Abstract
Background
Pharmacologic and animal studies have strongly implicated the norepinephrine transporter (NET) in the pathophysiology of attention-deficit/hyperactivity disorder (ADHD). We conducted a family-based study, with stratification based on sex and subtype, to test the association between 30 tag single-nucleotide polymorphisms (SNPs) within the gene encoding NET (SLC6A2) and ADHD.
Methods
Family-based association tests were conducted with the categorical diagnosis of ADHD, as well as quantitative phenotypes of clinical relevance (Conners Global Index for Teachers and Parents, and Child Behavior Checklist measures). Sliding window haplotype analysis was conducted with screening based on conditional power using PBAT.
Results
A previously reported association with rs3785143 was confirmed in this study. Further, extensive association was observed with haplotype blocks, with a differential pattern observed based on sex and subtype. The 5′ region of the gene (encompassing haplotype block 1 and including a functional promoter SNP, rs28386840) showed an association with ADHD in girls (irrespective of subtype). A different region of the gene (distributed around haplotype block 2) was associated with distinct behavioural phenotypes in boys. These findings are correlated with previously reported functional studies of gene variants in SLC6A2.
Limitations
The most important limitation of the study is the small size of the groups resulting from the stratification based on sex followed by subtype.
Conclusion
The results obtained in this family-based study suggest that haplotype blocks within different regions of SLC6A2 show differential association with the disorder based on sex and subtype. These associations may have been masked in previous studies when tests were conducted with pooled samples.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder in children, occurring in 8%–12% of the population.1 It is heterogeneous in its clinical expression, with the core symptoms being poor sustained attention, impulsivity and hyperactivity. Criteria in the Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV), recognize 3 behavioural subtypes of ADHD: primarily inattentive, primarily hyperactive/impulsive and the combined subtype.
A large body of literature (for a review, see Diamond2) suggests that the combined and inattentive subtypes are distinct disorders. Differentiating ADHD from attention-deficit disorder (ADD; inattentive, without hyperactivity), Diamond summarizes the differences between the 2. Whereas ADHD is associated with externalizing behaviours and good response to methylphenidate (MPH), ADD tends to be associated with internalizing behaviours and poor response to MPH. Comorbidity with conduct and oppositional defiant disorder is common in individuals with ADHD, whereas anxiety and depression are commonly present in those with ADD. Based on convergent evidence from neuropsychologic and imaging studies, Diamond suggests that distinct pathways may be involved in the 2 disorders. The primary deficit in individuals with ADHD appears to be response inhibition resulting from dysfuntion of the frontal-striatal circuits, whereas ADD likely results from deficits in working memory, with disturbance in the frontal-parietal neural circuits.
Attention-deficit/hyperactivity disorder is 3 to 4 times more common in boys than girls.3,4 Analysis on the nosological relations of the combined and inattentive subtypes has shown an important effect of sex. It has been noted that the relative risk for the 2 subtypes differs by sex,4 with girls requiring a higher risk loading than boys for development of the combined subtype.5 The sex ratio (female:male) is higher in the inattentive subtype than in the combined subtype, and the subtypes are more clearly distinguished in boys than girls.6 It has been suggested that etiologic factors for the development of the particular subtype may be different in boys and girls with ADHD.4,7 It has been noted that girls with ADHD had higher familial psychopathology load than boys.8 Attention-deficit/hyperactivity disorder has an important genetic component, with a mean heritability estimate of 76%.3 Yet no specific gene has been implicated beyond reasonable doubt, and it has been suggested that ADHD is caused by multiple genes, each having a small effect.9
The catecholamines dopamine (DA) and norepinephrine (NE) have been considered to be major players in the pathophysiology of ADHD. Pharmacologic studies provided the initial clues that these neurotransmitters are involved in the disorder.10 The psychostimulants MPH and amphetamine, which are widely used for the treatment of ADHD, block the DA and NE transporters, resulting in increased synaptic concentration of both these neurotransmitters.11–13 Further, pharmacologic agents that are selective for NE have been found to be effective in treating ADHD. Among these are the α2-adrenergic agonists clonidine and guanfacine, the NE antidepressant desipramine and the selective NE reuptake blocker atomoxetine.14 Neuroimaging15 and animal studies16 have provided further evidence for the role of DA and NE in ADHD.
Given the compelling evidence, it has been suggested that dysregulation of the noradrenergic system may be an important factor in ADHD.17,18 Noradrenaline/norepinephrine is known to be involved in visual attention, initiation of the adaptive response, sustained attention, learning and memory.19 Depletion of NE results in increased distractibility and motor hyperactivity in rodents. Conversely, studies in non-human primates have shown that stimulation of the NE system is associated with a decrease in distractibility and an improvement of cognitive function.
The norepinephrine transporter protein (NET) is a pivotal player in the noradrenergic system; it is involved in the reuptake of NE into presynaptic terminals, thereby playing a key role in controlling the intensity and duration of signal transduction. The NET is a transmembrane glycoprotein composed of 617 amino acids, and is a member of the sodium- and chloride-dependent neurotransmitter transporter family.20 It is encoded by NET1/SLC6A2, which has been localized to position 16q12.2.
