Abstract
Vitamin D deficiency and asthma are common conditions that share risk factors such as African American ethnicity, inner-city residence, and obesity. This review provides a critical examination of current experimental and epidemiologic evidence of a causal association between vitamin D status and asthma or asthma morbidity, including potential protective mechanisms such as antiviral effects and enhanced steroid responsiveness. Because most published epidemiologic studies of vitamin D and asthma or asthma morbidity are observational, a recommendation for or against vitamin D supplementation as preventive or secondary treatment for asthma is not advisable and must await results of ongoing clinical trials. Should these trials confirm a beneficial effect of vitamin D, others will be needed to assess the role of vitamin D supplementation to prevent or treat asthma in different groups such as infants, children of school age, and ethnic minorities.
Keywords: vitamin D, asthma, asthma morbidity
Asthma is a major public health problem in the United States (1) and worldwide (2). For unclear reasons, the prevalence of asthma increased from a period likely preceding the 1960s to at least the 1990s. Although there is recent evidence of no or modest further increase in asthma rates in countries with high disease prevalence (1–3), the causes of the “asthma epidemic” are incompletely understood.
Vitamin D is an essential nutrient with significant immunomodulatory effects (4, 5). The observation that vitamin D deficiency and asthma share risk factors such as urban residence (6, 7), obesity (8, 9), and African American ethnicity (10, 11) has generated interest in exploring a link between these two conditions. In this review, we discuss recent findings from experimental and human studies of vitamin D and asthma, critically assess current evidence for potential protective mechanisms of vitamin D against asthma and asthma morbidity, and provide general recommendations for future studies in this field.
Vitamin D Metabolism and Physiology
Sun exposure is the main source of vitamin D in humans. Solar UVB radiation photolyzes 7-dehydro-cholesterol in the skin to previtamin D3, which is then converted to vitamin D3 (cholecalciferol) (12). Cholecalciferol from the skin and diet is hydroxylated in the liver to 25-hydroxyvitamin D3 (25[OH]D) and stored. Parathyroid hormone controls calcium-phosphate homeostasis by regulating hydroxylation of 25(OH)D to its biologically active form (1,25[OH]2D3) in the kidney.
Vitamin D signaling predominantly occurs through binding of 1,25(OH)2D3 to the vitamin D receptor (VDR), formation of a heterodimer with retinoid X receptor, and subsequent regulation of gene expression by binding of this heterodimer to genomic sequences known as vitamin D response elements (VDREs). Hydroxylation of 25(OH)D in extrarenal sites (13) and differential expression of genes relevant to immune response and cancer in response to vitamin level suggest pleiotropic effects of vitamin D in humans (14). Adequacy of vitamin D level is assessed by measuring serum or plasma level of 25(OH)D, which is the major circulating form and is correlated with secondary hyperparathyroidism and skeletal diseases such as rickets (12).
Epidemiology of Vitamin D Deficiency
Vitamin D skin metabolism is influenced by melanin content of the skin, age, factors affecting sun exposure (latitude, season, time outdoors, and clothing), body fat, and sunscreen use (15). Dietary intake (mostly from oily fish, fortified grains, and dairy products) and supplements are a secondary source of vitamin D. On the basis of skeletal effects, vitamin D inadequacy (deficiency) was recently defined as a serum 25(OH)D < 20 ng/ml by a panel from the Institute of Medicine of the National Academy of Sciences (16). Vitamin D insufficiency has been previously defined as a serum 25(OH)D of 20 to 29 ng/ml (17), but the Institute of Medicine panel found inconclusive evidence for this threshold. This newly proposed definition of vitamin D sufficiency (i.e., ≥20 ng/ml) has generated great controversy (18, 19) largely because of its significant impact on the epidemiologic and clinical assessment of vitamin D insufficiency. Thus, there is no consensus on optimal vitamin D levels for nonmusculoskeletal health.
