Abstract
Background
During the postpartum period, some women might be under a considerable amount of stress and at increased risk for onset or exacerbation of obsessive–compulsive disorder (OCD). Little is known about the stress response correlates during the postpartum period and in patients with OCD. This study aimed to examine the cerebral, psychologic and endocrine correlates of the stress response in patients with OCD and during the postpartum period.
Methods
Women with postpartum OCD, healthy postpartum women and healthy mothers past the postpartum period underwent functional magnetic resonance imaging while facing a reliable psychosocial stressor (the Montreal Imaging Stress Task). Stress-related psychologic and endocrine responses (i.e., cortisol) were obtained.
Results
We enrolled 12 women with postpartum OCD, 16 healthy postpartum women and 11 healthy mothers past the postpartum period in our study. Compared with healthy postpartum counterparts, postpartum women with OCD had a heightened self-reported and endocrine stress response associated with a distinct brain activation pattern in response to psychosocial stress involving the orbitofrontal and temporal cortices. Moreover, compared with mothers assessed in a period of time beyond the postpartum period, healthy postpartum women did not differ in psychologic and cortisol response to stress, but recruited different brain regions, such as the dorsolateral pre-frontal cortex and the anterior cingulate cortex, during exposure to stress.
Limitations
Potential confounding factors, such as medication use, breastfeeding, parity and personality factors, may have modulated the stress-related endocrine response and could not be assessed in this study.
Conclusion
Obsessive–compulsive disorder and the postpartum period differentially influence the brain circuitry underlying psychosocial stress as well as the psychologic and endocrine responses.
Introduction
The perinatal period is known to be a period of great adaptation in a parent’s life, with increased demands and heightened responsibilities arising from the newborn’s needs. This challenging period is accompanied by great physiologic and mental stress as well as tremendous activation and rewiring of the brain circuitry linked to parental caregiving and attachment.1,2 It is therefore no surprise that the postpartum period is associated with increased risk for mood and anxiety disorders.3,4 To face these new or increased demands, some parents experience preoccupations centred on the newborn’s well-being, which some authors have suggested are similar to obsessive–compulsive symptoms.5 The preoccupations would be healthy and adaptive thoughts and behaviours engendered by hypervigilance for the infant’s safety and health.5,6 However, deregulation of what appears to be an evolutionarily useful pathway could cause parents to overestimate threats and responsibilities for causing or preventing harm, leading to clinical obsessive–compulsive symptoms.6,7 Moreover, while studying stressful life events and the onset of obsessive–compulsive disorder (OCD), Maina and colleagues8 observed that women identified the birth of a live child as the only precipitating/worsening stressful event. Reproductive milestones, especially menarche and the post-partum period, are frequently reported as being a time of onset or exacerbation of OCD symptoms in women.8–13 Recent evidence suggests that the prevalence of postpartum OCD (ppOCD) is greater than that of OCD in the general population, with prevalence rates between 4% and 9%14,15 compared with a lifetime prevalence of about 2%.16,17
Obsessive–compulsive disorder is characterized by intrusive and inappropriate recurrent thoughts, impulses or images (obsessions), repetitive behaviours and/or mental acts (compulsions). With postpartum onset or exacerbation of OCD, the obsessions and compulsions mainly focus on the newborn’s health and environment and create immense distress in affected mothers.7,9,14,15 Examples of post-natal obsessions are fear of harming the baby accidentally or intentionally, of the baby being contaminated and of infant death. Washing and checking rituals often mirror these obsessions.7,8,10,18,19
The psychologic or cognitive–behavioural model suggests that OCD symptoms arise from faulty appraisal of commonly experienced thoughts, images or situations. Obsessions or compulsions happen when an otherwise ordinary internal or external event is appraised as threatening.20 Recent evidence suggests that OCD is associated with dysfunction of cerebral regions mediating error detection and monitoring of the emotional system, including the orbitofrontal and anterior cingulate cortices (OFC and ACC) and the striatum.21–23 Psychologic stress also depends on the appraisal of a certain event. An important stress theory by Lazarus24 argues that for an event to be perceived as stressful, a person has to appraise the demands of this particular event as exceeding his or her available resources. Threats to social value, esteem and status, such as social evaluation, uncontrollability and high achievement ambiance, are also potent stressors.25
Psychologic stress is a powerful trigger of the hypothalamic–pituitary–adrenal (HPA) axis. However, only a few conflicting studies have investigated stress hormone regulation in patients with OCD. Some studies found higher basal cortisol levels in patients with OCD compared with controls, whereas results on the stress-related cortisol response were mixed.26–28 Likewise, limited studies exist on HPA axis function and hormonal stress response during the postpartum period, and again the results of these studies are mixed.29 In a recent pilot study, our group demonstrated that postpartum women with OCD have higher afternoon cortisol levels than healthy postpartum women, with a trend for greater cortisol increase observed in women with ppOCD when facing physiologic stress.30
Brain circuitry underlying the stress response shares a lot of similarities with the OCD orbitofrontal–striatal model, a widely accepted model of OCD brain dysfunction. Acute stress, on the other hand, is consistently associated with deactivation of the OFC and engagement of both the ACC and the medial prefrontal cortex (mPFC).31
The goal of the current study was to explore the cerebral and endocrine correlates of stress response during the post-partum period and in the presence of OCD symptoms. We hypothesized that women with OCD symptoms during their first 6 months postpartum would perceive the stressor as being even more stressful and that this perception of stress would be mirrored by a higher cortisol stress response and greater activation in the OFC in comparison with healthy postpartum mothers. Moreover, we hypothesized that healthy postpartum women would not differ in their psychologic and endocrine responses to stress, but would demonstrate a distinct pattern of activation involving limbic regions in comparison with healthy mothers past the postpartum period.