A number of family-based and case–control studies have been conducted to examine the association between specific polymorphisms within SLC6A2 and ADHD. Studies have been conducted with a limited number of single-nucleotide polymorphisms (SNPs) within SLC6A2,21–25 including a functional SNP in the promoter region,26–28 and arrays of tag SNPs covering the entire gene.29–33 To summarize previous findings and a recent meta-analysis,34 5 SNPs within SLC6A2 (rs3785157, rs998424, rs3785143, rs11568324, rs28386840) were shown to be associated with ADHD (details provided in Appendix 1, Table S1 and Fig. S1, available at cma.ca/jpn). Whereas some studies reported or confirmed an association, others were unable to replicate the findings.
We conducted a family-based study to test the association between a panel of 30 tag SNPs within SLC6A2 and ADHD, with particular attention to subtype and sex. In addition to the DSM-IV diagnosis of ADHD, clinically relevant dimensions of behaviour (e.g., child’s behaviour at home and in school) were used as quantitative phenotypes in the genetic analysis (the quantitative trait loci [QTL] approach). It has been suggested that this approach may be an important complement to the categorical diagnosis in molecular genetic studies.35–37 To maintain consistency among studies, tag SNPs selected by the International Multicentre ADHD Gene (IMAGE) project, excluding those with a minor allele frequency ≤ 0.02, were genotyped.30 In addition, 2 tag SNPs (rs15534, rs7188230) were selected to extend the 3′ flanking region examined. The functional promoter SNP, rs28386840, was also included in the panel.
Methods
Participants
Children between 6 and 12 years of age with a diagnosis of ADHD were recruited from the Disruptive Behaviour Disorders Program and the child psychiatry outpatient clinics at the Douglas Mental Health University Institute in Montréal, Canada. They were referred to these specialized care facilities by schools, community social workers, family doctors and pediatricians. The research protocol was approved by the Research Ethics Board of the Douglas Mental Health University Institute. Participants’ parents provided written consent after we explained the study protocol. Children (affected child and unaffected siblings) also consented to participate after we explained the study protocol.
Each child received a diagnosis of ADHD based on DSM-IV criteria. Details about diagnostic procedures have been described elsewhere.38 Briefly, the diagnosis was based on clinical interviews with the child and at least 1 parent conducted by a child psychiatrist. The diagnosis was corroborated with the Diagnostic Interview Schedule for Children, version IV (DISC-IV),39 based on the structured clinical interview of parents. The clinical examination was supplemented with school reports, including the Conners Global Index, Teacher version questionnaire.40 In most cases, mothers were the primary informants. A child was excluded from the study if he/she had an IQ less than 70, as measured with the Wechsler Intelligence Scale for Children III/IV, and/or a diagnosis of Tourette syndrome, pervasive developmental disorder and psychosis (including schizophrenia or bipolar disorder). Children with co-morbid learning disabilities were included in the study.
Clinical measures
In addition to the DSM-IV diagnosis of ADHD, the behaviour of the child was used as a quantitative trait in the genetic analysis. The behaviour of the child at home and in the classroom was evaluated by the parent(s) and teacher according to the Conners Global Index — Parents (CGI-P) and Conners Global Index — Teachers (CGI-T), respectively.40,41 In addition, the parents were asked to evaluate the behaviour of the child using the Child Behavior Checklist (CBCL).42 These evaluations represent ecologically relevant dimensions of the child’s behaviour at home (CGI-P and CBCL) and in school (CGI-T). The CGI scale has also been validated from a genetic point of view, with research showing that genetic factors account for 78% of its variance.43 The CBCL is a comprehensive rating scale (113-item questionnaire) that has been used extensively in clinical and research settings, having well-established metric characteristics and representative norms.
Information from both the important stakeholders (parents and teachers) was used to ensure a comprehensive assessment of the child’s behaviour. A large body of research has concluded that there is a low to moderate correlation between parent and teacher reports of ADHD symptoms, suggesting that each is an assessment of a different dimension of the child’s behaviour.37,44,45
Genotyping
The affected child, parents and unaffected siblings were invited to participate in the genetic component of the study. For each parent and child, DNA was extracted from a blood sample, a buccal swab or saliva sample if the participant was only amenable to the latter.
The tag SNPs within NET that had previously been examined in the IMAGE project were genotyped.30 However, tag SNPs with a very low minor allele frequency (MAF; ≤ 0.02) were excluded. The only tag SNP with a low MAF to be retained was rs11568324 (MAF = 0.01), since this SNP was shown to be associated with ADHD in the original IMAGE study30 and in a subsequent replication study.31 An additional SNP (rs28386840), which encodes a functional polymorphism in the upstream promoter region of NET and was reported to be associated with ADHD,26 was also included in the panel. We also selected 2 tag SNPs that were not genotyped in the IMAGE study. These 2 tag SNPs (rs15534, present in exon 14, and rs7188230, present in the 3′ intergenic region) extended the flanking region examined in NET compared with the IMAGE study.
The panel of SNPs was genotyped using Sequenom iPlex Gold Technology.46 Every plate included duplicates of 2 reference samples used to estimate genotyping error. Genotypes for these samples were read with 100% accuracy on each of the plates. Five tag SNPs in the original panel of SNPs genotyped in the IMAGE study (rs7201099, rs3760019, rs1362620, rs1861647, rs1566652) could not be genotyped on the Sequenom platform. Since these SNPs were in strong linkage disequilibrium with other SNPs in the panel and were not shown to be specifically associated in any of the previous studies, they were excluded from subsequent analysis. The genotype distribution at each of the markers analyzed in this study did not depart from Hardy–Weinberg equilibrium (p > 0.01; Appendix 1, Table S2). A linkage disequilibrium plot was constructed in Haploview version 4.0 using the genotype information from the current study.47 We used the default definition in Haploview48 to generate the plot. In this method, 95% confidence bounds on D’ are generated for each pairwise comparison. An SNP block is formed if 95% of the informative comparisons are in strong linkage disequilibrium with each other.
