Abstract
AIM: To investigate the relationship between Interleukin-8 (IL-8) and proliferation, adhesion, migration, invasion and chemosensitivity of gastric cancer (GC) cells.
METHODS: The IL-8 cDNA was stably transfected into human GC cell line MKN-45 and selected IL-8-secreting transfectants. The expression of IL-8 in human GC cell line KATO-III was inhibited by RNA interference. The expressions of mRNA and protein of IL-8 in GC cells were detected by real-time reverse transcription-polymerase chain reaction or enzyme-linked immunosorbent assay (ELISA).
RESULTS: The overexpression of IL-8 resulted in an increased cell adhesion, migration and invasion, and a significant resistance to oxaliplatin in MKN-45 cells. Inhibition of IL-8 expression with small interfering RNA decreased the adhesion, migration and invasion functions and oxaliplatin resistance in KATO-III cells. IL-8 increased NF-κB and Akt activities and adhesion molecules ICAM-1, VCAM-1, and CD44 expression in GC cells.
CONCLUSION: Overexpression of IL-8 promotes the adhesion, migration, invasion, and chemoresistance of GC cells, indicating that IL-8 is an important therapeutic target in GC.
Keywords: Interleukin-8, Gastric cancer, Adhesion, Migration, Invasion
INTRODUCTION
Gastric cancer (GC) is still a serious health problem and remains the second most common type of fatal cancer worldwide[1,2]. GC is one of the most aggressive tumors and is frequently associated with lymph node metastasis, peritoneal dissemination and hematogenous metastasis.
Interleukin-8 (IL-8), a cytokine of the CXC chemokine family that was originally classified as neutrophil chemoattractant, is now reported to play an important role in tumor progression and metastasis in a variety of human cancers[3]. It has been suggested that tumor cells produce IL-8 as an autocrine growth factor, which promotes tumor growth, tissue invasion and metastatic spread[4,5]. Moreover, IL-8 expression correlates with vascularity in gastric carcinoma[6]. In human moderately differentiated gastric adenocarcinoma cancer cell line SCG-7901[7] and poorly differentiated adenocarcinoma cancer cell line TMK-1[8], constitutive expression of IL-8 has been linked to tumorigenesis and angiogenesis in vitro and in vivo. However, the exact role of IL-8 in the progressive tumorigenesis of GC remains unclear.
The purpose of this study was to provide evidence for the role of IL-8 in determining the migration, invasion and chemosensitivity of human GC. We found that expression of IL-8 participated in the migration and invasion and was correlated with oxaliplatin resistance of GC cells in vitro. This study may provide the basis for the development of new therapies for GC by increasing chemosensitivity and decreasing the proliferation, migration and invasion of the cancer cells.
MATERIALS AND METHODS
Cell culture
Human GC cell lines MKN-45, and KATO-III, and human umbilical vein endothelial cells (HUVECs) were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). GC cells were cultured in RPMI-1640 medium (Invitrogen, United States) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 100 IU/mL penicillin and 100 μg/mL streptomycin (Invitrogen). HUVECs were cultured in Human Endothelial-SFM (Invitrogen). Cells were maintained at 37 °C in a humidified chamber containing 5% CO2.
Stable overexpression of interleukin-8 in MKN-45 cells
The empty plasmid vector pcDNA3.1 (Invitrogen) or the plasmid vector containing IL-8 cDNA was transfected into MKN-45 cells using Lipofectamine 2000 (Invitrogen). Multiple clones were selected in the presence of 0.75 mg/mL G418. IL-8-transfected clones were screened for IL-8 expression. Stably transfected clones were picked and maintained in the medium containing 0.1 mg/mL G418. To avoid clonal variations, six positive clones were pooled for further studies.