Methods
Participants
We recruited postpartum women presenting to the Women’s Health Concerns Clinic with symptoms of obsessive–compulsive disorder, postpartum controls and healthy mothers through outpatient clinics and flyers posted at St. Joseph’s Healthcare. Women were included if they were aged 18 years or older, within their first 6 months postpartum or more than 1 year postpartum, right-handed and able to communicate in English. We excluded women with premature or otherwise unwell babies; women with major physical conditions, current psychosis or history of psychotic disorder; heavy smokers; and alcohol/substance abusers. The study was approved by the Research Ethics Board of St. Joseph’s Healthcare, Hamilton, and written informed consent was obtained from all participants.
A primary diagnosis of OCD was established by experienced psychiatrists (M.S. or C.N.S.) and corroborated by the Composite International Diagnostic Interview for Women (CIDI-VENUS)32 and a severity score of 7 or greater on the Yale–Brown Obsessive Compulsive Scale (Y–BOCS).33 Post-partum women also completed an in-house questionnaire with proven validity, the Perinatal Obsessive–Compulsive Scale (POCS),34 to characterize the postpartum-specific obsessive–compulsive symptoms. To assess anxious and depressive symptoms, we used the Edinburgh Postnatal Depression Scale (EPDS),35 the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS)36 and the Spielberger State–Trait Anxiety Inventory (STAI).37 The EPDS, MADRS and STAI are easy to administer, reliable, valid and effective screening tools to measure depression and anxiety symptoms during and after the postnatal period.
The short version of the Childhood Trauma Questionnaire (CTQ),38 Rosenberg Self-Esteem questionnaire (RSE)39 and the Pittsburg Sleep Quality Index (PSQI)40 were also used to evaluate known stress-related confounding factors. To evaluate psychologic response to stress, we asked participants to complete the STAI state questionnaire and to rate how anxious and stressed they felt using visual analogue scales before and after being exposed to the stressor. Women were kept on their respective medications and/or psychologic treatments if applicable. Potential controls were excluded if they had a current or history of psychiatric diagnosis, as assessed by the CIDI-VENUS.
Experimental procedure and stress task
Sessions occurred between 1 pm and 4 pm to control for diurnal hormonal variations. After arrival at the laboratory, participants received standardized instructions from a female researcher. Resting periods of 30 minutes preceded and followed the magnetic resonance imaging (MRI) scanning session, during which participants were asked to complete questionnaires and/or read magazines. Participants were asked to complete the STAI state questionnaire and rate how anxious and stressed they were using a visual analogue scale. The STAI and the visual analogue scales were completed outside the scanner 15 minutes before and immediately after the stressor.
Stress task
To elicit a psychologic stress response, we applied a reliable stress paradigm while the participant lay in the scanner: the Montreal Imaging Stress Task (MIST).41 The MIST requires the performance of computerized challenging mental arithmetic in the presence of negative social evaluation. The arithmetic equations are presented on a computer screen, and the participants provide answers by choosing a 1-digit number from a rotary dial using a 3-button mouse. There are 3 conditions to the MIST: the rest condition where the interface is presented without arithmetic equations to complete; the control condition, which consists of simple mental arithmetic challenges; and the stress–experimental condition. During the stress condition, the difficulty of the arithmetic tasks adapts to the user’s performance to induce failure, preventing the participant from scoring higher than 50%–55%. To increase the socioevaluative threat, prior to the scan, participants are told that the task is usually completed with an average of 80%–90% correct answers. Moreover, while performing the stress condition, participants are provided with continuous negative feedback with a mock performance indicator suggesting poor performance on the part of the participant in comparison to the average female user. Negative feedback is also provided after each task, and participants are told that researchers outside the scanner are monitoring their performance. A time bar is also displayed below the arithmetic equation to increase time pressure. The MIST and its effect on physiologic parameters are explained in detail elsewhere.41,42
At the end of the experiment, once we collected all the saliva samples, we debriefed the participants about the purpose of the study and told them that the MIST requires deception. The experimenter explained to the participant that in order to have a stress response and study stress it was impossible to reveal fully the goal of the MIST at the study outset. Participants were told that it was impossible for them to perform well on the MIST owing to it being programmed to adapt to their performance and ensure that they did not score higher than 55%. They were also told that the feedback bar showing the performance of the average female user was not real and was purposely set higher than their score to provide negative feedback. They were asked to dismiss any negative feelings about their performance and were warmly thanked for their understanding. No participant wished to withdraw after being debriefed.
Saliva sampling and hormonal analysis
We collected 7 saliva samples throughout the experiment by putting 3 Sorbettes under each participant’s tongue for a minimum of 60 seconds at each sampling time. Saliva samples were collected outside the scanner before the stress task (−45 min), on the scanner bed (−25 min), before and after the first run of the MIST (0 min and +15 min) and outside the scanner after the second run of the MIST (+30, +40 and +60 min). After each collection, the Sorbettes were inserted tip-up in a labelled polystyrene conical tube and kept at room temperature until completion of the session. The tubes were then centrifuged for 15 minutes at 3000 rpm to extract saliva and kept in the tube at −80°C until analysis. We determined the fraction of free cortisol using enzyme immunoassays with proven reliability and validity (Salimetrics).