Statistical analyses
Family-based association tests (examining transmission disequilibrium of a specific allele/haplotype from parent to affected offspring) were conducted using the FBAT statistical software package (version 2.0.3).49 We performed all the analyses under the assumption of an additive model, with a null hypothesis of no linkage and no association. At the first level, family-based association test analysis was conducted with the total sample. However, given the results of earlier studies indicating that sex and ADHD subtype may be important confounds, further analysis was conducted in boys and girls separately, with subtype as the grouping variable.
Given the large number of markers and phenotypes being tested in the different subgroups, the association tests were subsequently conducted using PBAT software. By screening based on conditional power, PBAT allows for the control of type-I error, thereby helping to overcome the multiple comparison problem.50 We set the screening parameters as follows: the FBAT-GEE statistic (based on the generalized estimation equation) was selected, significance level was set at p = 0.05, and offset was selected to maximize power. We performed sliding window analysis to test the association with haplotype blocks. Serial analysis was conducted with a window size of 2 SNPs, then 3, 4, 5, 6, 7 and 8 (the maximum window size allowed in PBAT). Only haplotypes defined by adjacent markers were analyzed. For the analysis, the combined and hyperactive groups were analyzed as 1 group in contrast to the inattentive subtype.
Results
The study included 380 nuclear families having at least 1 child with a DSM-IV diagnosis of ADHD. Of the 380 families, 184 were trios with information from both parents, 18 were trios with 2 affected children, 49 were trios with information from 1 parent and 1 or more unaffected siblings, 115 were duos including the proband and 1 parent, and 14 were families with 2 affected siblings and 1 parent. A total of 412 children with ADHD were included in the study. The mean age of participants was 9 (standard deviation [SD] 1.8) years. Of the total number of affected children, 78.3% were boys and 85.5% were of white; 53.2% met DSM-IV criteria for the combined subtype, whereas 36.7% and 10.1% met the criteria for the inattentive and hyperactive subtypes, respectively. Among those with comorbid disorders, 40.4% had oppositional defiant disorder, 21.7% had conduct disorder, 44.1% had anxiety disorder (including phobias) and 8.3% had a mood disorder (major depressive episode, dysthymic disorder, manic episode and/or hypomanic episode). The mean (and SD) CBCL, CGI-P and CGI-T total scores were 68.8 (8.9), 73.3 (11.2) and 69.6 (12.5), respectively.
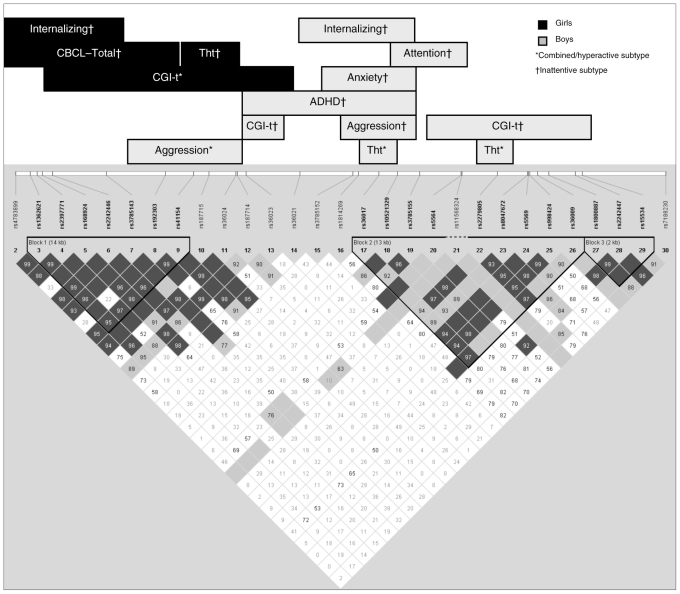
At the first level of analysis, the association between each of the 30 tag SNPs within SLC6A2 and ADHD as a diagnostic category was tested in the total sample using family-based association tests (Table 1). Two of the SNPs (rs3785143 and rs36021) showed a significant association with ADHD. The significant results of the sliding haplotype analysis are summarized in Tables 2 and 3 and depicted in Figure 1. We observed that different domains of SLC6A2 showed an association with different ADHD subtypes in boys and girls. In girls, a significant association with haplotype block 1, and a lack of association with blocks 2 and 3, was observed. The haplotype block at the 5′ end of the gene showed an association with different dimensions of ADHD in girls. In girls with the combined/hyperactive subtype, M03–14 showed an association with the CGI-T score. In this group, the A:G:T:T:C:G:G:A:C:C:C haplotype was undertransmitted in children with higher CGI-T scores, conferring a “protective” effect. In girls with the inattentive subtype, an overlapping region from M01–07 showed an association with the total score on the CBCL and with the CBCL–internalizing factor (if the power criteria was relaxed to ≥ 0.7). The A:T:A:G:T:T:C haplotype was undertransmitted in children with higher CBCL–total and CBCL–internalizing scores. Further, in this group of children, a different haplotype (A:A:T:T) of the region encompassing M09-M10-M11-M12 was overtransmitted in girls with higher scores on the CBCL–thought disorder subscale. This suggests that the A:A:T:T haplotype is a “risk” haplotype for this dimension of behaviour in girls.
Table 1.