Stable knockdown of interleukin-8 in KATO-III cells
The DNA sequence of RNAi of IL-8 was designed to hybridize and destroy human IL-8 mRNA (accession no. NM_000584) using the Web- based siRNA target finder and design tool provided at the Ambion website (Ambion). The DNA sequence of RNAi of IL-8 (sense, 5’-ACCACCGGAAGGAACCAUCdTdT-3’; antisense, 5’-GAUGGUUCCUUCCGGUGGUdTdT-3’) was synthesized and cloned into the pSilencer 2.1-U6 neo (Ambion) according to the manufacturer’s instructions. The human specific negative control siRNA was also designed with the sequences asfollows: sense: 5’-UUCUCCGAACGUGUACGUdTdT-3’; antisense: 5’-ACGUGACACGUUCGGAGAAdTdT-3’.
The negative control siRNA vector or the vector containing RNAi sequence of IL-8 was transfected into KATO-III cells using Lipofectamine 2000. Multiple clones were selected in the presence of 0.4 mg/mL G418. IL-8-RNAi clones were screened for IL-8 expression. Stable RNAi clones were picked and maintained in the medium containing 0.1 mg/mL G418. To avoid clonal variations, six positive clones were pooled for further studies.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed using commercial IL-8 ELISA kits from R and D Systems. Cells (1 × 106 cells per well) were plated in a 6-well plate and incubated at 37 °C for 72 h. An equal volume of cell culture supernatants was collected. The assays were done in triplicate, and the concentration of IL-8 in culture supernatants was determined by comparing their optical density with the standard curve.
3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
Cells growing exponentially were plated in 96-well plates at a density of 1 × 105 cells per well for 7 d. One hundred microliters of 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) stock solution (1 mg/mL) was added to each well daily, and the cells were further incubated at 37 °C for 4 h. The supernatant was discarded and 200 μL dimethyl sulfoxide was added. When the precipitates were completely dissolved, the absorbance at wavelength 595 nm was measured with a micro-ELISA reader.
Growth inhibition assay
Growth inhibition was measured as previously described[9]. Cells were trypsinized and seeded at 1 × 104 cells per well in 96-well plates. After 24 h, cells were exposed to oxaliplatin (Sigma, St. Louis, MO) for 72 h, at stepwise concentrations from 0 μg/mL to 10 μg/mL. The cells were quantified as described in MTT assay, to calculate the mean cell growth inhibition.
Cell adhesion assay
To measure the cell adhesion, monolayer adhesion assay and extracellular matrix component (ECM) adhesion assay were performed. For the monolayer adhesion assay, HUVECs were seeded onto 24-well plates (1 × 105 cells/well) 48 h before adhesion assay. Cells (1 × 105 cells/well) were seeded onto HUVEC monolayers and incubated at 37 °C. After 2 h, non-adherent cells were removed by washing with phosphate-buffered saline (PBS).
For ECM adhesion assay, 96-well plates were coated with human fibronectin (BD Biosciences) at a final concentration of 2 μg/cm2 overnight at 4 °C. Plates were washed with 1% bovine serum albumin in PBS to block nonspecific cell adhesion. Cells were seeded and incubated for 2 h. Nonadherent cells were washed up to three times with PBS.
The adherent cells were quantified as described in MTT assay, to calculate the mean cellular adhesion capability. The absorbance at 570 nm was measured by the ELX-800 ELISA plate reader (Bio-Tek Instruments, Winooski, VT). After subtracting background absorbance, results were calculated as the mean cellular adhesion rate.
Cell migration assay
To measure the cell migration activity, Transwell and wound-healing assays were performed. The Transwell cell migration assay was performed as previously described[10] using Transwells (8 μmol/L pore size polycarbonate membrane) obtained from Corning. Cells (1 × 105) in 0.5 mL serum-free medium were placed in the upper chamber, whereas the lower chamber was loaded with 0.8 mL medium containing 10% FBS. The total number of cells that migrated into the lower chamber was counted after 24 h of incubation at 37 °C with 5% CO2. Nonmigratory cells were removed. Migratory cells were stained with 0.2% crystal violet in 10% ethanol. To quantitate migratory cells, three independent fields of migratory cells per well were photographed under phase contrast microscope. The number of cells per field was counted and averaged.
