolated, the lysates centrifuged, and the supernatant collected. 30 μg of total protein was loaded per well, separated by 7.5% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes at 150 mA for 16 h at 4 °C according to the manufacturer’s instructions. The membranes were blocked and incubated with primary antibodies. Primary antibodies were as follows: anti-S100A4 (1:200 dilution) and anti-E-cadherin (1:200 dilution; all from Santa Cruz Biotechnology). The immunoblots were detected by using an electrochemiluminescence kit (Amasham, Piscataway, NJ) and exposed to X-OMATAR film.
Immunohistochemistry staining
The paraffin-embedded sections were stained with primary anti-S100A4 or anti-Ecadherin (Abcam, Cambridge, United Kingdom) antibody and horseradish peroxidase-labeled immunoglobulin (Boshide Biotech Co., Ltd, Wuhan, China). Images were obtained at × 200 magnification. Stained slides were scored by 2 blinded, independent observers. The results of the immunohistochemical stainings were evaluated by the percentage of positively stained carcinoma cells. Expression of S100A4 was determined as positive when cytoplasmic and/or perinuclear staining was seen in more than 10% of the tumour cells. Expression of S100A4 was considered negative when no cells or less than 10% of the tumour cells were stained. Expression of E-cadherin was determined as described in a previous study[23]. Briefly, the tumor cells that stained as strongly as normal epithelial cells were considered to be “preserved expression” (positive), and those which exhibited weaker staining than normal epithelial cells or showed completely negative staining were considered to be “reduced expression” (negative).
Cell invasion assays
Cell invasion assays were performed using 8-μm pore size Transwell Biocoat Control inserts (Becton Dickinson). In brief, 1 × 104 cells were seeded on a transwell containing numbers of 8-μm pores for invasion assay. The chamers were put into the incubator at 37 °C, 50 mL/L CO2. Cells on the top surface of the transwell were removed by scrubbing 24 h after incubation. The cells were fixed by 950 mL/L ethanol, and stained in 30 min by 1 mL/L crystal violet. We counted the number of transmembrane cells under an optical microscope, chose five high power fields by random, and chected each field of vision to evaluate the invasion and metastasis of tumor cells in vitro. Such invaded cells were counted and compared among groups. Individual experiments were done in duplicate and repeated four times.
Invasion study in vivo
All animals were maintained in a sterile environment and cared for within the laboratory animal regulations of the Ministry of Science and Technology of the People’s Republic of China (http://www.most.gov.cn/kytj/kytjzcwj/200411). Full details of the study approval by the ethics committee at the affiliated hospital of Tianjin medical university. After growth to subconfluency, transfected (pMD-S100A4 siRNA or mock siRNA) and nontransfected Eca-109 cells, stable transfected TE13 cells (pMD-S100A4 cDNA), mock transfected and nontransfected TE13 cells were injected into the pancreas under the envelope near spleen of nude mice (n = 8 for each variant). Twenty-eight days later, the mice were killed following the operation. The number of the seeded tumor naked in the liver and lung is used for assessment of metastases.
Statistical analysis
All statistical analyses were performed using SPSS 11.0 software. The results were presented as mean ± SD of three replicate assays. Differences between various groups were assessed using ANOVA or Dunnett t-test. P value (of < 0.05) was considered to indicate statistical significance.
RESULTS
Increased expression of S100A4 and E-cadherin is associated with lymph node metastasis
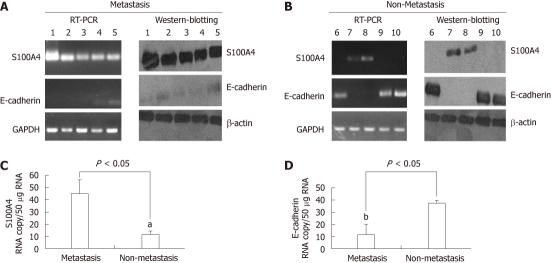
Results of immunohistochemistry (IHC) staining showed that S100A4 was weakly expressed in non-metastasis ESCC, whereas strongly expressed in metastatic ESCC (Table 1, P < 0.05). On the contrary, E-cadherin expression was strongly expressed in non-metastatic ESCC, whereas weak E-cadherin expression was detectable in metastatic ESCC (Table 1) Significantly, negative relationship was found between S100A4 and E-cadherin expression. The Western blotting, Q-PCR and reverse transcription-polymerase chain reaction (RT-PCR) analysis has the same results as IHC analysis. A six representive (5 non-metastatic ESCC cases and 5 metastatic ESCC cases) western blotting and RT-PCR results was shown in Figure 1A and B. In the present study, we also detected the levels of transcripts of S100A4 mRNA and E-cadherin mRNA in metastasis (M) tumor samples and non-metastasis tumor (N) samples (expressed as transcript copy number per 50 μg of messenger RNA and standardized with β-actin; Figure 1C and D). We found that transcript copy numbers for S100A4 were 46.3 ± 9.4 for M tumor and 10.5 ± 3.6 for N tumor (P = 0.036); for E-cadherin, they were 13.62 ± 2.3 for M tumor and 40.17 ± 3.91 for N tumor (P = 0.018); A significant negative relationship was observed between S100A4 mRNA and E-cadherin expression (P = 0.034). These results above indicates that ESCC metastasis is associated with significantly decreased expression of E-cadherin and increased S100A4 expression.