Overall association (using family-based association tests) between the 30 tag single-nucleotide polymorphisms within SLC6A2 and attention-deficit/hyperactivity disorder
| Marker no. | RS number | Location | Major allele (allele 1) | Minor allele | No. of informative families* | Z statistic (allele 1) | p value |
|---|---|---|---|---|---|---|---|
| 1 | rs28386840 | Upstream promoter | A | T | 147 | 0.71 | 0.48 |
| 2 | rs4783899 | 5′flanking | G | T | 183 | −1.69 | 0.09 |
| 3 | rs1362621 | 5′flanking | A | G | 148 | 1.13 | 0.26 |
| 4 | rs2397771 | 5′flanking | G | C | 173 | 1.85 | 0.06 |
| 5 | rs168924 | 5′flanking | T | C | 106 | 0.41 | 0.68 |
| 6 | rs2242446 | 5′flanking | T | C | 152 | 0.95 | 0.34 |
| 7 | rs3785143 | Intron 1 | C | T | 76 | 2.25 | 0.024 |
| 8 | rs192303 | Intron 1 | G | C | 156 | 1.11 | 0.27 |
| 9 | rs41154 | Intron 1 | A | G | 169 | −1.76 | 0.08 |
| 10 | rs187715 | Intron 2 | A | G | 41 | 0.61 | 0.54 |
| 11 | rs36024 | Intron 3 | C | T | 166 | 1.33 | 0.18 |
| 12 | rs187714 | Intron 3 | T | C | 173 | −1.55 | 0.12 |
| 13 | rs36023 | Intron 3 | C | T | 170 | 0.58 | 0.56 |
| 14 | rs36021 | Intron 3 | T | A | 163 | 2.64 | 0.008 |
| 15 | rs3785152 | Intron 3 | C | T | 73 | 1.46 | 0.14 |
| 16 | rs1814269 | Intron 3 | G | A | 162 | −1.66 | 0.10 |
| 17 | rs36017 | Intron 3 | C | G | 166 | −1.53 | 0.13 |
| 18 | rs10521329 | Intron 4 | C | A | 116 | 0.00 | > 0.99 |
| 19 | rs3785155 | Intron 4 | G | A | 94 | 0.03 | 0.98 |
| 20 | rs5564 | Intron 5 | T | C | 46 | 1.27 | 0.20 |
| 21 | rs11568324 | Intron 5 | C | T | 11 | 0.58 | 0.56 |
| 22 | rs2279805 | Intron 6 | T | C | 170 | −1.43 | 0.15 |
| 23 | rs8047672 | Intron 8 | G | A | 119 | −0.08 | 0.94 |
| 24 | rs5569 | Exon 9 | C | T | 153 | 1.72 | 0.08 |
| 25 | rs998424 | Intron9 | C | T | 151 | 1.76 | 0.08 |
| 26 | rs36009 | Intron 10 | G | A | 55 | 0.77 | 0.44 |
| 27 | rs1800887 | Intron 11 | T | C | 131 | 1.16 | 0.25 |
| 28 | rs2242447 | Intron 13 | T | C | 160 | 1.15 | 0.25 |
| 29 | rs15534 | Exon 14 | C | T | 102 | 0.76 | 0.45 |
| 30 | rs7188230 | 3′intergenic | A | G | 124 | 0.84 | 0.40 |
The number of informative families varies for each tag single-nucleotide polymorphism, depending on the heterozygocity of the marker.
Table 2.
Sliding window analysis of haplotype blocks in SLC6A2 (boys)*
| Group; markers in haplotype block | Haplotype | Freq. | No. | p value | Power | Quantitative trait |
|---|---|---|---|---|---|---|
| Combined and hyperactive | ||||||
| M08:M09 | C:G | 0.02 | 10 | 0.037 | 0.925 | CBCL–aggression |
| M07:M08:M09 | C:C:G | 0.02 | 9 | 0.037 | 0.946 | CBCL–aggression |
| M08:M09:M10:M11:M12 | C:G:A:C:C | 0.01 | 9 | 0.041 | 0.901 | CBCL–aggression |
| M07:M08:M09:M10:M11:M12 | C:C:G:A:C:C | 0.01 | 9 | 0.046 | 0.902 | CBCL–aggression |
| M22:M23 | T:A | 0.15 | 36 | −0.030 | 0.857 | CBCL–thought problems |
| M17:M18 | C:A | 0.16 | 33 | −0.016 | 0.712 | CBCL–thought problems |
| Inattentive subtype | ||||||
| M12:M13:M14:M15:M16:M17:M18 | T:C:A:C:G:C:C | 0.04 | 8 | −0.036 | 0.834 | ADHD |
| M12:M13:M14:M15:M16:M17:M18:M19 | T:C:A:C:G:C:C:G | 0.04 | 8 | −0.041 | 0.822 | ADHD |
| M14:M15:M16:M17:M18:M19 | A:C:G:C:C:G | 0.13 | 9 | −0.031 | 0.746 | CBCL–internalizing |
| M16:M17:M18:M19 | G:C:C:G | 0.28 | 12 | −0.020 | 0.858 | CBCL–aggression |
| M15:M16:M17:M18:M19 | C:G:C:C:G | 0.23 | 11 | −0.036 | 0.750 | CBCL–anxiety |
| M18:M19:M20:M21 | A:A:T:C | 0.12 | 7 | −0.039 | 0.855 | CBCL–attention |
| M12:M13 | C:C | 0.39 | 12 | −0.030 | 0.701 | CGI-T |
| M23:M24:M25:M26:M27 | G:C:C:G:T | 0.39 | 15 | −0.041 | 0.843 | CGI-T |
| M20:M21:M22:M23:M24:M25:M26 | T:C:C:G:C:C:G | 0.36 | 17 | −0.049 | 0.901 | CGI-T |
ADHD = attention-deficit/hyperactivity disorder; CBCL = Child Behavior Checklist;42 CGI-T = Conners Global Index — Teachers.41
Analysis was conducted in PBAT software, with conditioning on sufficient power. Family-based association test results (for each quantitative trait tested) with significance p < 0.05 and power > 0.7 are listed. The ADHD subtypes combined and hyperactive were combined into 1 group in comparison with the inattentive subtype. The RS numbers for each marker are provided in Table 1. The frequency of each haplotype (Freq.) and number of informative families (No.) is listed for each haplotype.