Regardless of the threshold used, vitamin D deficiency or insufficiency has likely increased in the United States over the last decades due to changes in behavior (e.g., less time outdoors) (20) and diet. In a recent study of 9,757 United States subjects 1 to 21 years of age, approximately 9% and approximately 61% of participants had vitamin D deficiency (defined by the authors as serum 25[OH]D < 15 ng/ml) and insufficiency (defined by the authors as serum 25[OH]D = 15–29 ng/ml), respectively (21). Predictors of vitamin D deficiency included older age, female gender, African- or Mexican-American ethnicity, obesity, the use of electronic devices, and reduced dairy intake (21). Reduced vitamin D levels have been found in populations living near the Equator (e.g., in Saudi Arabia, Israel, India, and Costa Rica) and in the southeastern United States (15, 22), suggesting that lifestyle can have major effects on vitamin D status regardless of latitude. Mounting evidence exists for a role of vitamin D in nonskeletal diseases (e.g., infectious illnesses, cancer, and T helper (Th)1-related autoimmune diseases, such as type I diabetes) (15, 17).
Vitamin D and The Immune System
Vitamin D has significant yet incompletely understood effects on innate and adaptive immunity (4, 15). The immune-modulatory role of vitamin D is supported by the presence of VDRs and the hydroxylation of 25(OH)D in relevant cell types, including macrophages and dendritic cells (23–26).
In experimental studies, vitamin D has been shown to inhibit proliferation of CD4+ T cells (27) and to reduce the production of Th1 cytokines (24, 27–30) and IL-17 (31, 32). Studies of vitamin D and Th2 cytokines have yielded inconsistent results (perhaps due to differences in target cell types and the timing and dose of vitamin administration) (15, 33). For example, vitamin D has been shown to enhance (34) and inhibit (35) IL-4 synthesis by cultured naive T cells. In mouse models of allergic airway inflammation (AAI), vitamin D (36) or UVB radiation (37) has been shown to inhibit AAI and to reduce IL-4 levels in bronchoalveolar lavage fluid. Consistent with potentially complex effects of vitamin D on asthma, VDR knock-out mice have elevated serum levels of IL-13 and IgE but do not develop AAI, supporting a key role for VDR lung expression in airway inflammation (38).
One way that vitamin D may influence asthma pathogenesis is through modulation of T regulatory cells (Tregs) (39). Vitamin D (alone or with glucocorticoids) has been reported to promote differentiation of naive T cells into IL-10–secreting Tregs (40, 41). Vitamin D has also been shown to increase serum levels of the immune-modulatory cytokines TGF-β and IL-10 in humans (40, 42) and to enhance the benefits of allergen immunotherapy in murine AAI by IL-10– and TGF-β–dependent mechanisms (43). In human T cells, vitamin D down-regulates dendritic cell Ox40L, which is required for Th2 priming, and up-regulates TGF-β. This leads to increased TGF-β–positive Tregs and lower Th2 cytokine levels (44).
Vitamin D and Asthma
Results of experimental studies (see above) and genetic association studies of the VDR (45, 46) have motivated observational studies of vitamin D and asthma in humans. These studies (summarized in Table 1) have differed in study design, sample size, and assessment of vitamin D status, which may explain their seemingly conflicting findings.
TABLE 1.