Imaging procedure
Images were acquired using a GE short-bore 3-T MRI scanner with an 8-channel phased array head coil (General Electric Healthcare). A T1-weighted 3-dimensional (3-D) fast spoiled gradient echo axial anatomic scan was performed (repetition time [TR] 9 ms, echo time [TE] 2.1 ms, flip angle 12°, field of view [FOV] 240 mm, slice thickness 2.0 mm, 140–168 slices, matrix size 320 × 192). We acquired functional images with a gradient-echo echo-planar imaging sequence covering 31 axial slices (4 mm thick, no gap), beginning at the cerebral vertex and encompassing the entire cerebrum (TR 2000 ms, TE 35 ms, FOV 240 mm, matrix 64 × 64, flip angle 90°). A block design was employed with 2 runs consisting of rest, control and experimental blocks, completed 3 times randomly within run and between participants. Each run consisted of 294 acquisitions lasting 9 minutes and 48 seconds, separated by a saliva sampling period.
Data analysis
Data were analyzed using the χ2 and Student t tests and analyses of variance (ANOVAs), as appropriate. We compared the demographic and clinical characteristics (age, weeks postpartum, parity, education, feeding mode) of participants in the 3 groups using the χ2 test and 1-way ANOVAs. We evaluated the questionnaire scores (Y–BOCS, POCS, EPDS, MADRS, STAI, CTQ, RSE and PSQI) using 1-way ANOVAs with post-hoc Tukey correction.
All subsequent analyses were performed separately to account for the postpartum period. The following analyses were repeated between groups as follows:
ppOCD versus postpartum controls to isolate OCD-related effects, and
postpartum controls versus non-postpartum controls to isolate the effect linked to the postpartum period itself.
We analyzed self-reported ratings of stress and anxiety (STAI score and visual analogue scales) using 2-way mixed-design repeated-measures ANOVAs (group × time). Subsequent analyses were conducted for salivary cortisol. Because the hormonal levels were not normally distributed, logarithmic conversions were performed before using them in mixed-design repeated-measures ANOVAs (group × time). To further explore the endocrine measures, the area under the curve (AUC) was computed43 with respect to ground and increase (AUCg and AUCi). We compared the mean AUC values per group using Student t tests. All statistical analyses were completed with the PASW Statistics software package 17.0 for Apple OS X.
The acquired MRI scans were transferred to a computer to be preprocessed and were analyzed using Brain Voyager QX version 2.1 (Brain Innovation B.V.). High-resolution T1-weighted 3-D anatomic MRI data sets were transformed into Talairach space, used for coregistration and averaged to generate a composite image, which was created using all 39 anatomic data sets. The functional data sets were temporally corrected, 3-D motion-corrected, realigned to the third frame of each run, smoothed using an 8-mm Gaussian kernel and normalized to Talairach space. Using a random-effects multiple general linear model, we set stress and control conditions as the explanatory variables accounting for differences in blood oxygen level–dependent (BOLD) signals within and between groups. We constructed t maps identifying clusters of activity associated with contrasts both within groups (stress v. control condition) and between groups (OCD [stress–control] v. postpartum controls [stress–control] and postpartum controls [stress–control] v. non-postpartum controls [stress–control]). Contrasts were corrected for multiple comparisons using the false discovery rate (FDR, set at p < 0.05) methodology implemented in Brain Voyager QX.
Results
Demographic and clinical characteristics
Twelve postpartum women with symptoms of OCD, 16 post-partum controls and 11 healthy mothers participated in this imaging study. Table 1 summarizes demographic and clinical characteristics of participants in the 3 groups. The OCD group met all diagnostic criteria for OCD and did not present with comorbid conditions. Women with OCD had significantly higher Y–BOCS, POCS, EPDS and STAI scores than women in the control groups. Women did not differ with regard to age, parity, years of education and marital status (data not shown, all women were living with the father of their most recent child). Postpartum women did not differ in breastfeeding status and weeks postpartum. On the POCS, all postpartum women endorsed postpartum-related obsessions highlighting the increase in maternal preoccupations during the postpartum period. However, only the women with diagnosed OCD had a clinically significant score on the Y–BOCS and POCS severity scales. At the time of testing, women with a diagnosis of OCD continued their medications. Eleven of 12 women were taking medication to alleviate their symptoms: escitalopram (n = 2), quetiapine (n = 3), lorazepam (n = 2), venlafaxine (n = 1), paroxetine (n = 1), sertraline (n = 1), fluvoxamine (n = 1) or citalopram (n = 2).
Table 1.