Table 3.
Sliding window analysis of haplotype blocks in SLC6A2 (girls)*
| Group; markers in haplotype block | Haplotype | Freq. | No. | p value | Power | Quantitative trait |
|---|---|---|---|---|---|---|
| Combined and hyperactive | ||||||
| M04:M05:M06:M07:M08 | G:T:T:C:G | 0.36 | 5 | −0.038 | 0.855 | CGI-T |
| M03:M04:M05:M06:M07:M08 | A:G:T:T:C:G | 0.36 | 5 | −0.038 | 0.855 | CGI-T |
| M04:M05:M06:M07:M08:M09 | G:T:T:C:G:G | 0.35 | 5 | −0.046 | 0.723 | CGI-T |
| M03:M04:M05:M06:M07:M08:M09 | A:G:T:T:C:G:G | 0.35 | 5 | −0.046 | 0.723 | CGI-T |
| M04:M05:M06:M07:M08:M09:M10 | G:T:T:C:G:G:A: | 0.35 | 5 | −0.046 | 0.723 | CGI-T |
| M10:M11:M12:M13 | A:C:C:C | 0.37 | 5 | −0.049 | 0.876 | CGI-T |
| M12:M13:M14 | C:C:T | 0.36 | 5 | −0.046 | 0.870 | CGI-T |
| Inattentive subtype | ||||||
| M01:M02:M03:M04:M05 | A:T:A:G:T | 0.45 | 9 | −0.046 | 0.845 | CBCL–total |
| M02:M03:M04:M05:M06 | T:A:G:T:T | 0.45 | 9 | −0.040 | 0.865 | CBCL–total |
| M01:M02:M03:M04:M05:M06 | A:T:A:G:T:T | 0.45 | 9 | −0.046 | 0.845 | CBCL–total |
| M02:M03:M04:M05:M06:M07 | T:A:G:T:T:C | 0.45 | 9 | −0.034 | 0.854 | CBCL–total |
| M01:M02:M03:M04:M05:M06:M07 | A:T:A:G:T:T:C | 0.45 | 9 | −0.039 | 0.834 | CBCL–total |
| M07:M08:M09 | C:G:A | 0.22 | 7 | 0.040 | 0.709 | CBCL–total |
| M02:M03:M04:M05 | T:A:G:T | 0.45 | 9 | −0.041 | 0.779 | CBCL–internalizing |
| M01:M02:M03:M04:M05 | A:T:A:G:T | 0.45 | 9 | −0.045 | 0.749 | CBCL–internalizing |
| M02:M03:M04:M05:M06 | T:A:G:T:T | 0.45 | 9 | −0.039 | 0.784 | CBCL–internalizing |
| M01:M02:M03:M04:M05:M06 | A:T:A:G:T:T | 0.45 | 9 | −0.045 | 0.749 | CBCL–internalizing |
| M02:M03:M04:M05:M06:M07 | T:A:G:T:T:C | 0.45 | 9 | −0.034 | 0.759 | CBCL–internalizing |
| M01:M02:M03:M04:M05:M06:M07 | A:T:A:G:T:T:C | 0.45 | 9 | −0.038 | 0.722 | CBCL–internalizing |
| M09:M10:M11:M12 | A:A:T:T: | 0.32 | 8 | 0.025 | 0.909 | CBCL–thought |
Analysis was conducted in PBAT software, with conditioning on sufficient power. Family-based association test results (for each quantitative trait tested) with significance p < 0.05 and power > 0.7 are listed. The ADHD subtypes combined and hyperactive were combined into 1 group in comparison with the inattentive subtype. The frequency of each haplotype (Freq.) and number of informative families (No.) is listed for each haplotype.
Fig. 1.
Summary of sliding window haplotype analyses of polymorphisms within SLC6A2. The significant findings (family-based association tests p < 0.05, power > 0.7) from the PBAT analysis are schematically represented. The Child Behavior Checklist (CBCL)42 subscale scores that were significant are presented; these include internalizing, aggressive behaviour, thought problems, attention problems and anxiety. The linkage disequilibrium plot was generated in Haploview version 4.0, as described in the Methods section. CGI-t = Conners Global Index —Teacher40 scores; Tht = CBCL–thought problems.
In boys, the region showing association with ADHD and the behavioural phenotypes was more extensive. However, a lack of association with the extreme 5′ and 3′ ends of the gene was noted. A greater heterogeneity was also observed in the dimensions of ADHD showing an association with SLC6A2 in the different subytpes. In boys with the combined/hyperactive subtype, M07-M08-M09-M10-M11-M12 showed an association with subscale CBCL scores for aggressive behaviour (C:C:G:A:C:C overtransmitted in children with higher scores).