OBSERVATIONAL STUDIES OF VITAMIN D AND ASTHMA
| Reference | Study Design | Main Findings | Study Limitations |
| Hypponen et al. (47) | Cross-sectional study of 7,648 Finnish adults at 31 yr of age | Vitamin D supplementation in the first year of life was associated with increased risk of asthma (OR, 1.33; 95% CI, 0.97–1.82) | 29.3% of subjects with data on vitamin D supplementation in infancy were lost to follow-upNo study visits between 4 and 31 yr of ageLack of serum vitamin D measures in infancy |
| Devereux et al. (48) | Case-control study of 160 adults in the United Kingdom | No significant association between serum vitamin D level and asthma | Small sample size, cross-sectional designLow vitamin D level common in all participantsInability to exclude vitamin D effects in early life |
| Freishtat et al. (49) | Case-control study of 106 African American subjects 6 to 20 yr of age | Vitamin D insufficiency or deficiency (<30 ng/ml) was associated with asthma (OR, 42; 95% CI, 4.4–399) | Small sample size/cross-sectional designInability to exclude selection bias (imbalanced numbers and characteristics for cases and controls) |
| Gale et al. (50) | Birth cohort study of 596 British mother-child pairs; 178 children assessed at 9 yr of age | Maternal serum vitamin D > 75 nmol/L during pregnancy was associated with 5.4-fold increased risk of childhood asthma (95% CI for OR, 1.1–26.7) at 9 yr of age | 70% of subjects lost to follow-upNo study visits between 9 mo and 9 yr of age |
| Devereux et al. (51) | Birth cohort study of 2,000 mother-child pairs; 1,212 children assessed at 5 yr of age | Compared with the lowest quintile, the highest quintile of maternal intake of vitamin D during pregnancy was associated with reduced risks of ever, current, and persistent (OR, 0.33; 95% CI, 0.11–0.98) wheeze | 39.4% of children not followed up to 5 yr of ageLack of serum vitamin D levels during pregnancy |
| Camargo et al. (52) | Birth cohort study of 2,128 children in Massachusetts, of whom 1,194 were assessed at 3 yr of age | Each 100-IU increment in vitamin D intake during pregnancy was associated with reduced risk of recurrent wheeze (OR, 0.81; 95% CI, 0.74–0.89) | 43.8% of children not followed up to 3 yr of ageShort duration of follow-up, uncertain diagnosis of asthmaLack of serum vitamin D levels during pregnancy |
| Miyake et al. (53) | Birth cohort study of 1,002 Japanese mother–child pairs; 763 children assessed at 16–24 mo of age | Maternal intake of vitamin D above the first quartile (≥172 IU/d) during pregnancy was associated with reduced risk of wheeze (OR, 0.64; 95% CI, 0.43–0.97) | 23.9% of children lost to follow-upNo serum vitamin D measures during pregnancyShort duration of follow-up, uncertain diagnosis of asthma |
| Erkkola et al. (54) | Birth cohort study of 3,565 children with HLA-DQB1–conferred susceptibility to type I diabetes; 1,669 children assessed at 5 yr of age | Compared with the bottom three quartiles, the highest quartile of total intake of vitamin D during pregnancy was associated with reduced risk of asthma (HR, 0.76; 95% CI, 0.59–0.99) | 53.2% of subjects not included in the analysis because of loss to follow-up or incomplete dataLack of serum vitamin D measures during pregnancyHighly selected cohort |
| Camargo et al. (55) | Birth cohort study of 1,105 children in New Zealand, of whom 823 (83.4%) were followed up to 5 yr of age | Cord blood levels of vitamin D were inversely associated with wheeze at all time points but not with incident asthma by 5 yr of age | 25.5% of children lost to follow-up at 5 yr of ageRelatively short duration of follow-up, uncertain diagnosis of asthma |
| Hollams et al. (56) | Birth cohort study of 2,834 mother–child pairs; 989 assessed at 6 yr of age and 1,380 children assessed at 14 yr of age (693 children seen at 6 and 14 yr of age) | No significant cross-sectional association between serum vitamin D and current asthma at 6 or 14 yr of age; vitamin D level at 6 yr of age was associated with asthma in boys at 14 yr of age | Analysis of vitamin D level at 6 yr of age and asthma at 14 yr of age was unadjustedSubstantial loss of follow-up |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; OR = odds ratio.
A cross-sectional study of Finnish adults found that vitamin D supplementation (assessed in infancy) was associated with increased risk of asthma (47). However, this study lacked vitamin D measures and had inadequate follow-up data on study participants (47). Case-control studies of serum vitamin D and asthma in British adults (48) and African American children and young adults (49) yielded conflicting findings (no association between vitamin D and asthma in the British study vs. a strong positive association between vitamin D insufficiency or deficiency and asthma in African Americans). Both studies were limited by lack of data on vitamin D status in early life and potential selection bias.