Demographic and clinical characteristics of postpartum women with obsessive–compulsive disorder, healthy postpartum controls and healthy non-postpartum controls
| Group; mean (SD)* |
||||||
|---|---|---|---|---|---|---|
| Characteristic | ppOCD, n = 12 | Postpartum controls, n = 16 | Non-postpartum controls, n = 11 | Statistical test | p value | Comparison |
| Age, yr | 33.0 (5.1) | 31.9 (4.6) | 34.8 (6.0) | F2,36 = 1.01 | 0.37 | |
| Postpartum, wk | 16.3 (8.9) | 15.9 (7.2) | 110.6(51.1) | F2,36 = 45.90 | < 0.001 | ppOCD = PP < non-PP |
| Parity | 1.7 (0.8) | 1.9 (1.0) | 2.2 (1.1) | F2,36 = 0.83 | 0.45 | |
| Breastfeeding, no. mo | 6 | 13 | 3 | χ2 = 8.01 | 0.016 | PP > non-PP |
| Years of schooling completed | 18.4 (3.4) | 17.0 (4.0) | 17.4 (2.9) | F2,37 = 0.50 | 0.61 | |
| Y–BOCS severity score | 14.8 (9.2) | 1.6 (3.5) | 2.0 (3.5) | F2,36 = 21.00 | < 0.001 | ppOCD > PP = non-PP |
| POCS severity score | 17.3 (8.7) | 3.5 (3.2) | F2,35 = 17.58 | < 0.001 | ||
| EPDS score | 7.9 (3.5) | 2.4 (2.5) | F2,33 = 19.31 | < 0.001 | ||
| MADRS score | 6.1 (3.4) | 2.2 (1.6) | 1.1 (1.2) | F2,34 = 15.39 | < 0.001 | ppOCD > PP = non-PP |
| STAI-trait score | 42.5 (10.4) | 30.1 (6.0) | 26.4 (4.6) | F2,36 = 15.71 | < 0.001 | ppOCD > PP = non-PP |
| CTQ score | 35.5 (10.8) | 35.1 (9.6) | 32.1 (7.0) | F2,33 = 0.41 | 0.67 | |
| RSE score | 18.3 (5.7) | 17.0 (5.0) | 13.5 (3.9) | F2,34 = 2.86 | 0.07 | |
| PSQI score | 10.6 (3.8) | 5.2 (2.8) | 3.3 (2.9) | F2,35 = 16.40 | < 0.001 | ppOCD > PP = non-PP |
CTQ = Childhood Trauma Questionnaire;38 EPDS = Edinburgh Postnatal Depression Scale;35 MADRS = Montgomery-Åsberg Depression Rating Scale;36 POCS = Postpartum Obsessive–Compulsive Scale;34 PP = postpartum; ppOCD = postpartum women with obsessive–compulsive disorder; PSQI = Pittsburgh Sleep Quality Index;40 RSE = Rosenberg Self-Esteem questionnaire;39 SD = standard deviation; STAI = Spielberger State-Trait Anxiety Inventory;37 Y–BOCS = Yale–Brown Obsessive–compulsive Symptoms.33
Unless otherwise indicated.
Psychologic and endocrine stress response
Postpartum OCD versus postpartum control
When comparing the 2 groups of postpartum women, we found a significant interaction effect for the STAI scores (F1,25 = 7.33, p = 0.012), with women with OCD having a larger increase in their STAI scores poststress. For the stress ratings (visual analogue scale stress) we found a significant main effect of time (F1,25 = 21.45, p < 0.001). There was no significant interaction, but there were significant main effects of group (F1,25 = 9.69, p = 0.005) and time (F1,25 = 8.47, p = 0.007) for the anxiety ratings (visual analogue scale anxiety).
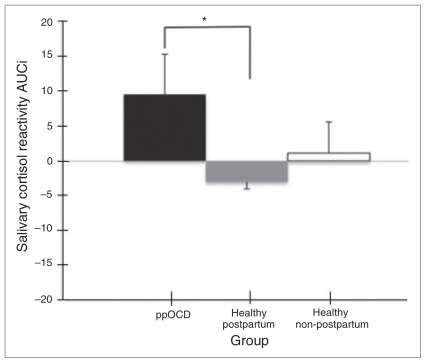
Postpartum women with OCD had a marginally greater cortisol stress response compared with the healthy post-partum women, with a group difference in cortisol AUCi composite measure that approached significance (t26 = 2.05, p = 0.05; Fig. 1). No significant effect emerged from the mixed-design ANOVA analysis or from the AUCg group comparison (all p > 0.05).
Fig. 1.
Mean and standard error of the mean of cortisol area under the curve increase (AUCi) per group. *Group trend toward differences in mean cortisol AUCi (p = 0.05) between postpartum women with obsessive–compulsive disorder (ppOCD) and healthy post-partum participants.
Postpartum versus non-postpartum controls
Both groups of healthy women had an increase in their stress ratings following the MIST, with a significant main effect of time only on the STAI score (F1,25 = 7.14, p = 0.013) and on the visual analogue scale stress score (F1,25 = 4.64, p = 0.042). We found no significant effect on the anxiety visual analogue scale score (p = 0.09).
No significant effect was found on the cortisol reactivity measures (no interaction, neither main effect of group nor time on the mixed-design ANOVA, and no group difference in AUCi and AUCg cortisol composite measures; all p > 0.05).
Stress-related brain activation
Imaging results are provided in Table 2. It summarizes stress-related brain activation (MIST: stress minus control conditions) in postpartum women with OCD compared with the postpartum controls as well as stress-related brain activation in postpartum compared with non-postpartum controls.
Table 2.