In boys with the inattentive subtype, 2 distinct haplotypes appeared to have 2 different effects. The haplotype T:C:A:C:G:C:C:G for block M12-M13-M14-M15-M16-M17-M18-M19 showed an overall undertransmission in boys with ADHD. Further, we noted that a subset of this haplotype block (M16-M17-M18-M19) showed an association with aggressive behaviour, with the G:C:C:G haplotype being undertransmitted, conferring a protective effect in this subgroup. In a partially overlapping block (M18-M19-M20-M21), a different haplotype (A:A:T:C) showed an undertransmission with inattention, and M20-M27 (T:C:C:G:C:C:G:T) was undertransmitted in children with higher CGI-T scores.
Discussion
Research on the genetics of ADHD, as with most psychiatric disorders, has been plagued by the lack of replication among studies. The same picture has emerged with SLC6A2 and ADHD. Five SNPs within SLC6A2 (rs3785157, rs998424, rs3785143, rs11568324, rs28386840) have shown an association with ADHD, though some studies have failed to replicate the findings. In the present study, a significant association was observed with rs3785143 (M07; p = 0.024), and a trend was observed with rs998424 (M25; p = 0.08). However, rs36017 (proxy marker in strong linkage disequilibrium with rs3785157, pairwise D′ = 1), rs11568324 and rs28386840 did not show a significant association. In addition, a novel SNP (rs36021) within Intron3 showed a significant association with ADHD (p = 0.008).
Detailed haplotype analysis of SLC6A2 was conducted with different quantitative dimensions of ADHD, while controlling for sex and subtype. Screening based on conditional power was implemented in PBAT software; only results meeting the dual criteria of low p value (p < 0.05) when the test had sufficient power (power > 0.8) were considered significant. We observed a complex picture of association results, with different regions of the gene important in boys and girls with the different subtypes (Fig. 1). We observed extensive association between haplotype blocks within SLC6A2 and clinical dimensions of ADHD. This is in stark contrast to all earlier reports, as well as results in the present study when testing individual SNPs (even if they were tag SNPs) for association with the disorder as a diagnostic entity defined by DSM-IV criteria. Most of this analysis has reported the association with only a limited number of SNPs, even when a large array of tag SNPs was tested, as in the IMAGE study.30
The results obtained in this family-based study strongly suggest that polymorphisms within different regions of SLC6A2 show differential association with ADHD. For example, in girls, a significant association with haplotype block 1, and a lack of association with blocks 2 and 3 was observed. In boys, most of the association was observed in haplotype block 2 and the region between blocks 2 and 3, with no association observed at the 5′ end of the gene. Further, different haplotypes showed a significant association with different dimensions of the disorder (Fig. 1). These results provide a map to help navigate through previous findings.
The association with rs3785143 has been reported in several studies and was noted in the present study. On close perusal, it is noted that rs3785173 was associated with aggressive behaviour in boys with the combined/hyperactive subtype. However, in girls it was associated with CGI-T scores, CBCL–internalizing scores and CBCL–total scores. If a pooled sample is examined, where reports on these different dimensions are pooled together, a significant result might be expected. Conversely, if different haplotypes are associated with opposing dimensions of ADHD, as in boys with the inattentive versus the combined/hyperactive subtypes, the effect might be missed when the samples are pooled together. This result is also consistent with that in a previous report showing a stronger effect of rs3785143 in girls compared with boys (odds ratio 2.11, p = 0.006 v. p = 0.37).29 In the present study, this SNP (and in fact the region encapsulated by haplotype block 1) showed an association in girls with all subtypes. However, in boys, the association with rs3785143 was limited.
These results are consistent with the large body of literature suggesting that the combined and inattentive ADHD subtypes are distinct disorders with different etiologies.2 Results of latent class analyses has suggested that there are shared as well as unique factors (including genetic determinants) that contribute to these 2 subtypes.51 We had reported earlier that a differential association with serotonin transporter–linked polymorphic region based on ADHD subtype was observed in this sample of children with ADHD.52 The frequency of the LL genotype in the inattentive group was lower than in the combined/hyperactive group. However, no differences were noted in the allele frequency of the DAT 3′UTR-VNTR and DRD4 variable number tandem repeat in the different subtypes. Further analysis on the nosologic relations of the combined and inattentive subtypes has shown an important effect of sex. The results of the present study examining polymorphisms and haplotype blocks within SLC6A2 support this hypothesis, with distinct regions implicated in boys compared with girls.
Finally, we attempt to explain how the polymorphisms in these chromosomal regions may translate into a different function of the protein, with an effect on brain function. Twenty SNPs have been identified in SLC6A2 that result in amino acid substitution (Appendix 1, Table S3 and Fig. S2). Some of these functional SNPs have been genotyped in earlier studies as well as the present study. However, most of these SNPs have a very low heterozygosity and corresponding minor allele frequency, making them relatively non-informative in family-based studies. The alternative approach has therefore been employed where SNPs (having a higher heterozygosity) in high linkage disequilibrium with the functional SNP are tested for association with ADHD. The region encapsulating intron 1, in combination with the promoter region, is responsible for high-level transcription of NET.53 Within the gene, the polymorphisms result in changes in activity of the enzyme. With girls, most of the association observed is in the upstream region of the gene, including the upstream promoter, whereas in boys the association is distributed throughout the gene, which likely translates to changes in the structure/function of the protein. However, this analysis is purely speculative, and further molecular and genetic association studies are required before firm conclusions can be reached.