To carry out the wound-healing assay, cells (5 × 105 cells per well) were plated in 6-well plates. After 24 h, the confluent monolayer cells were scratched manually with a plastic pipette tip, and after being washed with PBS, wounded monolayers of the cells were allowed to heal for 12-24 h. Each migration assay was done for at least three times independently.
Cell invasion assay
Cell invasion was measured using 8-μm pore BD BioCoat Matrigel Invasion Chambers (BD Biosciences) according to the manufacturer’s instructions. Cells (2.5 × 105) were added to chambers and incubated for 24 h at 37 °C. Matrigel and noninvasive cells were removed and chambers were stained as described above. To quantitate invasive cells, three independent fields of invasive cells per well were photographed under phase contrast microscope. The number of cells per field was counted and averaged. Each invasion assay was done at least three times independently.
RNA isolation and reverse transcription polymerase chain reaction
Total RNA was purified from cells using a Trizol reagent (Life Technol-ogies). First-strand cDNA was synthesized using 2.5 μg RNA and AMVretroviridase (Promega). Quantitative real-time polymerase chain reaction (PCR) was performed using the Bio-Rad iCycler iQ real-time PCR system (Bio-Rad) and following primers: IL-8, IL8-L: 5’-ATGACTTCCAAGCTGGCCGTGGCT-3’; IL8-R: 5’-TCTCAGCCCTCTTCAAAAACTTCT-3’. As a control, each cDNA sample was simultaneously subjected to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the primers: GAPDH-L: 5’-CCACCCATGGCAAATTCC ATGGCA-3’; GAPDH-R: 5’-TCTAGACGGCAGGTCA GGTCCACC-3’. The threshold cycle (Ct) of each sample was determined, and the relative level of a transcript (2ΔCt) was calculated by obtaining ΔCt (test Ct - GAPDH Ct) and then expressed as arbitrary units (1/2ΔCt 1 × 100) = fold difference.
Protein isolation and Western blotting
Cells at 80% culture confluence were harvested for Western blotting analysis. The harvested cells were lysed and their protein concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, IL). The cell lysates (50 μg protein each lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes (Hyclone, Logan, UT). The membranes were blocked with 5% (v/v) skim milk and probed with primary antibody at 4 °C overnight. Following washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. Primary antibodies were specific for phospho-NF-κB-p65, NF-κB-p65, phospho-Akt, Akt, ICAM-1, VCAM-1, CD44, and β-actin (Cell Signaling Technology, Inc., Danvers, MA). The bound antibodies were visualized using an electrochemiluminescence system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Statistical analysis
Data were presented as means ± SE. Differences of the variables between groups were analyzed by Student’s t test. Differences were considered significant when P < 0.05.
RESULTS
Stable overexpression and silencing expression of interleukin-8 in gastric cancer cells
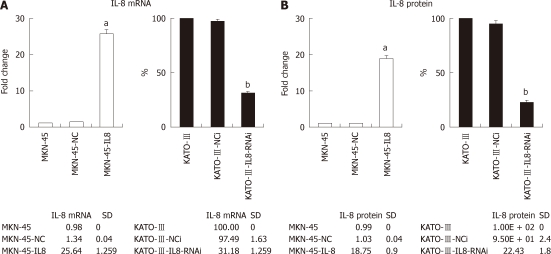
To investigate the effect of IL-8 in GC, the mRNA expression of IL-8 was quantified in three GC cells (Figure 1A). MKN-45 cell lines had lower expression of IL-8 in comparison with KATO-III cells, which is consistent with a previous study by Kitadai et al[11]. To increase the IL-8 expression, MKN-45 cells were stably transfected with full-length IL-8 expression construct. The RNAi technology was employed in order to stably silence the IL-8 expression in KATO-III cells.The mRNA expression of IL-8 in cells was examined by real-time PCR. The ELISA assay was performed to quantify secreted IL-8 present in the cell culture medium. After transfection and G418 selection, IL-8 mRNA expression level in the stably IL-8 transfected MKN-45 cells (MKN-45-IL8) was 25.6-folds higher than in MKN-45 cells (Figure 1A). As shown in Figure 1B, the secretion levels of IL-8 in MKN-45-IL8 cells were 18.4-folds higher than in MKN-45 cells. Endogenous IL-8 mRNA expression and protein secretion levels were significantly lowered by RNAi in KATO-III cells (KATO-III-IL8-RNAi) when compared with control transfections (KATO-III-NCi) and KATO-III cells (Figure 1).