Brain regions showing significant stress-related clusters of activation in postpartum women with OCD (n = 12) compared with postpartum controls (n = 16) and in postpartum controls (n = 16) compared with non-postpartum controls (n = 11) with Talairach coordinates at peak
| Group contrast; brain region | Talairach coordinates
|
t | p value | Cluster size, mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Postpartum OCD (stress–control conditions) minus postpartum controls (stress–control conditions) | ||||||
| Right STG, insula, OFC | 48 | 12 | −32 | 4.36 | 0.001 | 18 003 |
| Left STG, insula | −45 | −4 | −2 | 3.84 | 0.001 | 3 258 |
| Left STG temporal pole | −42 | 11 | −23 | 4.00 | 0.001 | 3 491 |
| Left ITG | −48 | −16 | −33 | 3.46 | 0.001 | 553 |
| Right superior frontal gyrus, mPFC | 6 | 36 | −17 | 3.46 | 0.001 | 201 |
| Left mPFC | −3 | 68 | 0 | 4.09 | 0.001 | 2 954 |
| Postpartum (stress–control conditions) minus non-postpartum controls (stress–control conditions) | ||||||
| Right mPFC | 6 | 56 | 7 | −3.68 | 0.001 | 2 858 |
| Left DLPFC | −36 | 23 | 49 | 3.33 | 0.001 | 833 |
| Right/left ACC | 19 | 23 | 19 | −3.76 | 0.001 | 652 |
ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; ITG = inferior temporal gyrus; mPFC = medial prefrontal cortex; OCD = obsessive–compulsive disorder; OFC = orbitofrontal cortex; STG = superior temporal gyrus.
To examine the directionality of these findings, we conducted within-group analyses in each of the 3 groups. Within-group analysis revealed that the response to stress in the ppOCD group was associated with activation in the OFC; dorsolateral prefrontal cortex (DLPFC); mPFC; superior temporal gyri (STG), including insular regions; and inferior temporal gyri (ITG). Additionally, deactivation was observed in the ACC and basal ganglia. Healthy postpartum women showed stress-related activation in the DLPFC and precuneus and deactivation in the OFC, mPFC, ACC, STG and basal ganglia. Healthy non-postpartum mothers activated the mPFC, ACC and precuneus and deactivated the OFC, left hippocampus, basal ganglia and bilateral insula in response to stress.
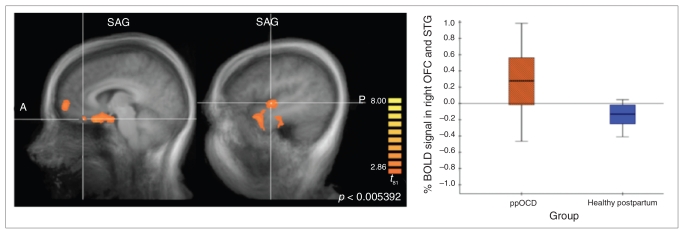
Between-group contrasts revealed significant brain activation clusters in the STG, OFC, insula, ITG and mPFC in the ppOCD group compared with postpartum controls (Fig. 2). Women with OCD activated these regions, whereas post-partum controls demonstrated deactivation patterns.
Fig. 2.
Between-group contrast for postpartum women with obsessive–compulsive disorder (ppOCD) compared with postpartum controls revealed significant clusters in the orbitofrontal cortex (OFC) extending to the superior temoporal gyrus (STG) and the insula (not shown) that reflected an increased blood oxygen level–dependent (BOLD) signal in women with OCD and deactivation in postpartum controls in response to stress (contrast = stress condition minus control condition).
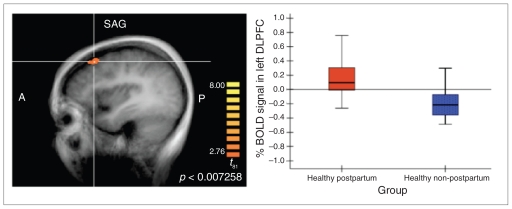
The group contrast between the healthy controls (post-partum v. non-postpartum) demonstrated significant clusters of activation in the DLPFC and clusters of deactivation in the mPFC and ACC. The DLPFC contrast demonstrates that postpartum controls activated this region in response to stress whereas non-postpartum controls deactivated it. The mPFC and ACC findings reflect deactivation in postpartum controls and activation in non-postpartum controls (Fig. 3).
Fig. 3.
Between-group contrast of healthy postpartum women and healthy non-postpartum mothers identified a significant cluster in the dorsolateral prefrontal cortex (DLPFC), reflecting heightened engagement of this region in women in the postpartum period in response to stress (contrast = stress condition minus control condition). BOLD = blood oxygen level–dependent.
Discussion
To our knowledge, this study is the first to investigate stress reactivity using functional neuroimaging in subgroups of women with OCD and healthy women during the post-partum period.
