Abstract
Background
The purpose of this study was to assess deep hypothermic circulatory arrest (DHCA) as a modifier of neurodevelopmental (ND) outcomes in preschool children after cardiac surgery in infancy for repair of congenital heart defects (CHD).
Methods
This is a planned analysis of infants enrolled in a prospective study of apolipoprotein E polymorphisms and ND outcome after cardiac surgery. The effect of DHCA was assessed in patients with single or biventricular CHD without aortic arch obstruction. Neurodevelopmental assessment at 4 years of age included cognition, language, attention, impulsivity, executive function, social competence, and visual-motor and fine-motor skills. Patient and procedural variables were evaluated in univariate and multivariate models.
Results
Neurodevelopmental testing was completed in 238 of 307 eligible patients (78%). Deep hypothermic circulatory arrest was used at the discretion of the surgeon at least once in 92 infants (38.6%) with a median cumulative duration of 36 minutes (range, 1 to 132 minutes). By univariate analysis, DHCA patients were more likely to have single-ventricle CHD (p = 0.013), lower socioeconomic status (p < 0.001), a higher incidence of preoperative ventilation (p < 0.001), and were younger and smaller at the first surgery (p < 0.001). By multivariate analysis, use of DHCA was not predictive of worse performance for any ND outcome.
Conclusions
In this cohort of children undergoing repair of CHD in infancy, patients who underwent DHCA had risk factors associated with worse ND outcomes. Despite these, use of DHCA for repair of single-ventricle and biventricular CHD without aortic arch obstruction was not predictive of worse performance for any ND domain tested at 4 years of age.
Cerebral ischemia during surgical repair of congenital heart disease (CHD) has been proposed to be a primary mechanism of central nervous system injury. Many observational studies and neuroprotective trials have focused on the surgical treatment of CHD, particularly intraoperative management strategies and the type of support used during the cardiac repair. Cardiopulmonary bypass (CPB) exposes blood to the foreign surfaces of the bypass circuit, initiating a systemic inflammatory response. When continuous CPB is used, perfusion to the body and brain are maintained, but the duration of blood exposure to the bypass circuit is increased. Use of deep hypothermic circulatory arrest (DHCA) provides a bloodless surgical field, which may facilitate completion of the best physiologic repair and decrease the duration of blood exposure to the bypass circuit but at the cost of a period of global cerebral ischemia. There remains considerable controversy as to which technique provides optimal neurodevelopmental outcomes.
Well-designed trials assessing the impact of changes in intraoperative management strategies have often provided conflicting data. The most comprehensive data were provided by the Boston Circulatory Arrest Study (BCAS), a randomized trial of DHCA and continuous CPB in children undergoing the arterial switch operation [1]. Deep hypothermic circulatory arrest was associated with worse outcomes in the immediate postoperative period and at 1-year follow-up. By the 8-year evaluation, however, the impact of use of DHCA was less significant [2]. Performance of the cohort for many neurodevelopmental domains was worse than population norms but was not related to treatment group assignment. Patient-specific factors, such as socioeconomic status (SES) (23.7%) and ventricular septal defect presence (3.2%), explained more of the variance in IQ than did treatment group (DHCA versus primarily low-flow CPB) assignment (0.3%). Treatment assignment resulted in different neurologic morbidities rather than reducing their frequency or severity. For example, motor and speech skills were lower in patients assigned to DHCA, whereas the low-flow CPB group demonstrated worse scores for impulsivity and behavior. Consistent with these findings, studies at our institution suggest that patient-specific factors (genetic syndromes, brain immaturity, and SES) contribute more substantially to the risk of adverse neurodevelopmental outcomes than intraoperative management strategies [3]. Despite these findings, many institutions altered their intraoperative management strategies to avoid DHCA. This has led to the introduction and widespread utilization of alternative support techniques, such as regional cerebral perfusion, despite no evidence of improvement in neurodevelopmental outcomes compared with DHCA. There are currently no data that justify the avoidance of DHCA in favor of alternative strategies.
In 1998 we initiated a prospective study to evaluate the association between neurodevelopmental outcome and polymorphisms of the apolipoprotein E (APOE) gene in neonates and infants undergoing repair of CHD [4]. The cohort has undergone formal neurodevelopmental evaluations at 1 and 4 years of age [3, 5, 6]. The APOE ε2 allele was associated with a significantly worse neurodevelopmental outcome at 1 year of age. At 4 years of age, the APOE ε2 allele was associated with a significantly increased prevalence of parent-reported behavioral symptoms including impaired social interactions, restricted behavior patterns, and inattention, as well as core language impairments. Conversely, the APOE ε4 allele was associated with more favorable outcomes. As part of the planned analysis at 4 years, we proposed to determine whether use of DHCA is a risk factor for adverse neurodevelopmental outcomes after cardiac surgery in infancy.