Table 1.
Immunohistochemistry staining of S100A4 and E-cadherin
| Groups | n |
S100A4 |
E-cadherin |
||||
| Positive | Negative | P value | Positive | Negative | P value | ||
| Metastasis | 28 | 19 | 9 | 0.027 | 10 | 18 | 0.036 |
| Non-metastasis | 20 | 7 | 13 | 12 | 8 | ||
Figure 1.
S100A4 and E-cadherin expression in metastasis and non-metastasis tissue. A: The representive reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting results for S100A4 and E-cadherin in metastasis tissue; B: The representive RT-PCR and Western blotting results for S100A4 and E-cadherin in non-metastasis tissue; C and D: Levels of transcripts of S100A4 and E-cadherin in M tumor samples in comparison to N tumor (expressed as transcript copynumber per 50 μg of messenger RNA and standardized with β-actin). The intensity of each band relative to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (α-tubulin) band was represented as the mean ± SD, aP < 0.05 vs metastasis group and bP < 0.05 vs non-metastasis group.
S100A4 and E-cadherin expression in EC109 and TE13 cell lines
Western blotting (Figure 2A) and RT-PCR (Figure 2B) analysis shown the levels of S100A4 were significantly higher in EC109 cells than that of in TE13 cells, and the levels of E-cadherin were significantly lower in EC109 cells than that of in TE13 cells. IHC analysis shown the same result as Western blotting and RT-PCR (data not shown).
Figure 2.
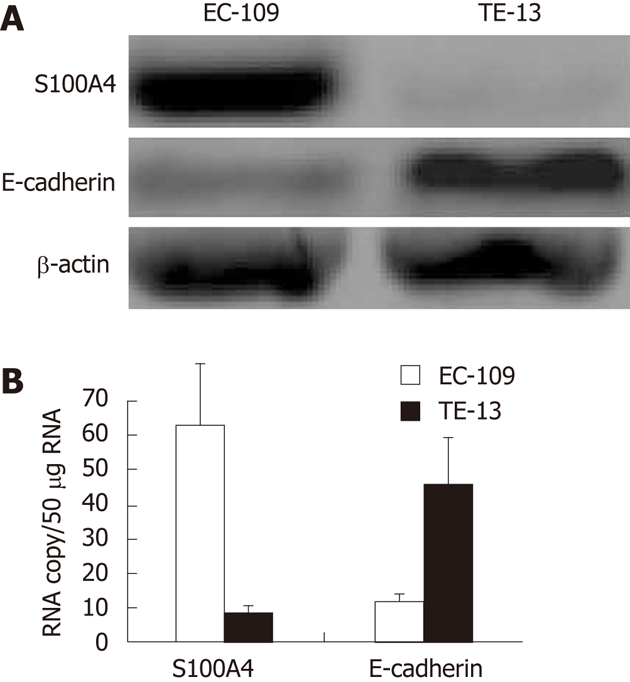
Analysis for S100A4 and E-cadherin in EC109 and TE13 cell lines. A: Western blotting analysis for S100A4 and E-cadherin in EC109 and TE13 cell lines; B: Reverse transcription-polymerase chain reaction analysis for S100A4 and E-cadherin in EC109 and TE13 cell lines. The levels of S100A4 were significantly higher in EC109 cells than that of in TE13 cells, and the levels of E-cadherin were significantly lower in EC109 cells than that of in TE13 cells.