Figure 1.
Interleukin-8 expression of gastric cancer cells. A: Real-time polymerase chain reaction determined the level of Interleukin-8 (IL-8) mRNA in gastric cancer (GC) cells. Expression levels were determined by the ΔΔCt method using glyceraldehyde-3-phosphate dehydrogenase as endogenous control. Histograms show fold change over the relative expression levels of IL-8 mRNA of control cells. Each bar represents the mean ± SD (aP < 0.05 vs MKN-45, bP < 0.05 vs KATO-III); B: IL-8 production in GC cells measured by enzyme-linked immunosorbent assay. Histograms show fold change in the relative expression levels of IL-8 protein of control cells. All experiments were repeated three times with similar results. Each bar represents the mean ± SD (aP < 0.05 vs MKN-45, bP < 0.05 vs KATO-III).
Interleukin-8 insignificantly affects proliferation of gastric cancer cells
Since IL-8 has been reported to be an autocrine growth factor[12], we examined its role in the growth of human GC cells. Overexpression or silencing expression of IL-8 in GC cells had insignificant effect on cancer cell proliferation as measured by the MTT assay (data not shown).
Interleukin-8 increases resistance to oxaliplatin in gastric cancer cells
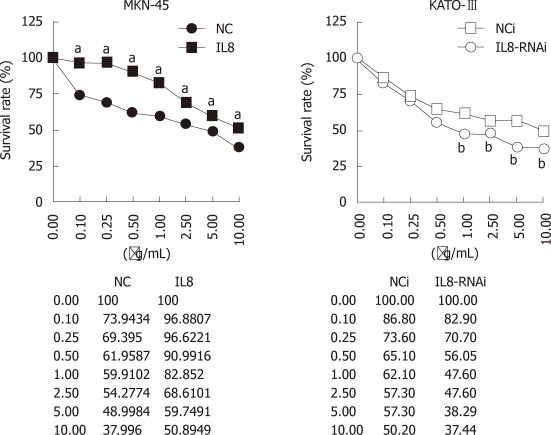
Previous reports indicated that increased IL-8 was associated with chemoresistance, such as oxaliplatin[13-15]. To investigate whether IL-8 is associated with chemosensitivity in GC cells, the effects of IL-8 expression on oxaliplatin sensitivity in IL-8-overexpressed MKN-45-IL8 cells and IL-8-silenced KATO-III-IL8-RNAi cells were tested using growth inhibition analyses.
As shown in Figure 2, MKN-45-NC and KATO-III-NCi cells were sensitive to oxaliplatin. In MKN-45-IL8 cells, the survival rate significantly increased than in the control cells (P < 0.05) after treatment with oxaliplatin at the concentrations from 0.1 μg/mL to 10 μg/mL (P < 0.05). On the contrary, when IL-8 expression was suppressed in KATO-III cells using RNAi, there was significant decrease in growth rate after treatment with 1.0-10 μg/mL oxaliplatin (P < 0.05). These results suggest that IL-8 expression in GC cells decreased the sensitivity to the cytotoxic effects of oxaliplatin.
Figure 2.
Growth inhibition assay of gastric cancer cells treated with oxaliplatin. Survival of gastric cancer cells is tested by 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Each bar represents the mean ± SD. All experiments were repeated three times with similar results (aP < 0.05 vs MKN-45-NC, bP < 0.05 vs KATO-III-NCi).