Patients and Methods
Patient Population
This is a subgroup analysis of patients enrolled in a prospective trial assessing the effects of polymorphisms of the APOE gene on neurobehavioral outcomes at 4 years of age after infant cardiac surgery. Exclusion criteria for the overall study included (1) multiple congenital anomalies, (2) recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletion syndrome, and (3) language other than English spoken in the home. Both premature infants and low birth weight neonates were enrolled, as were infants with functional single-ventricle CHD. Deep hypothermic circulatory arrest was uniformly used for aortic arch reconstruction for patients in the APOE cohort. Therefore, the subgroup for this analysis comprises patients with single-ventricle or biventricular CHD without aortic arch obstruction in whom the use of DHCA was considered optional. This study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from the parent or guardian.
Operative Management
Operations were performed by 5 cardiac surgeons with a dedicated team of cardiac anesthesiologists. Alpha-stat blood gas management was used. Pump flow rates were not standardized for this study. In general, during normothermic or mild hypothermic CPB (nasopharyngeal [NP] temperature >28°C), the pump flow rate was maintained at 100 to 150 mL · kg−1 · min−1 to achieve a mean arterial pressure of 30 to 55 mm Hg. When moderate hypothermia (NP temperature 22° to 28°C) was used, the pump flow rate was maintained at 100 mL · kg−1 · min−1 with a target mean arterial pressure greater than 30 mm Hg. For deep hypothermic continuous CPB (NP temperature <22°C), a pump flow rate of 25 to 50 mL · kg−1 · min−1 was used. These were general guidelines that were modified according to the clinical situation. Deep hypothermic circulatory arrest was selectively used at the surgeon’s discretion, not according to a predetermined protocol. Before DHCA, patients underwent core and surface cooling, with topical hypothermia of the head, to a NP temperature of 18°C. Modified ultrafiltration was performed in all patients after CPB. Postoperatively, patients were managed in the cardiac intensive care unit by a dedicated team of cardiologists, intensivists, nurses, and surgeons.
Data Collection
Preoperative factors that might independently affect neurobehavioral outcomes, including gestational age, birth head circumference, and birth weight, were obtained from birth and hospital records. Weight and age at surgery were recorded for the initial operation and subsequent procedures with CPB. Operative variables were recorded, including the duration of CPB and DHCA, lowest NP temperature, and hematocrit after hemodilution.
Genetic Evaluation
All patients were examined by a genetic dysmorphologist at 1 or 4 years of age. Because recognition of genetic syndromes in neonates may be difficult, some children with genetic abnormalities had been enrolled as infants. Genetic testing was performed as indicated. Patients were classified as no definite genetic syndrome or chromosomal abnormality (normal), suspected genetic syndrome (suspect), or definite genetic or chromosomal abnormality (abnormal).
Apolipoprotein E Genotype Determination
Whole blood was obtained before the operation and stored at 4°C. Genomic DNA was prepared and used to determine APOE genotypes by using a previously published method [6].
Four-Year Evaluation and Neurodevelopmental Testing
Children were evaluated between their fourth and fifth birthdays. Growth measurements were obtained, including weight, length, and head circumference. Maternal education and the child’s ethnicity were assessed by parental report. The familial SES was assessed by parental report according to the Hollingshead scale. A health history was obtained, focusing on the incidence of interim illnesses, rehospitalizations, neurologic events or interim evaluations, current medication use, and parental concerns about health. Parents completed behavior questionnaires and rating scales.
To provide a broad assessment of neurodevelopmental status, multiple domains were tested including cognition; language; fine-motor and visual-motor skills; executive function; inattention and impulsivity; and social skills. Quantitative testing was use to assess cognition, core language skills, fine-motor and visual-motor skills, and executive function. Cognitive skills were assessed with Full-Scale IQ (FSIQ) from the Wechsler Preschool and Primary Scale of Intelligence-3rd Edition (WPPSI-III). Core language competence was assessed using the Pre-school Language Test-4 Total Language Score (PLS4-TLS). Fine-motor skills were tested with the Wide Range Assessment of Visual Motor Abilities (WRAVMA) pegboard, a manipulative dexterity test. Visual-motor integration was assessed with the Developmental Test of Visual Motor Integration (VMI), a simple copying task that assesses the child’s fine-motor and visual-motor coordination skills. Executive function was assessed with the NEPSY (NEuro-PSYchology) Attention/Executive Functions Core Domain Score, which reflects performance on measures of selective attention and executive functions including motor and task persistence, inhibition, planning, and mental flexibility. If a child was judged to be too developmentally impaired to complete the tasks, a score was imputed by assigning him or her the lowest possible score for the specific test.