As expected, these findings indicate that women with OCD have a heightened psychologic and endocrine stress response associated with a distinct brain activation pattern in response to psychosocial stress involving the OFC and temporal cortex compared with healthy counterparts. Moreover, compared with mothers assessed in a period of time beyond the postpartum period, healthy postpartum women do not differ in their self-reported stress and cortisol response, but recruit different brain regions, such as the DLPFC and ACC, during exposure to psychologic stress. Our findings corroborate and provide additional support to the hypothesis of OFC dysfunction or hyperactivity in patients with OCD. Furthermore, our brain imaging results are in accordance with those from previous studies using the MIST or similar stressors.31
The MIST is known to be an effective but moderate psychologic stressor, inducing modest cortisol increases compared with the widely used Trier Social Stress Test. In previous MIST studies, on average 50% of the participants demonstrated an increase in cortisol secretion and were thus classified as responders.31,42,44 In the present study, 58% (7 of 12) of the ppOCD group and 56% (6 of 11) of the non-postpartum healthy mothers had a cortisol increase in response to the MIST compared with only 38% (6 of 16) of the healthy postpartum women. Although the increase was not significant in healthy mothers, these observations argue in favour of women physiologically reacting to the MIST. At this point, we cannot disentangle the effect of stress perception from the potential blunting effect of breastfeeding29 because 13 of 16 women were exclusively breastfeeding. Despite a putative hypothetical protective effect of the post-partum period and lactation in particular on acute cortisol secretion, OCD and/or pathological anxiety might override this effect since postpartum women with OCD had a significant increase in reactive cortisol. These results showed great variability, highlighting the importance of further investigating potential confounding factors, such as appraisal, stressor saliency (child- v. non–child related threat), breastfeeding, parity, personality traits, sex hormone levels and severity of OCD symptoms. There are ongoing challenges to investigate the neural correlates of the stress response in a neuroimaging environment. A stress task that yields more consistent and significant cortisol increases still needs to be developed.31 Nonetheless, the MIST significantly increased stress/anxiety ratings, which sufficed to uncover stress-related neural correlates specific to OCD and the postpartum period.
Neuroimaging studies on brain correlates of acute stress invariably report either no change or deactivation of the OFC in response to stress.31 It has been hypothesized that the deactivation of the limbic system, including the OFC, is linked to a significant stress response from the HPA axis, differentiating the cortisol responders from nonresponders.31,42,45,46 To date, only 2 published studies have examined the brain under stress using a validated stress paradigm among anxious patients (i.e., social phobia),47,48 and to our knowledge no studies focused on the stress response in the postpartum period.
In the present study, the response to stress in women with OCD was associated with significant activation of the OFC, STG, insula and mPFC in contrast to the healthy postpartum women who demonstrated deactivation in these regions. Interestingly, both postpartum groups activated the DLPFC and deactivated the ACC. On the other hand, healthy postpartum women had reverse stress-related brain correlates compared with the non-postpartum healthy mothers, with the non-postpartum mothers activating the ACC and the mPFC whereas postpartum mothers deactivated these regions while activating the DLPFC.
Brain correlates of acute stress in women with OCD
Our results contrast with the hypothesis that decreased activity in the OFC would be linked to increased cortisol reactivity; the observed extensive activation cluster, including the OFC, insula and STG, described here has never been reported in relation to stress and may be unique to OCD. Functionally, the OFC is involved in decision-making, error detection and cognitive appraisal to determine the significance of stimuli and the appropriate emotional response to them. The OFC is well known to be overactive in people with OCD.20–22 Anterior cingulate cortex hyperactivity has also been linked to OCD, with increased activity during error-processing.49 The STG has dense connections with both the OFC and ACC. It has also been linked to OCD pathophysiology, with patients with OCD having higher regional cerebral blood flow at rest and during presentation of neutral and symptom-related stimuli50 and increased BOLD signal in response to symptom provocation.51–53 Rotge and colleagues53 suggested that the left STG activation was related to the anxiety manifestations rather than an engagement of the neural circuitry underlying OCD-related symptoms. It is thought that cerebral hyperactivity seen in patients with OCD might mediate the formation of improper error detection and contribute to uncontrollable OCD symptoms, such as excessive doubt and compulsive checking. Taken together, the present results suggest that OCD is associated with heightened activity within brain regions associated with vigilance, threat detection and emotional reactivity.
Brain correlates of acute stress during the postpartum period
The DLPFC activation seen in both groups of postpartum mothers might reflect the engagement of network regions common to both the affective/error-processing elements of the stress response and the processes involved in anticipatory anxiety and worrying. Thus, when facing a neutral non-postpartum/baby-related stressor, healthy postpartum women would recruit the same regions as postpartum women with OCD with regard to error-processing, worrying and normal anxiety. However, in contrast to postpartum women with OCD, healthy postpartum women would not recruit the OFC, potentially reflecting a more adapted emotional response to error and stress. Activation of the DLPFC in conjunction with the expected deactivations in limbic brain areas in response to stress in healthy postpartum women would thus be linked to a more efficient gating and inhibition of the limbic activity compared with their OCD counterparts.
The postpartum period has been described as a time during which there is a re-evaluation of the sense of self, an assessment of personal identity relative to others and increasing preoccupations that centre on the baby’s well-being. Interestingly, DLPFC activity has been observed to correlate with high levels of self-criticism, therefore linking error-processing and resolution to self-critical thinking.54 The potential self-critical response to the novel, uncontrollable, unpredictable and ego-threatening events happening during the postpartum period might render the DLPFC more active in response to personal mistakes and stress. Since the MIST is a socioevaluative stressor, inducing failures, the DLPFC activation seen in both groups of postpartum women compared with non-postpartum women might reflect higher levels of self-criticism while performing the task.
Brain correlates of the stress response
The mPFC has been involved in the appraisal and regulation of stress and emotions with a possible modulatory control over the physiologic stress responses.46,55,56 Neuroimaging studies tend to find the mPFC to be activated in response to a stressor.42,46,55 In the present study, postpartum mothers with OCD and healthy non-postpartum mothers activated the mPFC, whereas the healthy postpartum controls deactivated this region; both groups of women with increased cortisol demonstrated activation of the mPFC.