Limitations
The most important limitation of the study is the small size of the groups given the stratification based on sex and subtype. In addition, a relaxed criterion of p = 0.05 was set for significance. If a more stringent criteria of p ≤ 0.01 is set, with power > 0.8, none of the reported results would remain significant. These results must therefore be considered exploratory and may help to inform related studies of children with ADHD. Finally, a word of caution is called for, given the instability of ADHD subtypes. A longitudinal, follow-up study noted that a significant proportion (about 50%) of children with diagnoses of the inattentive subtype of ADHD in preschool years, met criteria for other ADHD subtypes at least twice during 6 follow-up assessments.54 Given these limitations, the value of this study is that it provides insight into the complex heterogeneity of the disorder, which translates into a complex association between genotype and phenotype. However, the results need to be confirmed in a larger group before firm conclusions can be reached.
Conclusion
The results obtained in this family-based study suggest that haplotype blocks within different regions of SLC6A2 show differential association with ADHD based on sex and subtype. These associations may have been masked in previous studies when tests were conducted with pooled samples.
Acknowledgments
This work was supported in part by grants from the Fonds de la recherche en santé du Québec and the Canadian Institutes of Health Research to R. Joober and N. Grizenko. S.M. Sengupta is a recipient of the 2008 NARSAD Young Investigator and 2009 Dr. Mortimer D. Sackler Developmental Psychology Investigator Awards. We thank Jacqueline Richard, Matthew Lebaron and Nicole Pawliuk for technical and clinical assistance. A special word of thanks to the families who participated in the research.
Footnotes
Contributors: N. Grizenko and R. Joober designed the study. N. Grizenko, J. Bellingham, R. DeGuzman, S. Robinson, A. Poloskia, S.M. Shaheen, M.-E. Fortier, Z. Choudhry and R. Joober acquired the data. S.M. Sengupta, G.A. Thakur, M. TerStepanian, A. Poloskia and R. Joober analyzed the data. S.M. Sengupta and R. Joober wrote the article. N. Grizenko, G.A. Thakur, J. Bellingham, R. DeGuzman, S. Robinson, M. TerStepanian, A. Poloskia, S.M. Shaheen, M.-E. Fortier, Z. Choudhry and R. Joober reviewed the article. All authors approved its publication.
Conflict of interest: As above. N. Grizeko, G. Thakur, J. Bellingham, R. DeGuzman, S. Robinson, Z. Choudhry, M. TerStepanian, A. Poloskia, M.-E. Fortier and S.M. Shaheen declare having received travel support from the Canadian Institutes of Health Research. R. Joober sits on the advisory boards and speakers’ bureaus of Pfizer Canada, Janssen Ortho Canada and Shire Canada; he has received grant funding from them and from AstraZeneca and BMS Canada. He has received honoraria from Janssen Ortho Canada for CME presentations and royalties for Henry Stewart talks.
References
- 1.Faraone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2:104–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): a neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity) Dev Psychopathol. 2005;17:807–25. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 4.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–45. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SH, Waldman ID, Hay DA, et al. Sex differences in genetic and environmental influences on DSM-III-R attention-deficit/hyperactivity disorder. J Abnorm Psychol. 1999;108:24–41. doi: 10.1037/0021-843X.108.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Carlson CL, Mann M. Attention-deficit/hyperactivity disorder, predominantly inattentive subtype. Child Adolesc Psychiatr Clin N Am. 2000;9:499–510. [PubMed] [Google Scholar]
- 7.Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5:143–54. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- 8.Smalley SL, McGough JJ, Del’Homme M, et al. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:1135–43. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Pliszka SR. The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1385–90. doi: 10.1016/j.biopsych.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Krause KH, Dresel SH, Krause J, et al. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285:107–10. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 12.Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biederman J, Spencer T. Methylphenidate in treatment of adults with attention-deficit/hyperactivity disorder. J Atten Disord. 2002;6(Suppl 1):S101–7. doi: 10.1177/070674370200601s12. [DOI] [PubMed] [Google Scholar]
- 15.Del CN, Chamberlain SR, Sahakian BJ, et al. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211–6. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–46. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148:564–77. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- 19.Ordway GA, Schwartz MA, Frazer A. Brain norepinephrine: neurobiology and therapeutics. Cambridge (UK): Cambridge University Press; 2007. [Google Scholar]
- 20.Uhl GR, Johnson PS. Neurotransmitter transporters: three important gene families for neuronal function. J Exp Biol. 1994;196:229–36. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- 21.Barr CL, Kroft J, Feng Y, et al. The norepinephrine transporter gene and attention-deficit hyperactivity disorder. Am J Med Genet. 2002;114:255–9. doi: 10.1002/ajmg.10193. [DOI] [PubMed] [Google Scholar]
- 22.Bobb AJ, Addington AM, Sidransky E, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 23.De Luca V, Muglia P, Jain U, et al. No evidence of linkage or association between the norepinephrine transporter (NET) gene MnlI polymorphism and adult ADHD. Am J Med Genet. 2004;124:38–40. doi: 10.1002/ajmg.b.20075. [DOI] [PubMed] [Google Scholar]
- 24.McEvoy B, Hawi Z, Fitzgerald M, et al. No evidence of linkage or association between the norepinephrine transporter (NET) gene polymorphisms and ADHD in the Irish population. Am J Med Genet B Neuropsychiatr Genet. 2002;114:665–6. doi: 10.1002/ajmg.10416. [DOI] [PubMed] [Google Scholar]