Knockdown of S100A4 inhibits invasion in EC109 cell by upregulation of E-cadherin
S100A4 has been implicated in the malignant phenotype of tumor cells, including cell motility, however the biological function is hardly known. Many studys found that S100A4-induced invasiveness in malignant tumor cells is caused or partially caused by down-regulation of E-cadherin[24-29]. Kwak et al[30] has reported there was no significant association between S100A4 and clinicopathological parameters such as tumor differentiation or TNM stage, and also no correlation between the reactivity and E-cadherin. In the present study, we found that knockdown of S100A4 inhibited invasion in EC109 cells, followed by increased E-cadherin expression in EC109 cells. As shown in Figure 3A, suppression of the S100A4 caused a over 60% reduction (P < 0.05) in the number of cells that traversed the membrane versus nonsilencing control or mock control in EC109 cells. Western blotting, RT-PCR and Q-PCR analysis shown E-cadherin mRNA and protein expression was upregulated when the S100A4 expression was knockdown (Figure 3B). When the stable transfectants (S100A4 siRNA) were transfected with S100A4 cDNA for 48h, S100A4 expression was observed to be very high 48 h after transfection (data not shown) to restore the expression of S100A4 in the stable transfected EC109 cells, only to find significantly increased invasion ability in EC109 cells (Figure 3A) followed by the downregulation of E-cadherin expression (Figure 3C). These data suggest that knockdown of S100A4 suppressed the invasion ability of human EC109 cells by upregulation of E-cadherin expression.
Figure 3.
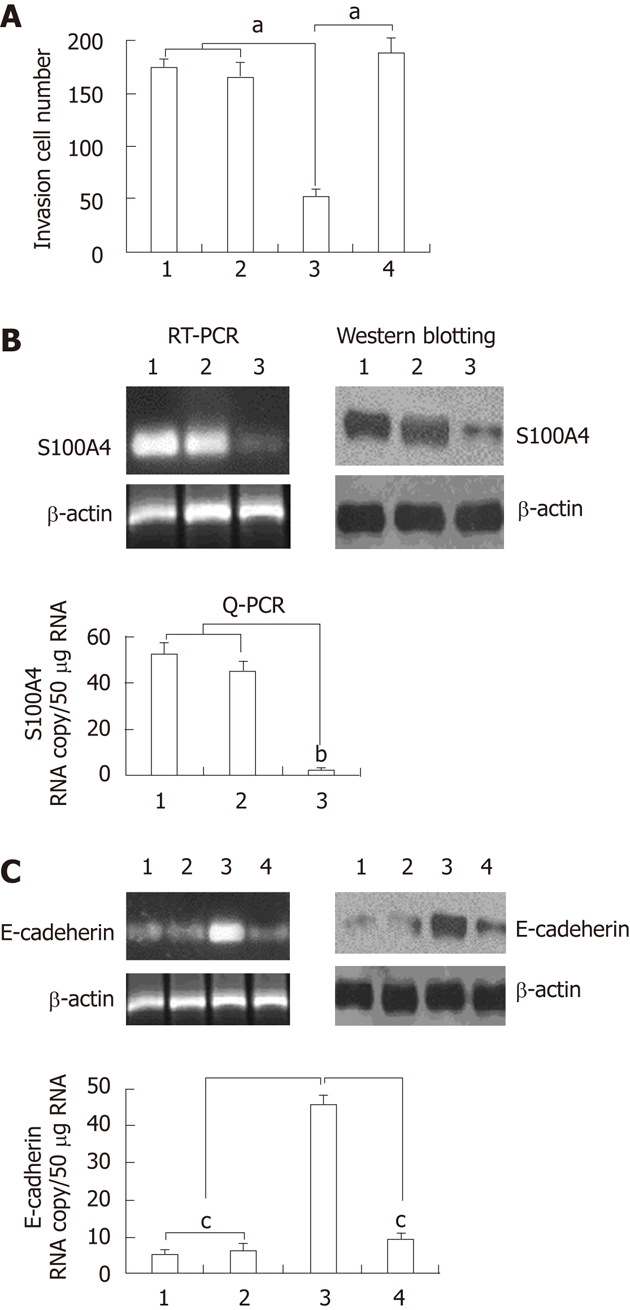
Knockdown of S100A4 inhibits invasive capability of EC109 cells. A: Transmigration cells of control, mock siRNA, S100A4 siRNA and S100A4 siRNA + S100A4 cDNA cells was calculated from three independent experiments. The indicated cells (1 × 104) were seeded on 8-mm porous transwell chambers. After 24 h of plating, transmigration cells were fixed and stained with crystal violet. Transmigration cells were counted for each of the indicated cells. Columns, mean number of cells obtained in three independent experiments; bars, SD; aP < 0.05 vs control or mock siRNA group; B: Western blotting, reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real time PCR (Q-PCR) analysis for S100A4 and mRNA and protein expression, bP < 0.05 vs control or mock siRNA group; C: Western blotting, RT-PCR and Q-PCR analysis for E-cadherin and mRNA and protein expression. cP < 0.05 vs S100A4 siRNA group. 1: Control; 2: Mock siRNA; 3: S100A4 siRNA; 4: S100A4 siRNA + E-cadherin siRNA.