Birth cohort studies allow us to prospectively assess the relation between an exposure and an outcome of interest. Although a birth cohort study of British children reported a strong association between serum vitamin D levels in late pregnancy and asthma at 9 years of age, it had inadequate follow-up of participating children (50). Birth cohort studies in Boston, Scotland, Japan, and Finland (each including ≥750 mother–child pairs) have shown that maternal dietary intake of vitamin D (assessed by food frequency questionnaires) during pregnancy is inversely associated with wheeze and recurrent wheeze (51–53) or asthma (54) in early childhood. All studies were limited by relatively short duration (from 1.3 to 5 yr, making a diagnosis of asthma challenging), significant loss to follow-up, and lack of serum vitamin D measures during pregnancy or in infancy. An additional birth cohort study of children in New Zealand found that vitamin D level in cord blood was inversely associated with wheeze but not with incident asthma by 5 years of age (55). Although a birth cohort study of Australian children found an association between vitamin D level at 6 years of age and asthma in boys at 14 years of age, it lacked vitamin D measures in early life and had substantial loss to follow–up, and the analyses were unadjusted for potential confounders (56).
In summary, there is insufficient evidence of a causal association between vitamin D status and asthma per se. The inverse association between maternal intake of vitamin D during pregnancy or cord blood level of vitamin D and childhood wheeze, reported in the best available observational (birth cohort) studies (51–53, 55), merits further assessment in ongoing clinical trials.
Vitamin D, Asthma Morbidity, and Asthma Exacerbations
In addition to a potential role in the primary prevention of asthma, there is considerable interest in assessing whether vitamin D protects against or reduces asthma morbidity. Table 2 summarizes the main results of studies of vitamin D and asthma morbidity or asthma control.
TABLE 2.
STUDIES OF VITAMIN D AND ASTHMA MORBIDITY, ASTHMA CONTROL, OR STEROID RESPONSIVENESS
| Reference | Study Design | Main Findings | Study Limitations |
| Brehm et al. (22) | Cross-sectional study of 616 children with asthma in Costa Rica | Serum vitamin D level was inversely associated with indicators of asthma morbidity or severity, including hospitalizations, use of antiinflammatory medications (OR, 0.18; 95% CI, 0.05–0.67), and airway hyperresponsiveness | Inability to fully exclude “reverse causation” (e.g., reduced vitamin D levels due to reduced sun exposure in children with severe asthma) |
| Chinellato et al. (59) | Cross-sectional study of 75 Italian children with asthma | Vitamin D level was positively correlated with the Childhood Asthma Control Test (r = 0.28; P = 0.01) and was higher in children with controlled asthma than in those without (P = 0.02 for trend) | Inability to exclude reverse causationLack of adjustment for confoundersSmall sample size |
| Chinellato et al. (60) | Cross-sectional study of 45 Italian children with mild intermittent asthma | Serum vitamin D level was lower in children with exercise-induced bronchoconstriction than in those without | Small sample sizeLack of adjustment for confoundersInability to exclude reverse causation |
| Searing et al. (78) | Cross-sectional study of 100 children with asthma in Denver, Colorado | Serum vitamin D was positively correlated with lung function and enhanced glucocorticoid action in peripheral blood mononuclear cells; vitamin D was inversely correlated with total IgE, degree of atopy, and use of inhaled or oral steroids | Lack of adjustment for potential confoundersInability to exclude reverse causation |
| Sutherland et al. (79) | Cross-sectional study of 54 adults with persistent asthma in Denver, Colorado | Serum vitamin D was positively correlated with FEV1 and glucocorticoid response; vitamin D insufficiency or deficiency (< 30 ng/ml) was associated with airway hyperresponsiveness | Small sample sizeInability to exclude reverse causationLack of adjustment for potential confounders other than age, sex, and body mass index |
| Brehm et al. (57) | Longitudinal study of 1,024 North American children with mild to moderate persistent asthma | Vitamin D insufficiency or deficiency (25[OH]D ≤ 30 ng/ml) at baseline was associated with increased risk of severe asthma exacerbations (≥1 hospitalization or Emergency Department visit) over 4 yr of follow-up (OR, 1.5; 95% CI, 1.1–1.9) | Lack of repeated measures of serum vitamin D over timeNo information about vitamin D supplementation |
| Majak et al. (58) | 6-mo trial of vitamin D3 (500 IU/d) as adjuvant therapy to ICS to reduce asthma morbidity in 48 Polish children | Children who received vitamin D supplementation and ICS had a lower frequency of asthma exacerbations (4 or 16.7%) than those who received ICS and placebo (11 or 45.8%) | No difference in vitamin D level between treatment groups, likely due to the low dose usedAsthma exacerbation not defined according to current standards |
| No objective markers of viral infection |
Definition of abbreviations: CI = confidence interval; ICS = inhaled corticosteroid; OR = odds ratio.