Inattention, impulsivity, and social skills were assessed by parental report. Inattention and impulsivity were also assessed by the Impulsivity and Inattention Scales of the Attention Deficit/Hyperactivity Disorder (ADHD) Rating Scale-IV Preschool Version. Social competence was assessed by the Preschool and Kindergarten Behavior Rating Scales (PKBS) Social Skills Total Score, which details social cooperation, social interaction, and social independence as reported by parents.
Data Analysis and Statistical Methods
Subjects were characterized on the basis of exposure to DHCA. Patients in whom DHCA of any duration was used before the 4-year evaluation were categorized as DHCA patients. Those in whom DHCA was never used were categorized as No DHCA. Ethnicity was classified as Asian/Pacific Islander, African American, Hispanic, Native American, white, or other. APOE genotype was coded for presence of the ε2 or ε4 allele. Subjects were categorized in three groups: ε2 (ε2ε2 and ε2ε3), ε3 (ε3ε3), and ε4 (ε3ε4 and ε4ε4). Subjects with ε2ε4 genotype (n = 14) were excluded from analysis of APOE genotype effect. The APOE group was coded as a dummy variable, with group ε3 as the reference. Comparisons of covariates and unadjusted outcomes among groups of subjects were performed using Fisher’s exact test or Student’s t test.
To identify risk factors for adverse neurodevelopmental outcomes, the variables listed in Table 1 were tested in a univariate manner for association with the results for each of the neurodevelopmental domains by using linear and logistic regression analyses. Categorical data were coded as dummy variables for the regression analyses, with the most common category being used as the reference group, and the overall model probability values were reported. After univariate analyses, variables associated with an outcome with a probability of less than 0.1 in the univariate models were included in multivariate analyses. In the multivariate analyses, we considered forward and backward stepwise regression models, with the most parsimonious set of significant predictors being reported in the final model for each outcome. All analyses used SPSS version 10.0 for Windows (SPSS Inc, Chicago, IL) and the R statistical software environment (R Project for Statistical Computing, Vuebba, Austria).
Table 1.
Baseline and Management Covariatesa
| Variable | No DHCA (n = 146) | DHCA (n = 92) | p Value |
|---|---|---|---|
| Sex | 0.717 | ||
| Male | 86 (58.9%) | 52 (56.5%) | |
| Female | 60 (41.1%) | 40 (43.5%) | |
| Race | 0.403 | ||
| American Indian/Alaskan | 5 (3.4%) | 0 | |
| Asian | 7 (4.8%) | 5 (5.4%) | |
| Black | 26 (17.8%) | 20 (21.7%) | |
| Hispanic | 7 (4.8%) | 6 (6.5%) | |
| White | 101 (69.2%) | 61 (66.3%) | |
| Maternal education | 0.101 | ||
| Less than high school | 9 (6.2%) | 4 (4.4%) | |
| High school/some college | 55 (37.7%) | 50 (54.3%) | |
| College | 51 (34.9%) | 25 (21.2%) | |
| Graduate | 30 (20.5%) | 13 (14.1%) | |
| SES class | 0.013 | ||
| 1 | 3 (2.1%) | 5 (5.4%) | |
| 2 | 11 (7.5%) | 7 (7.6%) | |
| 3 | 26 (17.8%) | 22 (23.9%) | |
| 4 | 43 (29.5%) | 38 (41.3%) | |
| 5 | 63 (43.2%) | 20 (21.7%) | |
| Genetic evaluation | 0.410 | ||
| Normal | 110 (75.3%) | 62 (67.4%) | |