Overexpression of S100A4 promotes invasion in TE-13 cell by downregulation of E-cadherin
TE-13 cells stablely transfected with pMD-S100A4 cDNA plasmid displayed a significantly increased S100A4 expression as compared with vector control. The overexpression of S100A4 was confirmed by performing Western blotting analysis (Figure 4B). We analyzed the effect of S100A4 overexpression on the invasive ability of TE13 cells. As shown in Figure 4A, overexpression of S100A4 significantly increased the number of invasive cells (P < 0.05), followed by the downregulation of E-cadherin (Figure 4C). However, when the stable transfectants (pMD-S100A4 cDNA plasmid) were transfected with pMD-E-cadherin cDNA for 48 h [E-cadherin expression was observed to be very high 48 h after transfection (data not shown)] to restore the expression of E-cadherin in the stable transfected TE-13 cells, only to find significantly decreased invasion ability in the TE-13 cells (pMD-S100A4 cDNA plasmid-transfected) (Figure 4A). These data suggest that the S100A4 gene controls the invasion ability of human TE-13 cells by regulation of E-cadherin. These data further support our hypothesis that S100A4 confers the invasive characteristics to cells during human ESCC development.
Figure 4.
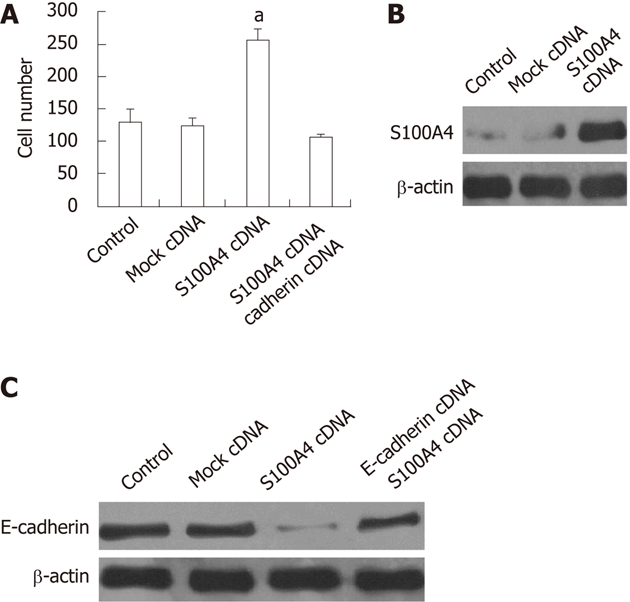
S100A4 overexpression promotes invasive capability of TE13 cells. A: Transmigration cells of control, mock cDNA, S100A4 cDNA and S100A4 cDNA + E-cadherin cDNA cells was calculated from three independent experiments. The indicated cells (1 × 104) were seeded on 8-mm porous transwell chambers. After 24 h of plating, transmigration cells were fixed and stained with crystal violet. Transmigration cells were counted for each of the indicated cells. Columns, mean number of cells obtained in three independent experiments; bars, SD; aP < 0.05 vs other 3 groups respectively; B: Western blotting, analysis for S100A4 protein expression; C: Western blotting analysis for E-cadherin protein expression.