Limitations
The present study is not without limitations. The heterogeneity in the cortisol response to the MIST paradigm, possibly owing to the relatively small sample size, might have prevented us from finding a significant difference in the stress-related cortisol response over time and between groups. Other potential confounding factors, such as medication use, breastfeeding, parity and personality factors, may have modulated the stress-related endocrine response and could not be assessed in the present study.
The study of a clinical population in the postpartum period is particularly challenging given the increased responsibilities in a woman’s life at this point in time. On the other hand, the OCD clinical presentation appears to be more homogeneous during the postpartum period than in the general population, so this time in a woman’s life might offer an opportunity to investigate neuroimaging correlates and underlying cerebral mechanisms in a more cohesive sample.
Conclusion
Our results uncover the unique stress-related brain activation patterns in women with OCD and during the postpartum period while providing additional support to the prevailing hypothesis of OFC–STG dysfunction in patients with OCD, with a novel approach to the postpartum population. Future studies could investigate the role of self-criticism and self-reassurance in the psychologic, endocrine and brain response to acute stress as well as during the postpartum period to better understand the “top–down” stress-regulation process.
Acknowledgments
We thank Dalia Bibr, Amber Rieder, Carmen McPherson, Patricia Dabek, Dawn Gore, Karen Jansen and Stephanie Bissell for their help with participant recruitment and testing. Thanks to Margaret Coote for her laboratory support and guidance. A special thank you to all the mothers who accepted to participate in the study. Catherine Lord’s involvement in this study was supported by the Fonds de la Recherche en Santé du Québec (FRSQ). Funding for this study was provided by the Wiesz family and Effort Trust.
Footnotes
Contributors: C. Lord, M. Steiner and G.B. Hall designed the study. C. Lord, C.N. Soares C.L. Carew and G.B. Hall acquired and analyzed the data. All authors wrote and reviewed the article and approved its publication.
Competing interests: None declared for C. Lord, C.L. Carew and G.B. Hall. C.N. Soares has received research grants from Eli Lilly, AstraZeneca, Physicians Services Incorporated (PSI) Foundation, Allergen National Centre of Excellence, Hamilton Community Foundation, Lundbeck, Wyeth and the Canadian Institutes of Health Research. He has worked as a research consultant for Wyeth, Pfizer, Lundbeck and Bayer Healthcare Pharmaceuticals; he has participated as a member of the speakers’ bureaus of AstraZeneca, Wyeth, Pfizer, Lundbeck, Bayer Healthcare Pharmaceuticals and as a member of advisory boards for AstraZeneca, Wyeth, Pfizer, Lundbeck and Bayer Healthcare Pharmaceuticals. M. Steiner has received grant/research support from the Canadian Institutes of Health Research, Physicians Services Inc., Wyeth Pharmaceuticals, Astra-Zeneca, Lundbeck, Eli Lilly, Azevan Pharmaceuticals and Bayer Shering Pharmaceuticals; he is a consultant for Wyeth Pharmaceuticals, Bayer Shering Pharmaceuticals, AstraZeneca, Azevan Pharmaceuticals, Servier and Pfizer; he is a member of the speakers’ bureaus of AstraZeneca and Pfizer.
References
- 1.Swain JE, Lorberbaum JP, Kose S, et al. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- 3.Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(Suppl 2):29–33. [PubMed] [Google Scholar]
- 4.Breitkopf CR, Primeau LA, Levine RE, et al. Anxiety symptoms during pregnancy and postpartum. J Psychosom Obstet Gynaecol. 2006;27:157–62. doi: 10.1080/01674820500523521. [DOI] [PubMed] [Google Scholar]
- 5.Leckman JF, Mayes LC, Feldman R, et al. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- 6.Fairbrother N, Abramowitz JS. New parenthood as a risk factor for the development of obsessional problems. Behav Res Ther. 2007;45:2155–63. doi: 10.1016/j.brat.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Abramowitz JS, Schwartz SA, Moore KM, et al. Obsessive-compulsive symptoms in pregnancy and the puerperium: a review of the literature. J Anxiety Disord. 2003;17:461–78. doi: 10.1016/s0887-6185(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 8.Maina G, Albert U, Bogetto F, et al. Recent life events and obsessive-compulsive disorder (OCD): the role of pregnancy/delivery. Psychiatry Res. 1999;89:49–58. doi: 10.1016/s0165-1781(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 9.Brandes M, Soares CN, Cohen LS. Postpartum onset obsessive-compulsive disorder: diagnosis and management. Arch Womens Ment Health. 2004;7:99–110. doi: 10.1007/s00737-003-0035-3. [DOI] [PubMed] [Google Scholar]
- 10.Labad J, Menchon JM, Alonso P, et al. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry. 2005;66:428–35. doi: 10.4088/jcp.v66n0404. quiz 546. [DOI] [PubMed] [Google Scholar]
- 11.Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061–8. doi: 10.4088/JCP.09m05381blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vulink NC, Denys D, Bus L, et al. Female hormones affect symptom severity in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2006;21:171–5. doi: 10.1097/01.yic.0000199454.62423.99. [DOI] [PubMed] [Google Scholar]
- 13.Williams KE, Koran LM. Obsessive-compulsive disorder in pregnancy, the puerperium, and the premenstruum. J Clin Psychiatry. 1997;58:330–4. doi: 10.4088/jcp.v58n0709. quiz 335–6. [DOI] [PubMed] [Google Scholar]