Vitamin D insufficiency or deficiency (defined as a 25[OH]D level < 30 ng/ml) was present in 175 (28%) of 616 children with asthma in Costa Rica (22), in whom serum vitamin D level was inversely associated with total IgE, eosinophil count, hospitalizations for asthma, use of anti-inflammatory medications, and airway hyperresponsiveness (22). A temporal and causal relation between vitamin D and asthma morbidity cannot be established from that cross-sectional study. To follow up on those results, Brehm and colleagues conducted a longitudinal study of serum vitamin D and severe asthma exacerbations (defined as at least one hospitalization or visit to the Emergency Department) in 1,024 North American children with mild to moderate persistent asthma (57). In that study, vitamin D insufficiency or deficiency (a 25[OH]D level < 30 ng/ml) at baseline was associated with increased risk of severe asthma exacerbations during 4 years of follow-up. The magnitude of the observed association was greater in children who did not receive inhaled corticosteroids (ICS) and who had vitamin D insufficiency than in children who received ICS but had vitamin D insufficiency or in those who did not receive ICS but had sufficient levels of vitamin D. This finding and others (see below) suggest that vitamin D enhances steroid responsiveness.
Further evidence that vitamin D may protect against asthma exacerbations is provided by a recent 6-month clinical trial of vitamin D3 supplementation (500 IU/d) as adjuvant therapy to ICS to reduce asthma morbidity in 48 Polish children (58). In that study, there was no difference in serum vitamin D level between treatment groups, likely due to an insufficient dose of vitamin D. However, children in the intervention group were less likely to have a vitamin D level that decreased during the trial, and there were significantly fewer children with an asthma exacerbation (n = 4 or 16.7%) in the vitamin D group than in the placebo group (n = 11 or 45.8%). Findings from this small clinical trial must be interpreted with caution due to nonstandardized assessment of asthma exacerbations and lack of significant improvement in lung function or symptom score.
Two small cross-sectional studies have found that vitamin D level is positively correlated with asthma control (59) and inversely correlated with exercise-induced bronchoconstriction (60) in Italian children with asthma. However, reverse causation and confounding cannot be excluded as alternative explanations for these findings.
In brief, promising and consistent evidence from observational studies and a small clinical trial suggest that vitamin D protects against asthma exacerbations.
Ongoing Clinical Trials
Given the suggestive evidence of protective effects of vitamin D from observational studies, several ongoing clinical trials are testing whether vitamin D supplementation prevents asthma or reduces asthma morbidity. Table 3 summarizes the main characteristics of the ongoing trials that are attempting to enroll at least 250 subjects and that are registered in http://clinicaltrials.gov. Two of these trials are testing whether vitamin D supplementation during pregnancy prevents asthma by 3 years of age (NCT00920621 and NCT00856947) and will be completed in 2014; continued follow-up of participants will be needed to adequately assess asthma. Three additional trials (to be completed in 2013 and 2014) are testing whether vitamin D supplementation prevents moderate asthma exacerbations in adults when added to low-dose ICS in adults with asthma and persistent symptoms (NCT01248065), reduces the incidence of upper respiratory infections (URIs) and asthma exacerbations in children (1–5 yr of age) with and without asthma (NCT01419262), or delays the time to a first URI or a severe disease exacerbation in adolescents and adults with asthma (NCT00978315). In addition to treatment efficacy, ongoing trials should yield valuable additional information about dosing, monitoring toxicity, and the safety of vitamin D supplementation in different age groups.
TABLE 3.