Effect of S100A4 on metastasis in vivo model
EC109 cells (S100A4 siRNA-transfected) or TE-13 cells (S100A4 cDNA-transfected) and their control cells were injected into the pancreas under the envelope near spleen of nude mice (n = 6 for each variant). Twenty-one days latter, the mice were killed , autopsy was carried out to remove organs. The number of the seeded tumor naked in the speen, liver, pancreas and lung is used for assessment of metastases. Less sleep, liver, pancreas and lung metastasis nodes were found in S100A4 siRNA-transfected groups. The total nodes in S100A4 siRNA-transfected groups was significantly fewer than that of in mock siRNA or control (P < 0.01) (Figure 5A). However, more seeded nodes were found in S100A4 cDNA transfected groups than that of in mock cDNA or control groups, although the difference was not significant (Figure 5B).
Figure 5.
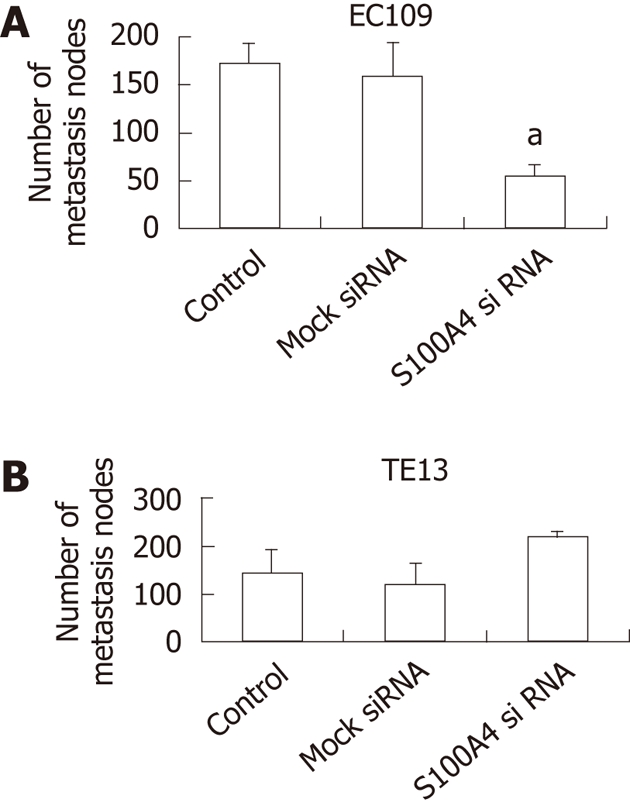
Knockdown of S100A4 inhibits metastasis of xenograft tumors (n = 6 per group). A: The total metastasis nodes in EC109 tumors (aP < 0.05 vs control or mock siRNA); B: The metastasis nodes in TE13 tumors.
DISCUSSION
Metastasis is a complex cascade of events involving a finely tuned interplay between malignant cells and multiple host factors. The transition from benign tumor growth to malignancy is manifested by the ability of tumor cells to traverse tissue barriers and invade surrounding tissues[31]. Among a multitude of factors playing a role, the small calcium-binding protein S100A4 has been found to add to the invasive and metastatic capacity of cancer cells[32,33]. Recent studies have shown S100A4 is up-regulated in many types of epithelial cancers, including esophageal squamous cell[17-20]. S100A4S plays an important role in tumor progression and invasion. However, the exact molecular function or mechanism by which S100A4 exerts its putative metastasis-promoting effects has not been fully elucidated, and the protein is most likely involved in several aspects of tumor progression[34,35].
EMT is a crucial process during morphogenesis of multi-cellular organisms. EMT not only is a normal developmental process but also plays a role in tumor invasion and metastasis[36]. Currently, EMT is thought to be a key step for cancer metastasis[37]. One of the key features of EMT is the down-regulation of the expression of the cell adhesion molecule E-cadherin, a critical event in tumor invasion. Many reports have found E-cadherin expression was significantly reduced in ESCCs, and lower expression of E-cadherin followed by increased lymph node metastasis and poor procession[2,38-40]. Several studies have recently described a direct interaction and/or reciprocal influence between S100A4 and E-cadherin[24-30].
In the present study, we found that expression of S100A4 was significantly associated with nodal metastasis in ESCC. The ESCC tissue with a high S100A4 expression had a weak E-cadherin expression, and the expression of S100A4 was significantly associated with decreased E-cadherin. It is suggestd S100A4 promotes migration and invasion may correlate with the downregulation of E-cadherin expression.
Several studies found knockdown of S100A4 inhibits invasion and proliferation in carcinoma cells, and overexpression of S100A4 promotes invasion and proliferation[41-44]. To test the significance of S100A4 expression in ESCC, we transfected the S100A4 siRNA into EC109 cells to knockdown of S100A4. After transfection, the invasive ability of EC109 cells decreased dramatically. Our results were consequent with the resent report[45].