- 14.Uguz F, Akman C, Kaya N, et al. Postpartum-onset obsessive-compulsive disorder: incidence, clinical features, and related factors. J Clin Psychiatry. 2007;68:132–8. doi: 10.4088/jcp.v68n0118. [DOI] [PubMed] [Google Scholar]
- 15.Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50:503–9. doi: 10.1016/j.comppsych.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Sasson Y, Zohar J, Chopra M, et al. Epidemiology of obsessive-compulsive disorder: a world view. J Clin Psychiatry. 1997;58(Suppl 12):7–10. [PubMed] [Google Scholar]
- 17.Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockington IF, Macdonald E, Wainscott G. Anxiety, obsessions and morbid preoccupations in pregnancy and the puerperium. Arch Womens Ment Health. 2006;9:253–63. doi: 10.1007/s00737-006-0134-z. [DOI] [PubMed] [Google Scholar]
- 19.Sichel DA, Cohen LS, Rosenbaum JF, et al. Postpartum onset of obsessive-compulsive disorder. Psychosomatics. 1993;34:277–9. doi: 10.1016/S0033-3182(93)71893-9. [DOI] [PubMed] [Google Scholar]
- 20.Abramowitz JS, Taylor S, McKay D. Obsessive-compulsive disorder. Lancet. 2009;374:491–9. doi: 10.1016/S0140-6736(09)60240-3. [DOI] [PubMed] [Google Scholar]
- 21.Fineberg NA, Potenza MN, Chamberlain SR, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huey ED, Zahn R, Krueger F, et al. A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2008;20:390–408. doi: 10.1176/appi.neuropsych.20.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu Rev Psychol. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson PE, Gustafsson PA, Ivarsson T, et al. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. 2008;57:14–21. doi: 10.1159/000123117. [DOI] [PubMed] [Google Scholar]
- 27.Kluge M, Schussler P, Kunzel HE, et al. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J Psychiatr Res. 2007;41:928–33. doi: 10.1016/j.jpsychires.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Monteleone P, Catapano F, Tortorella A, et al. Cortisol response to d-fenfluramine in patients with obsessive-compulsive disorder and in healthy subjects: evidence for a gender-related effect. Neuropsychobiology. 1997;36:8–12. doi: 10.1159/000119352. [DOI] [PubMed] [Google Scholar]
- 29.Tu MT, Lupien SJ, Walker CD. Measuring stress responses in post-partum mothers: perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- 30.Lord C, Hall G, Soares CN, et al. Physiological stress response in postpartum women with obsessive-compulsive disorder: a pilot study. Psychoneuroendocrinology. 2011;36:133–8. doi: 10.1016/j.psyneuen.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Dedovic K, D’Aguiar C, Pruessner JC. What stress does to your brain: a review of neuroimaging studies. Can J Psychiatry. 2009;54:6–15. doi: 10.1177/070674370905400104. [DOI] [PubMed] [Google Scholar]
- 32.Martini J, Wittchen HU, Soares CN, et al. New women-specific diagnostic modules: the Composite International Diagnostic Interview for Women (CIDI-VENUS) Arch Womens Ment Health. 2009;12:281–9. doi: 10.1007/s00737-009-0077-2. [DOI] [PubMed] [Google Scholar]
- 33.Steketee G, Frost R, Bogart K. The Yale-Brown Obsessive Compulsive Scale: interview versus self-report. Behav Res Ther. 1996;34:675–84. doi: 10.1016/0005-7967(96)00036-8. [DOI] [PubMed] [Google Scholar]
- 34.Lord C, Rieder A, Hall GB, et al. Perinatal Obsessive-Compulsive Scale (POCS): development and validation. J Anxiety Disord. 2011 Jul 19; doi: 10.1016/j.janxdis.2011.07.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- 38.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg M. Society and the adolescent self-image. Princeton (NJ): Princeton University Press; 1965. [Google Scholar]
- 40.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Dedovic K, Renwick R, Mahani NK, et al. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- 42.Pruessner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 44.Dedovic K, Rexroth M, Wolff E, et al. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Dedovic K, Duchesne A, Andrews J, et al. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–9. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52:1113–9. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- 48.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–6. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald KD, Welsh RC, Gehring WJ, et al. Error-related hyper-activity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–94. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 50.Cottraux J, Gerard D, Cinotti L, et al. A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res. 1996;60:101–12. doi: 10.1016/0165-1781(96)02697-2. [DOI] [PubMed] [Google Scholar]
- 51.Adler CM, McDonough-Ryan P, Sax KW, et al. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–24. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 52.Sanematsu H, Nakao T, Yoshiura T, et al. Predictors of treatment response to fluvoxamine in obsessive-compulsive disorder: an fMRI study. J Psychiatr Res. 2010;44:193–200. doi: 10.1016/j.jpsychires.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Rotge JY, Guehl D, Dilharreguy B, et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 54.Longe O, Maratos FA, Gilbert P, et al. Having a word with yourself: neural correlates of self-criticism and self-reassurance. Neuroimage. 2010;49:1849–56. doi: 10.1016/j.neuroimage.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Kern S, Oakes TR, Stone CK, et al. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–29. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchanan TW, Driscoll D, Mowrer SM, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]