Interleukin-8 is involved in gastric cancer cell adhesion
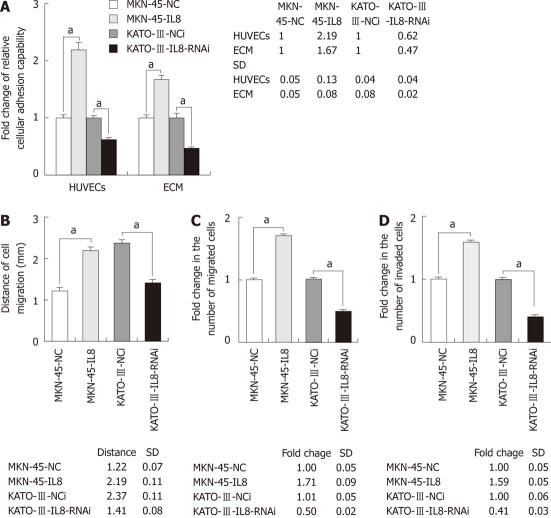
To determine the role of IL-8 expression in GC cell adhesion, adhesion capacity of GC cells to endothelium or extracellular matrix components was evaluated (Figure 3A). The 2-h adhesion capability of GC cells to a monolayer of HUVECs or ECM components was significantly increased in MKN-45-IL8 cells as compared with the control cells. Silencing expression of IL-8 in KATO-III cells significantly reduced the cell adhesion capability (P < 0.05).
Figure 3.
Interleukin-8 involved in gastric cancer cell adhesion, migration and invasion. A: Effect of Interleukin-8 (IL-8) on gastric cancer (GC) cell adhesion to human endothelial cells and extracellular matrix components. Histograms show fold change in the relative OD value of control cells quantified by cell proliferation assay to calculate the mean cellular adhesion capability; B: Effect of IL-8 on GC cell migration tested by wound-healing assays. Histograms show distances of cell migration; C: Effect of IL-8 on GC cell migration tested by Transwell assays. Histograms show fold change in the number of migrated cells vs the control cells; D: Effect of IL-8 on GC cell invasion. Histograms show fold change in the number of invaded cells vs the control cells. Each bar represents the mean ± SD. All experiments were repeated three times with similar results (aP < 0.05). HUVEC: Human umbilical vein endothelial cell.
Interleukin-8 is involved in gastric cancer cell migration and invasion
To measure the cell migration activity, Transwell and wound-healing assays were performed. As shown in Figure 3B and C, expression of IL-8 could significantly promote GC cell migration into the cell-free region and migration through cell culture inserts. The invasive potential of GC cells was investigated using in vitro Matrigel invasion assay. Cell invasion was similar to cell migration. MKN-45-IL8 cells exhibited significant increase in invasion capacity whereas KATO-III-IL8-RNAi cells exhibited significant decrease (Figure 3D). Therefore, IL-8 overexpression was sufficient to increase the rate of GC cell migration and in vitro invasion.
Interleukin-8 increases NF-κB and Akt activities, adhesion molecules ICAM-1 and VCAM-1, and CD44 expression in gastric cancer cells
Recent studies have reported that induction of IL-8 signaling increased NF-κB transcriptional activity, and activated the phosphoinositide-3-kinase and cascade. As shown in Figure 4, the levels of phospho-NF-κB-p65 and phospho-Akt were upregulated in IL-8 overexpressed MKN-45-IL8 cells compared with the control cells, and were downregulated in KATO-III-IL8-RNAi cells. These data suggest that constitutive IL-8 expression strongly increased NF-κB and Akt activation.
Figure 4.
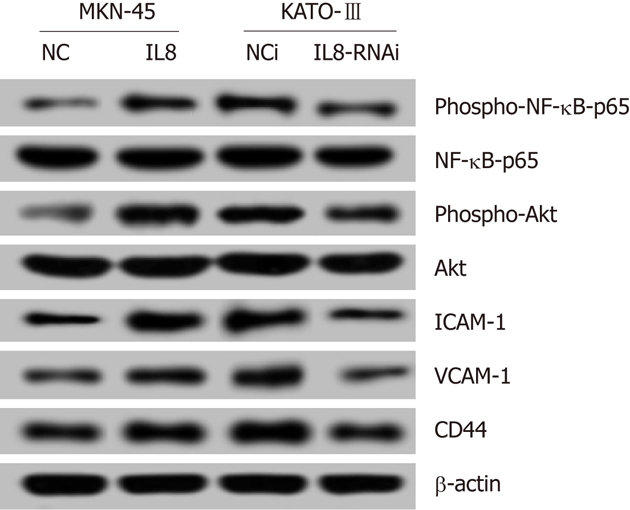
Effect of Interleukin-8 on NF-κB and Akt activities and adhesion molecules ICAM-1 and VCAM-1, and CD44 expression in gastric cancer cells. The protein expression levels of phospho-NF-κB-p65, NF-κB-p65, phospho-Akt, Akt, ICAM-1, VCAM-1, and CD44 were determined by Western blotting. All experiments were repeated three times with similar results.