| Suspect | 17 (11.6%) | 14 (15.2%) | |
| Confirmed | 19 (13.0%) | 16 (17.3%) | |
| APOE | 0.546 | ||
| ε2ε2 | 0 | 1 (1.1%) | |
| ε2ε3 | 15 (10.3%) | 12 (13.0%) | |
| ε2ε4 | 4 (2.7%) | 1 (1.1%) | |
| ε3ε3 | 90 (61.6%) | 55 (59.8%) | |
| ε3ε4 | 31 (21.2%) | 21 (22.8%) | |
| ε4ε4 | 5 (3.4%) | 1 (1.1%) | |
| Birth weight (kg) | 3.1 ± 0.6 (145) | 3.0 ± 0.7 (91) | 0.155 |
| Birth head circumference (cm) | 33.5 ± 2.0 (141) | 33.2 ± 2.3 (89) | 0.253 |
| Gestational age (wk) | 38.5 ± 2.1 (145) | 38.2 ± 2.6 (90) | 0.344 |
| Preoperative mechanical ventilation | <0.001 | ||
| Not intubated | 118 (80.8%) | 55 (59.8%) | |
| Intubated | 28 (19.2%) | 37 (40.2%) | |
| Diagnostic class | <0.001 | ||
| Two ventricles | 135 (92.5%) | 67 (72.8%) | |
| Single ventricle | 11(7.5%) | 25 (27.2%) | |
| Age at first operation (days) | 72.8 ± 59.4 (146) | 42.9 ± 50.8 (92) | <0.001 |
| Weight at first operation (kg) | 4.5 ± 1.3 (146) | 3.7 ± 1.3 (92) | <0.001 |
| Total CPB duration at first operation (min) | 83.3 ± 47.3 (146) | 50.1 ± 23.6 (92) | <0.001 |
| HCT after hemodilution at first operation (%) | 27.0 ± 4.1 (146) | 28.2 ± 3.9 (92) | 0.022 |
| Delayed sternal closure | 0.304 | ||
| No | 137 (93.8%) | 83 (90.2%) | |
| Yes | 9 (6.2%) | 9 (9.8%) | |
| Postoperative length of stay (days) | 8.5 ± 11.0 (146) | 13.1 ± 15.9 (92) | 0.016 |
| Use of ECMO or LVAD | 0.322 | ||
| No | 144 (98.6%) | 89 (96.7%) | |
| Yes | 2 (1.4%) | 3 (3.3%) | |
| Number of additional operations before age 4 | <0.001 | ||
| None | 126 (86.3%) | 59 (64.1%) | |
| One | 18 (12.3%) | 20 (21.8%) | |
| Two or more | 2 (1.4%) | 13 (14.1%) | |
| Total additional CPB time before age 4 | 11.4 ± 34 (146) | 28.0 ± 45.3 (92) | 0.003 |
Results are numbers with percentages in parentheses, or mean standard deviation with number in parentheses.
APOE = apolipoprotein E; CPB = cardiopulmonary bypass; DHCA = deep hypothermic circulatory arrest; ECMO = extracorporeal membrane oxygenation; HCT = hematocrit; LVAD = left ventricular assist device; SES = socioeconomic status.
Results
Between September 1998 and April 2003, 675 eligible infants underwent cardiac surgery. Twenty-three infants died before consent. Parents of 102 declined participation, and 550 (81%) were enrolled. The study population was 65% white, 23% African American, and 12% other ethnic origins; 58% were male. Four hundred eighty-six patients were alive and eligible for the 4-year evaluation, which was completed by 381 patients (78% of eligible). The only difference in baseline characteristics between returning and nonreturning patients was underrepresentation of African Americans in the returning patients (21% versus 29%).
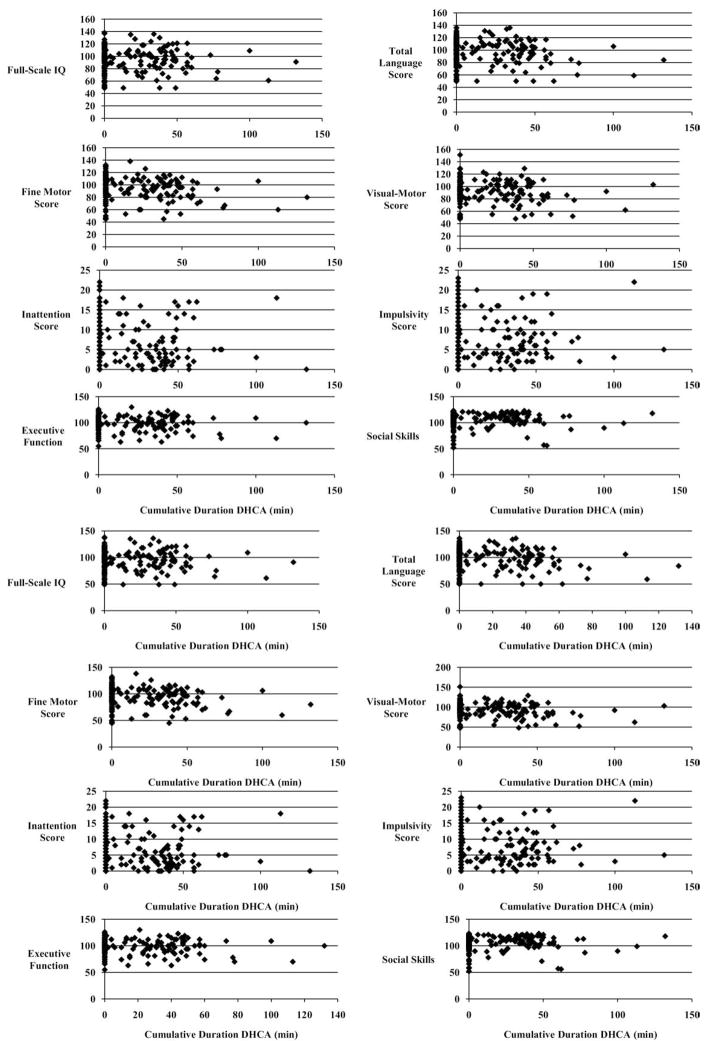
In the entire cohort, 329 patients (60%) met the anatomic criterion of single-ventricle or biventricular CHD without aortic arch obstruction. Twenty-two patients died before the 4-year evaluation, and 69 patients (22%) did not return for the 4-year evaluation. Two hundred thirty-eight patients (78%) underwent neurodevelopmental testing. Of these, 202 (85%) had biventricular CHD, with the majority being tetralogy of Fallot, transposition of the great arteries, or ventricular septal defect (Fig 1).