We also observed that S100A4 gene suppression significantly increased the expression of E-cadherin. When we restored the expression of S100A4 in the stable transfected EC109 cells, only to find significantly increased invasion ability in EC109 cells followed by the downregulation of E-cadherin expression. To prove that overexpression of S100A4 promotes invasion again, the TE-13 cells (less S100A4 expression) was transfected with S100A4 cDNA, only to find significantly increased the invasion ability of TE-13 cells, followed by the downregulation of E-cadherin. However, when the S100A4 cDNA transfected TE13 cells were transfected with E-cadherin cDNA to restore the expression of E-cadherin in the TE-13 cells, only to find significantly decreased invasion ability in the TE-13 cells. These data suggest that the S100A4 gene controls the invasion ability of ESCC by regulation of E-cadherin.
In vivo, we found S100A4 knockdown can synergistically reduce the metastatic burden using a EC109 pseudometastatic model in immunodeficient mice, and the effects reached statistical significance. We also observed that overexpression of S100A4 increased the metastatic burden in a TE13 pseudometastatic model.
In summary, we demonstrated that the S100A4 gene controls invasion ability of human ESCC cells through the regulation of E-cadherin gene. We suggest that control of invasion and metastasis through suppression of the S100A4 gene may contribute to a novel therapeutic approach against ESCC. This approach could be realized through development of specific S100A4 inhibitors or use of a gene therapy approach.
COMMENTS
Background
Esophagus squamous cell carcinoma (ESCC) is one of the most deadly malignances because of its high frequency of metastasis. S100A4 possesses a wide range of biological functions, such as regulation of angiogenesis, cell survival, motility and invasion. In the study, the authors explored a potential role of S100A4 in ESCC metastasis.
Research frontiers
S100A4 was overexpressed or silenced by S100A4 siRNA in TE-13 or Eca-109 cells in vitro and in vivo, and to explore the influence of S100A4 on esophageal cancer invasion and metastasis.
Innovations and breakthroughs
It has reported S100A4 may play an important role in promoting metastasis and the early step of tumorigenesis of human cancers. Furthermore, S100A4 silencing could suppress invasion and metastasis in many cancer cells.
Applications
S100A4 may be involved in ESCC progression, RNA interference (RNAi) targeting S100A4 is a potential therapeutic method for human ESCCs.
Terminology
S100A4, also known as mts1, p9Ka, FSP1, CAPL, calvasculin, pEL98, metastasin, 18A2, and 42A, was cloned in the 1980s and early 1990s from various cell systems. S100A4 is localized in the nucleus, cytoplasm, and extracellular space and possesses a wide range of biological functions, such as regulation of angiogenesis, cell survival, motility, and invasion.
Peer review
The study investigates the role of S100A4 oncoprotein in esophageal cancer samples and cell lines. The study clearly shows that the oncoprotein mediates tumor invasion by down-regulating CDH1.