Adhesion molecules play an important role in cell-cell, cell-ECM interactions in cancer invasion and metastasis. As shown in Figure 4, IL-8 increased the protein expression levels of adhesion molecules ICAM-1 and VCAM-1, and CD44 expression in GC cells.
DISCUSSION
It has been shown that IL-8 modulates proliferation and migration of tumor cells, including melanoma[16-18], prostate cancer[14,19], breast cancer[20], and colon cancer[13]. A study confirmed that IL-8 increases angiogenesis of human gastric carcinoma[6]. Our goal was to evaluate whether IL-8 is involved in proliferation, adhesion, migration, invasion and the sensitivity to chemotherapeutics in human GC cell lines. We found that IL-8 overexpression in GC cells was associated with increased adhesion, migration, and invasion activity and resistance to oxaliplatin, suggesting that IL-8 is a promising therapeutic target.
In this study, we examined the biological role of IL-8 in adhesion, migration, and invasion of GC cells with either overexpression or knocked down expression of IL-8. The stable IL-8 transfectants and IL-8 RNAi were successfully generated in MGC803 cells. The constitutive expression of IL-8 in MGC803 cells increased cell adhesion, migration and invasion, which was opposed by silencing IL-8 expression in KATO-III cells using RNAi. Our findings suggest that expression of IL-8 plays an important role in modulating cell adhesion, migration, and invasion of GC cells. This observation is supported by the study of Ju et al[7], which showed that recombinant interleukin-8 promoted the adhesion, migration and invasion of GC SCG-7901 cells and up-regulated the expression of matrix metalloproteinase-9, intercellular adhesion molecule-1 and E-cad in vitro. However, IL-8 in GC cells had insignificant effect on cell proliferation according to the previous studies[6,7].
The NF-κB and Akt signaling pathway activations are involved in cellular transformation, survival, proliferation, invasion, angiogenesis, metastasis and inflammation in cancers. It has been reported that NF-κB stimulates IL-8 production, and endogenous IL-8 causes constitutive activation of NF-κB (p65) in colon cancer[13] and prostate cancer cells[14]. In this study, we found that IL-8 overexpression caused activation of NF-κB and Akt signaling in GC cells.
It is widely accepted that the invasion and metastasis of caner is dependent on the capacity of cancer cell adhesion and migration[21]. In this study, we found that GC cells overexpressing IL-8 produced increased adhesion and migration activity with upregulated ICAM-1, VCAM-1, and CD44. Further investigations are warranted to elucidate the regulative mechanism of IL-8 as a migratory and invasive factor in GC cells.
In conclusion, our studies provide significant evidence that IL-8 expression contributes to GC cell adhesion, migration and invasion, and leads to resistance to oxaliplatin. These findings may help develop novel IL-8-targeted therapies for GC.
COMMENTS
Background
Gastric cancer (GC) is still a serious health problem and remains the second most common type of fatal cancer worldwide. Interleukin-8 (IL-8), a cytokine of the CXC chemokine family that was originally classified as neutrophil chemoattractant, is now reported to play an important role in tumor progression and metastasis in a variety of human cancers.
Research frontiers
The exact role of IL-8 in the progressive tumorigenesis of GC remains unclear. The purpose of this study was to provide evidence for the role and molecular mechanism of IL-8 in determining the migration, invasion and chemosensitivity of human GC.