Fig 1.
Unadjusted outcomes related to duration of deep hypothermic circulatory arrest (DHCA).
Deep hypothermic circulatory arrest was used at least once in 92 infants (39%) with a median cumulative duration of 36 minutes (range, 1 to 132 minutes) and was never used in 146 patients (61%). Deep hypothermic circulatory arrest was used at the first operation in 78 of 92 (85%) patients, 43 of whom were younger than 30 days at the initial operation. Eighteen patients underwent multiple episodes of DHCA. The median maximal duration for a single episode of DHCA was 32 minutes (range, 1 to 73 minutes). The maximal duration of a single episode of DHCA was greater than 40 minutes for 26 patients and greater than 60 minutes for 2 patients. Cooling to a target temperature of 18°C was achieved on average during a length of 15 to 22 minutes in all patients.
There were significant differences between the DHCA and No DHCA groups for both patient-specific factors and other management characteristics. Deep hypothermic circulatory arrest patients were more likely to have single-ventricle CHD (27% versus 8%; p = 0.013; Fig 1). The SES was lower in the DHCA group (p < 0.001). Preoperative mechanical ventilation was more common in the DHCA patients (37 of 92; 40.2% versus 28 of 146; 19.2%; p < 0.001). There were no differences between groups for sex, race, maternal education level, genetic anomalies, APOE genotype, birth weight, birth head circumference, or gestational age (Table 1).
Patients who underwent DHCA were both younger and smaller at the first operation (both p < 0.001). The mean duration of CPB at the first operation was significantly shorter in the DHCA group (50 ± 24 versus 83 ± 47 minutes; p < 0.001). The hematocrit after hemodilution was greater in DHCA patients (28% ± 4%) compared with the No DHCA patients (27.0% ± 4%; p = 0.022). There were no differences between DHCA and No DHCA groups for delayed sternal closure or use of mechanical support (extracorporeal membrane oxygenation and ventricular assist device). Postoperative hospital length of stay was longer in DHCA patients (13 ± 16 days) compared with No DHCA patients (9 ± 11 days; p = 0.016). The DHCA group was more likely to have additional operations with CPB before age 4 (p < 0.001) and thus, longer additional exposure to CPB (p = 0.003; Table 1).
Consistent with previous reports, mean scores for the entire cohort are in the normal to low-average range for all domains tested (Table 2). Despite the increased prevalence of risk factors previously associated with adverse neurodevelopmental outcomes in the DHCA group, including lower SES, younger age, lower weight at the first surgery, preoperative mechanical ventilation, longer length of stay, and single-ventricle physiology, there were no differences in unadjusted mean outcomes between the DHCA and No DHCA groups. However, group mean scores may underestimate the proportion of children with significant impairments. Again, despite the increased prevalence of risk factors previously associated with adverse neurodevelopmental outcomes in the DHCA group, the proportion of children with moderate to severe impairment based on unadjusted scores is similar between groups for all domains (Table 3).
Table 2.
Unadjusted Neurodevelopmental Outcomes
| Domain | All
|
No DHCA
|
DHCA
|
DHCA vs No DHCA p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (Range) | SD | N | Mean | SD | N | Mean | SD | ||
| Cognition | 235 | 96.4 (49–138) | 19.5 | 145 | 97.6 | 20 | 90 | 94.6 | 18.7 | 0.239 |
| Core language | 234 | 97.5 (50–136) | 19.3 | 144 | 98 | 19.4 | 90 | 96.8 | 19.4 | 0.643 |
| Fine-motor skills | 237 | 95.1 (45–138) | 19.2 | 145 | 96.7 | 20.2 | 92 | 92.5 | 17.2 | 0.086 |
| Visual-motor integration | 237 | 93 (48–151) | 17.8 | 145 | 94.2 | 18 | 92 | 91.2 | 17.3 | 0.192 |
| Executive function | 220 | 99.7 (55–130) | 14.2 | 135 | 100.9 | 13.7 | 85 | 97.7 | 14.7 | 0.109 |
| Inattention | 235 | 6.1 (0–22) | 5 | 144 | 6.1 | 5 | 91 | 6.1 | 5.1 | 0.951 |
| Impulsivity | 235 | 6.9 (0–23) | 5.2 | 144 | 6.8 | 5.3 | 91 | 7.1 | 5.1 | 0.729 |
| Social competence | 234 | 107 (52–123) | 13.3 | 146 | 106.8 | 13.4 | 88 | 107.2 | 13.1 | 0.812 |
DHCA = deep hypothermic circulatory arrest; SD = standard deviation.