ONGOING CLINICAL TRIALS OF VITAMIN D SUPPLEMENTATION TO PREVENT ASTHMA OR REDUCE ASTHMA MORBIDITY
| Title; ID no.* | Target Date for Completion | Type/ Design | Study population | Study Hypothesis/Primary Outcome(s) | Treatment Arms | Secondary Outcomes | Limitations |
| Maternal Vitamin D Supplementation to Prevent Childhood Asthma (VDAART); NCT00920621 | June, 2014 | Multicenter (US), randomized, double-blind, placebo-controlled | 870 pregnant women (18–39 yr of age) and their offspringParticipating women must report a history of asthma or allergies in themselves or the child's father | Adequate vitamin D supplementation in the pregnant mother is associated with reduced incidence of asthma in the child during the first 3 yr of life | Vitamin D3 (4,000 IU/d) plus prenatal multivitamins vs. placebo plus prenatal multivitamins | Eczema, allergic sensitization, and LRIsVitamin D status in mother and childPrematurity and other perinatal complications | High-risk cohortShort duration of follow-upNonassessment of postnatal vitamin D supplementation |
| Vitamin D Supplementation During Pregnancy for Prevention of Asthma in Childhood (ABCvitaminD); NCT00856947 | March, 2014 | Single site (Denmark), randomized, double-blind, placebo-controlled | 600 pregnant women older than 17 yr and their children | Vitamin D supplementation during pregnancy and 1 wk after delivery will prevent asthma symptoms (recurrent wheeze) in the first 3 yr of life | Vitamin D (2,400 IU/d) vs. placeboTreatment arms stratified by a second (fish oil) intervention | Eczema, allergy, and LRIs/URIsVitamin D status in mother and childGrowth | Length of follow-up not sufficient to assess an effect on asthma per seStatistical power may be inadequateNonassessment of postnatal vitamin D supplementation |
| Study of the Effect of Vitamin D as an Add-on Therapy to Corticosteroids in Asthma (VIDA); NCT01248065 | December, 2012 | Multicenter (US), randomized, double-blind, placebo-controlled | 400 subjects, 18 yr old with serum 25(OH)D < 30 ng/ml who have asthma with persistent symptoms despite low-dose ICS | Adding vitamin D supplementation to a controller medication (ICS) helps prevent worsening of asthma symptoms and asthma attacks (treatment failure/moderate exacerbation) over a 28-wk period | Vitamin D3 (100,000 IU loading dose followed by 4,000 IU/d) plus low-dose ICS (cyclesonide, 160 μg bid) vs. placebo plus low-dose ICS | Lung function changes from baseline | Non assessment of viral infections |
| DO IT Trial: Vitamin D Outcomes and Interventions In Toddlers; NCT01419262 | May, 2013 | Multicenter (Canada), randomized, double-blind, controlled | 400 children 1 to 5 yr of age who do and do not have asthma | Preschoolers receiving “high-dose” vitamin D supplementation during the wintertime will be less likely to have (laboratory-confirmed) URIs | Vitamin D3 (2,000 IU/d) vs. vitamin D3 (400 IU/d) | URIs by parental reportAsthma exacerbationsVitamin D status | No-assessment of atopyPotential misclassification of asthma |
| Trial of Vitamin D Supplementation in Asthma (ViDiAs); NCT00978315 | August, 2013 | Multicenter (UK), randomized, double-blind, placebo-controlled | 250 subjects >15 to <81 yr of age who have physician-diagnosed asthma | Vitamin D supplementation will influence time to URIs and time to severe asthma exacerbation in adult and adolescent patients over 1 yr of follow-up | Vitamin D3 (as 2-monthly oral doses of Vigantol oil) vs. 2-monthly oral doses of Miglyol oil (placebo) | Asthma Control Test scoreTime to healthcare use for URI or severe asthma exacerbation | Potential misclassification of asthma and URIs |
Definition of abbreviations: ICS = inhaled corticosteroids; URI = upper respiratory infections; LRI = lower respiratory infections.
Clinicaltrials.gov identifier.
How Could Vitamin D Protect Against Asthma Morbidity?
Antiviral Properties
Airway epithelial cells can hydroxylate 25(OH)D to its active form (1,25(OH)2D3) (61), leading to increased differentiation and recruitment of macrophages (28), enhanced production of cathelicidin and CD14, and potentiation of host defenses against Mycobacterium tuberculosis and other bacteria, fungi, and viruses (62–65).