Innovations and breakthroughs
This study demonstrated that IL-8 expression is associated with cell adhesion, migration, and invasion in GC. Overexpression of IL-8 promotes invasion phenotype of GC cells with activated NF-κB and Akt and increased expression of adhesion molecules ICAM-1, VCAM-1, and CD44 in vitro.
Applications
The findings help clarify the molecular mechanisms of IL-8 involved in GC invasion and indicate that IL-8 may be an important therapeutic target in GC.
Peer review
The authors have shown that overexpression of IL-8 in vitro promotes the adhesion, migration, invasion, and chemoresistance of some gastric cancer cell lines, thus indicating IL-8 as possible therapeutic target in gastric cancer. The work addresses an interesting topic with the proper methodological approach.
Footnotes
Supported by The Fund of Nanjing Medical University Science and Technology Development, No. 09NJMUZ30
Peer reviewers: Tim Tak Kwok, Associate Professor, School of Biomedical Sciences, The Chinese University of Hong Kong, Shatin, NT, Hong Kong, China; Gabriele Grassi, Department of Medical, Technological and Tran, University Hospital of Cattinara, Trieste 34100, Italy
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
References
- 1.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley RR, Villares G, Sanguino A, Lutgendorf SK, Lopez-Berestein gastric cancer ing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100:359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kido S, Kitadai Y, Hattori N, Haruma K, Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y, et al. Interleukin 8 and vascular endothelial growth factor -- prognostic factors in human gastric carcinomas? Eur J Cancer. 2001;37:1482–1487. doi: 10.1016/s0959-8049(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 7.Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2010:[Epub ahead of print]. doi: 10.1007/s12032-010-9780-0. [DOI] [PubMed] [Google Scholar]
- 8.Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, et al. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647–653. doi: 10.1038/sj.bjc.6690742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriguchi M, Nonaka Y, Yanagie H, Yoshizaki I, Takeda Y, Sekiguchi M. A molecular biological study of anti-tumor mechanisms of an anti-cancer agent Oxaliplatin against established human gastric cancer cell lines. Biomed Pharmacother. 2003;57:412–415. doi: 10.1016/s0753-3322(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem. 1998;273:8502–8507. doi: 10.1074/jbc.273.14.8502. [DOI] [PubMed] [Google Scholar]
- 11.Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- 12.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 15.Tamatani T, Azuma M, Ashida Y, Motegi K, Takashima R, Harada K, Kawaguchi S, Sato M. Enhanced radiosensitization and chemosensitization in NF-kappaB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108:912–921. doi: 10.1002/ijc.11640. [DOI] [PubMed] [Google Scholar]
- 16.Araki K, Shimura T, Yajima T, Tsutsumi S, Suzuki H, Okada K, Kobayashi T, Raz A, Kuwano H. Phosphoglucose isomerase/autocrine motility factor promotes melanoma cell migration through ERK activation dependent on autocrine production of interleukin-8. J Biol Chem. 2009;284:32305–32311. doi: 10.1074/jbc.M109.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel PS, Varney ML, Dave BJ, Singh RK. Regulation of constitutive and induced NF-kappaB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J Interferon Cytokine Res. 2002;22:427–435. doi: 10.1089/10799900252952217. [DOI] [PubMed] [Google Scholar]
- 18.Satyamoorthy K, Li G, Vaidya B, Kalabis J, Herlyn M. Insulin-like growth factor-I-induced migration of melanoma cells is mediated by interleukin-8 induction. Cell Growth Differ. 2002;13:87–93. [PubMed] [Google Scholar]
- 19.Singh RK, Lokeshwar BL. Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol Cancer. 2009;8:57. doi: 10.1186/1476-4598-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavey C, Mühlbauer M, Bossard C, Freund A, Durand S, Jorgensen C, Jobin C, Lazennec G. Interleukin-8 expression is regulated by histone deacetylases through the nuclear factor-kappaB pathway in breast cancer. Mol Pharmacol. 2008;74:1359–1366. doi: 10.1124/mol.108.047332. [DOI] [PubMed] [Google Scholar]
- 21.Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci. 2011;16:898–936. doi: 10.2741/3726. [DOI] [PubMed] [Google Scholar]