Table 3.
Severity of Impairment (Unadjusted Outcomes)
| Domain | No DHCA
|
DHCA
|
||
|---|---|---|---|---|
| Moderate Impairment | Severe Impairment | Moderate Impairment | Severe Impairment | |
| Cognition | 19/145 (13%) | 17/145 (12%) | 18/90 (20%) | 9/90 (10%) |
| Language skills | 19/144 (13%) | 14/144 (10%) | 14/90 (16%) | 9/90 (10%) |
| Fine-motor skills | 18/145 (12%) | 18/145 (12%) | 19/92 (21%) | 11/92 (12%) |
| Visual-motor integration | 19/134 (14%) | 13/145 (9%) | 22/92 (24%) | 11/92 (12%) |
| Executive function | 10/135 (7%) | 6/135 (4%) | 13/85 (15%) | 5/85 (6%) |
| Inattention | 40/144 (28%) | NA | 25/91 (27%) | NA |
| Impulsivity | 27/144 (19%) | NA | 19/91 (21%) | NA |
| Social Skills | 6/146 (4%) | 5/146 (3%) | 4/88 (5%) | 3/88 (3%) |
For cognition, language skills, fine-motor and visual motor skills, and executive function, moderate impairment is defined as a score ≥1 standard deviation (SD) but <2 SD below the mean and severe impairment is a score ≥2 SD below the mean. For inattention and impulsivity, a clinically significant is above 80%. For social skills, a moderate deficit is ≤89 but >71, and a significant deficit is ≤71. These cutoffs are at the 25th and 2nd percentiles, respectively.
DHCA = deep hypothermic circulatory arrest; NA = not applicable.
Stepwise regression identified multiple predictors of neurodevelopmental outcomes (Table 4). Consistent with prior reports from our institution and others, patient-specific factors were more important determinants of neurodevelopmental outcome than were operative management strategies. Specifically, use of DHCA was not a significant predictor of any neurodevelopmental outcomes (Fig 1). Presence of a genetic anomaly, lower SES and maternal education, and younger gestational age were all associated with worse outcomes. As in previous studies, preoperative mechanical ventilation and longer postoperative length of stay were associated with worse outcomes.
Table 4.
Predictors of Neurodevelopmental Outcomes
| Predictor | Cognition
|
Language
|
Fine-Motor
|
Visual-Motor
|
Executive Function
|
Inattention
|
Impulsivity
|
Social Skills
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Confirmed genetic anomaly | −20.20 | <0.001 | −18.73 | <0.001 | −17.69 | <0.001 | −11.17 | <0.001 | −15.06 | <0.001 | −5.63 | 0.019 | ||||
| SES | 3.56 | 0.003 | 5.38 | <0.001 | 3.31 | 0.001 | 4.48 | <0.001 | −1.03 | 0.001 | 1.93 | 0.005 | ||||
| Maternal education | 4.46 | 0.004 | ||||||||||||||
| Gestational age | 1.21 | 0.12 | 1.83 | <0.001 | 1.15 | 0.016 | ||||||||||
| White | 4.67 | 0.003 | 3.56 | <0.001 | ||||||||||||
| African American | −7.69 | 0.001 | ||||||||||||||
| Birth weight | 3.54 | 0.042 | −1.65 | 0.001 | −1.19 | 0.024 | 4.25 | 0.002 | ||||||||
| Single-ventricle CHD | 1.03 | 0.021 | ||||||||||||||
| Male sex | −5.11 | 0.004 | 1.38 | 0.04 | ||||||||||||
| Preoperative mechanical ventilation | −4.45 | 0.024 | ||||||||||||||
| APOE ε4 allele | 5.52 | 0.007 | 5.04 | 0.01 | ||||||||||||
| Additional operations with CPB | −6.50 | 0.001 | −5.35 | 0.03 | ||||||||||||
APOE = apolipoprotein E; CHD = congenital heart defect; CPB = cardiopulmonary bypass; SES = socioeconomic status.