- 25.Retz W, Rosler M, Kissling C, et al. Norepinephrine transporter and catecholamine-O-methyltransferase gene variants and attention-deficit/hyperactivity disorder symptoms in adults. J Neural Transm. 2008;115:323–9. doi: 10.1007/s00702-007-0822-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Hahn MK, Joung Y, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci U S A. 2006;103:19164–9. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CH, Waldman ID, Blakely RD, et al. Functional gene variation in the human norepinephrine transporter: association with attention deficit hyperactivity disorder. Ann N Y Acad Sci. 2008;1129:256–60. doi: 10.1196/annals.1417.023. [DOI] [PubMed] [Google Scholar]
- 28.Cho SC, Kim JW, Kim BN, et al. No evidence of an association between norepinephrine transporter gene polymorphisms and attention deficit hyperactivity disorder: a family-based and case-control association study in a Korean sample. Neuropsychobiology. 2008;57:131–8. doi: 10.1159/000138916. [DOI] [PubMed] [Google Scholar]
- 29.Biederman J, Kim JW, Doyle AE, et al. Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1511–8. doi: 10.1002/ajmg.b.30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–53. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 31.Kim JW, Biederman J, McGrath CL, et al. Further evidence of association between two NET single-nucleotide polymorphisms with ADHD. Mol Psychiatry. 2008;13:624–30. doi: 10.1038/sj.mp.4002090. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Knight J, Brookes K, et al. DNA pooling analysis of 21 nor-epinephrine transporter gene SNPs with attention deficit hyperactivity disorder: no evidence for association. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:115–8. doi: 10.1002/ajmg.b.30160. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Hawi Z, Brookes KJ, et al. Replication of a rare protective allele in the noradrenaline transporter gene and ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1564–7. doi: 10.1002/ajmg.b.30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forero DA, Arboleda GH, Vasquez R, et al. Candidate genes involved in neural plasticity and the risk for attention-deficit hyperactivity disorder: a meta-analysis of 8 common variants. J Psychiatry Neurosci. 2009;34:361–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Kuntsi J, Rijsdijk F, Ronald A, et al. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry. 2005;57:647–54. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Lasky-Su J, Neale BM, Franke B, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- 37.Thapar A, Langley K, O’Donovan M, et al. Refining the attention deficit hyperactivity disorder phenotype for molecular genetic studies. Mol Psychiatry. 2006;11:714–20. doi: 10.1038/sj.mp.4001831. [DOI] [PubMed] [Google Scholar]
- 38.Grizenko N, Kovacina B, Amor LB, et al. Relationship between response to methylphenidate treatment in children with ADHD and psychopathology in their families. J Am Acad Child Adolesc Psychiatry. 2006;45:47–53. doi: 10.1097/01.chi.0000184932.64294.d9. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer D, Fisher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH Drosoph Inf ServC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Conners CK, Sitarenios G, Parker JD, et al. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–91. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 41.Conners CK, Sitarenios G, Parker JD, et al. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 42.Achenbach TM. Manual for the Child Behavioral Checklist/4–18 and 1991 Profile. Burlington (VT): University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 43.Hudziak JJ, Derks EM, Althoff RR, et al. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ Rating Scales–Revised. Am J Psychiatry. 2005;162:1614–20. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- 44.Touliatos J, Lindholm BW. Congruence of parents’ and teachers’ ratings of children’s behavior problems. J Abnorm Child Psychol. 1981;9:347–54. doi: 10.1007/BF00916839. [DOI] [PubMed] [Google Scholar]
- 45.Mitsis EM, McKay KE, Schulz KP, et al. Parent-teacher concordance for DSM-IV attention-deficit/hyperactivity disorder in a clinic-referred sample. J Am Acad Child Adolesc Psychiatry. 2000;39:308–13. doi: 10.1097/00004583-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Ehrich M, Bocker S, van den BD. Multiplexed discovery of sequence polymorphisms using base-specific cleavage and MALDI-TOF MS. Nucleic Acids Res. 2005;33:e38. doi: 10.1093/nar/gni038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 49.Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype–phenotype associations. Eur J Hum Genet. 2001;9:301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- 50.Lange C, DeMeo D, Silverman EK, et al. Using the noninformative families in family-based association tests: a powerful new testing strategy. Am J Hum Genet. 2003;73:801–11. doi: 10.1086/378591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stawicki JA, Nigg JT, von EA. Family psychiatric history evidence on the nosological relations of DSM-IV ADHD combined and inattentive subtypes: new data and meta-analysis. J Child Psychol Psychiatry. 2006;47:935–45. doi: 10.1111/j.1469-7610.2006.01628.x. [DOI] [PubMed] [Google Scholar]
- 52.Grizenko N, Paci M, Joober R. Is the inattentive subtype of ADHD different from the combined/hyperactive subtype? J Atten Disord. 2010;13:649–57. doi: 10.1177/1087054709347200. [DOI] [PubMed] [Google Scholar]
- 53.Kim CH, Kim HS, Cubells JF, et al. A previously undescribed intron and extensive 5′ upstream sequence, but not Phox2a-mediated transactivation, are necessary for high level cell type-specific expression of the human norepinephrine transporter gene. J Biol Chem. 1999;274:6507–18. doi: 10.1074/jbc.274.10.6507. [DOI] [PubMed] [Google Scholar]
- 54.Lahey BB, Pelham WE, Loney J, et al. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]