Footnotes
Peer reviewers: Francesco Crea, MD, PhD, Division of Pharmacology, University of Pisa, Via Roma 55, Pisa 56100, Italy; Lin Zhang, PhD, Associate Professor, Department of Pharmacology and Chemical Biology, University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, UPCI Research Pavilion, Room 2.42d, Hillman Cancer Center, 5 117 Centre Ave., Pittsburgh, PA 15213-1863, United States
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
References
- 1.Vallböhmer D, Brabender J, Metzger R, Hölscher AH. Genetics in the pathogenesis of esophageal cancer: possible predictive and prognostic factors. J Gastrointest Surg. 2010;14 Suppl 1:S75–S80. doi: 10.1007/s11605-009-1021-5. [DOI] [PubMed] [Google Scholar]
- 2.Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Dis Esophagus. 2009;22:9–20. doi: 10.1111/j.1442-2050.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Gavert N, Ben-Ze’ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 6.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251–258. doi: 10.1016/j.humpath.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Kuwabara Y, Yamada T, Yamazaki K, Du WL, Banno K, Aoki D, Sakamoto M. Establishment of an ovarian metastasis model and possible involvement of E-cadherin down-regulation in the metastasis. Cancer Sci. 2008;99:1933–1939. doi: 10.1111/j.1349-7006.2008.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura S, Shiozaki H, Miyata M, Kadowaki T, Inoue M, Matsui S, Iwazawa T, Takayama T, Takeichi M, Monden M. Decreased E-cadherin expression is associated with haematogenous recurrence and poor prognosis in patients with squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1608–1614. doi: 10.1002/bjs.1800831138. [DOI] [PubMed] [Google Scholar]
- 10.Miyata M, Shiozaki H, Kobayashi K, Yano H, Tamura S, Tahara H, Mori T. [Correlation between expression of E-cadherin and metastases in human esophageal cancer: preliminary report] Nihon Geka Gakkai Zasshi. 1990;91:1761. [PubMed] [Google Scholar]
- 11.Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 12.Sherbet GV, Lakshmi MS. S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Res. 1998;18:2415–2421. [PubMed] [Google Scholar]
- 13.Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS. Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene. 1993;8:999–1008. [PubMed] [Google Scholar]
- 14.Cho YG, Nam SW, Kim TY, Kim YS, Kim CJ, Park JY, Lee JH, Kim HS, Lee JW, Park CH, et al. Overexpression of S100A4 is closely related to the aggressiveness of gastric cancer. APMIS. 2003;111:539–545. doi: 10.1034/j.1600-0463.2003.1110502.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim MA, Lee HS, Lee HE, Kim JH, Yang HK, Kim WH. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442–451. doi: 10.1111/j.1365-2559.2009.03247.x. [DOI] [PubMed] [Google Scholar]
- 16.Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Schlag PM. Diagnostic and prognostic value of metastasis inducer S100A4 transcripts in plasma of colon, rectal, and gastric cancer patients. J Mol Diagn. 2011;13:189–198. doi: 10.1016/j.jmoldx.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotta K. [Role of Japanese antibiotics in world development of penicillin] Jpn J Antibiot. 2010;63:179–204. [PubMed] [Google Scholar]
- 18.Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH, Yoo NJ, Lee JY, Park WS. Overexpression of S100A4 is closely associated with progression of colorectal cancer. World J Gastroenterol. 2005;11:4852–4856. doi: 10.3748/wjg.v11.i31.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen K, Mori H, Fata J, Bascom J, Oyjord T, Mælandsmo GM, Bissell M. The metastasis-promoting protein S100A4 regulates mammary branching morphogenesis. Dev Biol. 2011;352:181–190. doi: 10.1016/j.ydbio.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ninomiya I, Ohta T, Fushida S, Endo Y, Hashimoto T, Yagi M, Fujimura T, Nishimura G, Tani T, Shimizu K, et al. Increased expression of S100A4 and its prognostic significance in esophageal squamous cell carcinoma. Int J Oncol. 2001;18:715–720. doi: 10.3892/ijo.18.4.715. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K, Zhang S, Jiao X, Wang H, Zhang D, Niu Z, Shen Y, Lv L, Zhou Y. Slug regulates proliferation and invasiveness of esophageal adenocarcinoma cells in vitro and in vivo. Med Oncol. 2010;28:1089–1100. doi: 10.1007/s12032-010-9652-7. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Vitali R, Mancini C, Cesi V, Tanno B, Mancuso M, Bossi G, Zhang Y, Martinez RV, Calabretta B, Dominici C, et al. Slug (SNAI2) down-regulation by RNA interference facilitates apoptosis and inhibits invasive growth in neuroblastoma preclinical models. Clin Cancer Res. 2008;14:4622–4630. doi: 10.1158/1078-0432.CCR-07-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriyama-Kita M, Endo Y, Yonemura Y, Heizmann CW, Miyamori H, Sato H, Yamamoto E, Sasaki T. S100A4 regulates E-cadherin expression in oral squamous cell carcinoma. Cancer Lett. 2005;230:211–218. doi: 10.1016/j.canlet.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Endo Y, Yonemura Y, Heizmann CW, Schafer BW, Watanabe Y, Sasaki T. Clinical significance of S100A4 and E-cadherin-related adhesion molecules in non-small cell lung cancer. Int J Oncol. 2000;16:1125–1131. doi: 10.3892/ijo.16.6.1125. [DOI] [PubMed] [Google Scholar]
- 26.Yonemura Y, Endou Y, Kimura K, Fushida S, Bandou E, Taniguchi K, Kinoshita K, Ninomiya I, Sugiyama K, Heizmann CW, et al. Inverse expression of S100A4 and E-cadherin is associated with metastatic potential in gastric cancer. Clin Cancer Res. 2000;6:4234–4242. [PubMed] [Google Scholar]
- 27.Pedersen KB, Nesland JM, Fodstad Ø, Maelandsmo GM. Expression of S100A4, E-cadherin, alpha- and beta-catenin in breast cancer biopsies. Br J Cancer. 2002;87:1281–1286. doi: 10.1038/sj.bjc.6600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon CS, Hyung WJ, Lee JH, Chae YS, Won NH, Yeom BW, Choi JS. Expression of S100A4, E-cadherin, alpha- and beta-catenin in gastric adenocarcinoma. Hepatogastroenterology. 2008;55:1916–1920. [PubMed] [Google Scholar]
- 29.Ikoma N, Yamazaki H, Abe Y, Oida Y, Ohnishi Y, Suemizu H, Matsumoto H, Matsuyama T, Ohta Y, Ozawa A, et al. S100A4 expression with reduced E-cadherin expression predicts distant metastasis of human malignant melanoma cell lines in the NOD/SCID/gammaCnull (NOG) mouse model. Oncol Rep. 2005;14:633–637. [PubMed] [Google Scholar]
- 30.Kwak JM, Lee HJ, Kim SH, Kim HK, Mok YJ, Park YT, Choi JS, Moon HY. Expression of protein S100A4 is a predictor of recurrence in colorectal cancer. World J Gastroenterol. 2010;16:3897–3904. doi: 10.3748/wjg.v16.i31.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 34.Sherbet GV. Metastasis promoter S100A4 is a potentially valuable molecular target for cancer therapy. Cancer Lett. 2009;280:15–30. doi: 10.1016/j.canlet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Tarabykina S, Griffiths TR, Tulchinsky E, Mellon JK, Bronstein IB, Kriajevska M. Metastasis-associated protein S100A4: spotlight on its role in cell migration. Curr Cancer Drug Targets. 2007;7:217–228. doi: 10.2174/156800907780618329. [DOI] [PubMed] [Google Scholar]
- 36.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 37.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 38.Salahshor S, Naidoo R, Serra S, Shih W, Tsao MS, Chetty R, Woodgett JR. Frequent accumulation of nuclear E-cadherin and alterations in the Wnt signaling pathway in esophageal squamous cell carcinomas. Mod Pathol. 2008;21:271–281. doi: 10.1038/modpathol.3800990. [DOI] [PubMed] [Google Scholar]
- 39.Chung Y, Lam AK, LUnited Kingdom JM, Law S, Chan KW, Lee PY, Wong J. Altered E-cadherin expression and p120 catenin localization in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3260–3267. doi: 10.1245/s10434-007-9511-8. [DOI] [PubMed] [Google Scholar]
- 40.Chibana Y, Fujii S, Ichikawa K, Fujita M, Ono Y, Tomita S, Imura J, Kawamata H, Terano A, Fujimori T. Tumor cell dissociation score highly correlates with lymph node metastasis in superficial esophageal carcinoma. J Gastroenterol Hepatol. 2005;20:1371–1378. doi: 10.1111/j.1440-1746.2005.03858.x. [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Yang Y, Wang Y, An G, Lv G. Small interfering RNA-directed knockdown of S100A4 decreases proliferation and invasiveness of osteosarcoma cells. Cancer Lett. 2010;299:171–181. doi: 10.1016/j.canlet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Guo Y, Fu S, Yang M, Sun KL, Fu WN. Hypomethylation-induced expression of S100A4 increases the invasiveness of laryngeal squamous cell carcinoma. Oncol Rep. 2010;23:1101–1107. [PubMed] [Google Scholar]
- 43.Hua J, Chen D, Fu H, Zhang R, Shen W, Liu S, Sun K, Sun X. Short hairpin RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses proliferation of BGC823 gastric cancer cells in vitro and in vivo. Cancer Lett. 2010;292:41–47. doi: 10.1016/j.canlet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang HY, Zheng XZ, Wang XH, Xuan XY, Wang F, Li SS. S100A4 mediated cell invasion and metastasis of esophageal squamous cell carcinoma via the regulation of MMP-2 and E-cadherin activity. Mol Biol Rep. 2012;39:199–208. doi: 10.1007/s11033-011-0726-1. [DOI] [PubMed] [Google Scholar]