Comment
Use of DHCA as a support technique during cardiac surgery in infancy has many advantages. These include ease of cannulation, decreased duration of exposure of blood to the foreign surfaces of the bypass circuit, decreased inflammatory response, and a bloodless, uncluttered surgical field. Operation with DHCA may facilitate a superior repair resulting in improved postoperative hemodynamics. However, there has been lingering concern that DHCA increases the risk of acute brain injury and adverse long-term neurodevelopmental outcomes. In this observational study of a large cohort of prospectively followed survivors of infant heart surgery, we evaluated the use of DHCA as a predictor of adverse neurodevelopmental outcomes. Deep hypothermic circulatory arrest was used selectively at the surgeon’s discretion. Children in whom DHCA was used were more likely to have single-ventricle CHD, were younger and smaller at the time of the initial surgery, and were more likely to undergo additional surgery with CPB. They were more likely to have been mechanically ventilated before surgery and had longer postoperative length of stay. Despite the increased prevalence of these previously identified risk factors in the DHCA group, there was no difference for unadjusted outcomes between the DHCA and No DHCA patients for any neurodevelopmental domain tested. Even after covariate adjustment using multivariable linear regression, use of DHCA was not predictive of a worse outcome for any domain tested. These findings support the hypothesis that selective use of DHCA during cardiac surgery in infancy is not associated with worse neurodevelopmental outcomes and does not need to be avoided.
Improved survival after repair of complex CHD in infancy has led to an increasing focus on longer term outcomes, particularly neurobehavioral. Neurodevelopmental dysfunction has been recognized as the most common and potentially most disabling outcome of CHD and its treatment. Multiple studies have evaluated potential mechanisms of neurodevelopmental dysfunction and potential protective therapies. However, well-designed trials assessing the impact of changes in intra-operative management strategies, specifically avoidance of DHCA, have yielded conflicting data. Despite the inconclusive evidence and because of theoretic concerns over use of DHCA, many institutions have altered their intraoperative management strategies to avoid all DHCA. This desire to avoid DHCA has led to the introduction and widespread utilization of new support techniques such as regional cerebral perfusion despite a lack of data showing superiority of regional cerebral perfusion to DHCA [7–9].
The most comprehensive data concerning the use of CPB (with and without DHCA) and neurodevelopmental outcomes are provided by the BCAS [1, 2, 10–15]. This randomized trial examined the incidence of acute postoperative neurologic injury as well as subsequent cognitive and behavioral outcomes at 1, 4, and 8 years postoperatively. Assignment to DHCA was associated with an increased incidence of seizures in the early postoperative period [16]. Evaluation at 1 year of age demonstrated that the children assigned to DHCA had lower scores for the Psychomotor Developmental Index of the Bayley Scales of Infant Development. At 4 years of age, assignment to DHCA was associated with impaired gross and fine-motor skills, as well as speech apraxia. Despite cognitive function assessed by full scale, verbal, and performance IQ being significantly lower for the entire cohort compared with the general population, there was no treatment group effect. Social class predicted a larger percentage of the variation in IQ (24%) than did treatment group (DHCA versus primarily low-flow CPB) assignment (3%). Similarly, at the 8-year evaluation, performance of the cohort for many neurodevelopmental domains was worse than population norms, with no effect of treatment group assignment. However, there were treatment group–specific effects. Motor and speech skills were lower in patients assigned to DHCA, whereas the low-flow CPB group demonstrated worse scores for impulsivity and behavior. As at the 4-year evaluation, SES (23.7%) and ventricular septal defect status (3.2%) explained more of the variance in IQ than did treatment group (DHCA versus low-flow CPB) assignment (0.3%). Treatment assignment resulted in different neurologic morbidities rather than globally reducing their frequency or severity. The investigators also examined the relationship between duration of DHCA and later performance on neurodevelopmental testing. It was concluded that there was little influence of DHCA at shorter durations (<40 to 45 minutes) on long-term outcomes. The investigators suggested that “it may not be necessary to take special measures to avoid deep hypothermic circulatory arrest periods of shorter duration.”
In three subsequent randomized trials of blood gas management or hemodilution during CPB, these same investigators also evaluated the effect of use of DHCA on neurodevelopmental outcomes [13, 17]. The patient population for all three studies included infants with a variety of biventricular CHD undergoing reparative surgery. Neurodevelopmental outcomes were evaluated using the Bayley Scales of Infant Development. Depending on the specific study, DHCA was used in 42% to 52% of patients, a greater proportion than in our study. In all of the trials, use of DHCA was not associated with worse outcomes. Consistent with the current study, patient factors such as birth weight, preoperative mechanical ventilation, and SES were more important predictors of neurodevelopmental outcomes than was use of DHCA.
Other investigators have reported similar findings. In an observational study, Sharma and associates [18] evaluated neurodevelopmental outcomes at 2.5 to 5.5 years of age after cardiac surgery in infancy in a cohort of 100 patients with a variety of biventricular CHD. As in the current study, DHCA or continuous CPB was selectively used at the surgeon’s discretion. The patients in whom DHCA was used were younger and smaller, and had lower preoperative oxygen saturations than the continuous CPB patients. Consistent with previous reports, performance of the entire cohort was worse than population norms. However, there was no difference in performance between the DHCA and the continuous CPB patients.
The Western Canadian Complex Pediatric Therapies Project Follow-Up Group reported neurodevelopmental outcomes at 18 to 24 months of age in 82 infants with transposition of the great arteries after the arterial switch operation with selective use of DHCA. Anatomic complexity and CPB and DHCA times were not predictive of developmental outcome [19]. When the same cohort was evaluated at 5 years of age, use of DHCA was not predictive of worse outcome. These investigators also evaluated outcomes at 5 years in 61 children with complex CHD including both single-ventricle and biventricular anatomy. They found that genetic and patient-specific factors were more important predictors of neurodevelopmental outcome than operative support techniques [20]. Use of DHCA was not associated with worse outcomes.
Strengths of the current study include the prospective design and the large, heterogeneous patient population. The BCAS excluded some patients included in the current study such as those with CHD other than transposition of the great arteries, birthweight less than 2.5 kg, significant extracardiac anomalies, and additional procedures with CPB. Thus, our cohort is more diverse than the BCAS subjects in terms of sex, race, and cardiac defect. This increases the potential for generalization of our findings to the larger CHD population. In the BCAS, children were randomly assigned to an operative strategy of either predominately DHCA or continuous low-flow CPB. However, even those patients randomly assigned to predominantly continuous CPB did undergo a brief period of DHCA. Thus, the study does not compare use of DHCA with no DHCA. The current study compares children with any exposure to DHCA, regardless of duration, with those who were never exposed to DHCA. The primary limitation is that it is not a randomized trial.
In conclusion, in this heterogeneous cohort of patients with CHD without aortic arch obstruction, selective use of DHCA was not associated with adverse neurodevelopmental outcomes. Use of DHCA as a support technique during cardiac surgery in infancy has many advantages: ease of cannulation (especially in small infants), decreased exposure of blood to the foreign surfaces of the bypass circuit, decreased inflammatory response, and a bloodless, uncluttered surgical field. The current study suggests that it is not necessary to sacrifice these advantages merely to avoid use of DHCA. Given that our study adds to the growing body of literature showing no adverse influence of limited periods of DHCA, new support techniques, such as regional cerebral perfusion, must be carefully evaluated before widespread acceptance to confirm they are not inferior to conventional management strategies.
Acknowledgments
This research was supported by grants from the American Heart Association, the National Institutes of Health, and the Washington Life Sciences Discovery Fund Award to the Northwest Institute of Genetic Medicine.
Footnotes
Presented at the Forty-sixth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 25–27, 2010. Winner of the J. Maxwell Chamberlain Memorial Award for Congenital Heart Surgery.
References
- 1.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery [See comment] N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, duDuplessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial [See comment] J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–53. doi: 10.1016/j.jtcvs.2006.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–45. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 5.Fuller S, Nord AS, Gerdes M, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36:40–7. doi: 10.1016/j.ejcts.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Gaynor JW, Nord A, Wernovsky G, et al. Apolipoprotein E genotype modifies the risk of behavior problems in pre-school children following neonatal and infant cardiac surgery. Pediatrics. 2009;124:241–50. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–7. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–7. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Visconti KJ, Rimmer D, Gavreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–13. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger D, Rappaport L, Wypij D, Wernovsky G, New-burger J. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr. 1997;18:75–83. doi: 10.1097/00004703-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 11.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114:e572–6. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]
- 12.Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial [See comment] J Thorac Cardiovasc Surg. 2003;126:1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 13.Bellinger D, Wypij D, du Plessis A, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–83. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger D, Newburger JW, Wypij D, Kuban K, Du Plessis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungerleider RM, Gaynor JW. The Boston Circulatory Arrest Study: an analysis. J Thorac Cardiovasc Surg. 2004;127:1256–61. doi: 10.1016/j.jtcvs.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 17.Jonas R, Wypij D, Roth S, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–74. doi: 10.1016/j.jtcvs.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R, Choudhary SK, Mohan MR, et al. Neurological evaluation and intelligence testing in the child with operated congenital heart disease. Ann Thorac Surg. 2000;70:575–81. doi: 10.1016/s0003-4975(00)01397-7. [DOI] [PubMed] [Google Scholar]
- 19.Freed DH, Robertson CMT, Sauve RS, et al. Intermediate-term outcomes of the arterial switch operation for transposition of the great arteries in neonates: alive but well? J Thorac Cardiovasc Surg. 2006;132:845–52. doi: 10.1016/j.jtcvs.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Creighton DE, Robertson CMT, Sauve RS, et al. Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120:e478–86. doi: 10.1542/peds.2006-3250. [DOI] [PubMed] [Google Scholar]