Vitamin D deficiency and influenza epidemics follow similar seasonal patterns (66–68), and vitamin D level has been inversely associated with the risk of respiratory illnesses in observational studies of children and adults (69–73). A U.S. cross-sectional study of approximately 19,000 subjects 12 years of age or older showed that reduced serum vitamin D was associated with an increased risk of self-reported upper respiratory infections, particularly in subjects with chronic obstructive pulmonary disease or asthma (69). Two clinical trials of vitamin D to prevent (74) or reduce the severity of (71, 74) respiratory infections in adults showed no (74) or modest (71) effects. Limitations of those trials include short follow-up or low vitamin D dose and nonmicrobiologic assessment of respiratory illnesses (74, 71). A recent trial showed that vitamin D3 supplementation (1,200 IU/d) during winter reduced the incidence of influenza A (diagnosed by antigen testing in nasopharyngeal swabs) but not influenza B in 167 Japanese schoolchildren (relative risk [RR], 0.58; 95% confidence interval [CI], 0.34–0.99) (75). A subgroup (exploratory) analysis showed that vitamin D supplementation reduced the risk of disease exacerbations in children with asthma (RR, 0.17; 95% CI, 0.04–0.73). The antiviral properties of vitamin D are further supported by a recent observational study of 284 Finnish infants hospitalized with a wheezing illness, in whom vitamin D level was inversely associated with coinfection with respiratory syncytial virus or rhinovirus (OR, 0.92; 95% CI, 0.84–0.99) (76).
Enhanced Steroid Responsiveness
Xystrakis and colleagues showed that adding vitamin D to cell cultures increases glucocorticoid-induced secretion of IL-10 by Tregs, with similar effects ex vivo in patients with steroid-resistant asthma (77). Consistent with a role of vitamin D on enhancing steroid responsiveness, cross-sectional studies of children (78) and adults (79) (Table 2) have shown that a low vitamin D level is associated with impaired lung function (78, 79) and increased steroid use (78) or decreased in vitro steroid response (albeit by a seemingly IL-10–independent mechanism) (79).
Down-regulation of Atopy
Experimental findings suggest complex and incompletely understood effects of vitamin D on innate and adaptive immune responses (see above). A large cross-sectional United States study reported that serum vitamin D level was positively associated with an increased risk of physician-diagnosed allergic rhinitis (but not allergic sensitization) in non-Hispanic whites and African Americans younger than 20 years of age (80). A cross-sectional study of Finnish adults reported that vitamin D supplementation during infancy was associated with increased risks of allergic sensitization and allergic rhinitis at 31 years of age (47). That study had inadequate follow-up of participants. In contrast, a birth cohort study of Finnish children at risk for type I diabetes mellitus found an inverse association between maternal intake of vitamin D during pregnancy and allergic rhinitis (diagnosed by questionnaire: HR, 0.85; 95% CI, 0.75–0.97) (54) and sensitization to food allergens (81) at 5 years of age.
Similar to the conflicting findings reported for allergic sensitization or allergic rhinitis, cross-sectional studies of children and adults have shown a U-shaped relation between serum vitamin D and total IgE at 45 years of age (82), an inverse association between serum vitamin D and total IgE at school age (22), or no association at school age (57).
There is insufficient and weak evidence for an association between vitamin D status and atopy or atopic diseases other than asthma. Interpretation of the available studies of vitamin D and atopy is limited by the inability to exclude confounding or selection bias (due to differential loss of follow-up) as alternative explanations for the observed results and by a lack of adequate assessment of vitamin D status or allergic sensitization.
Other Potential Mechanisms
The relation between vitamin D status and obesity is complex and bidirectional. In obese individuals, serum vitamin D level is inversely correlated with total body fat, which is partly explained by increased storage of vitamin D in adipose tissue. On the other hand, vitamin D may influence lipofibroblast differentiation and adipogenesis in utero (83, 84), and factors correlated with reduced vitamin D levels in mothers (obesity) and their neonates (birth during winter) have been associated with increased birth weight and subsequent obesity in childhood (85, 86). A recent study of Colombian schoolchildren found that reduced vitamin D level at baseline was associated with an increased risk of developing greater adiposity over 2 years of follow-up (87). Given that being overweight or obesity has been associated with asthma and increased asthma severity in children and adults (8), it is reasonable but highly speculative to postulate that vitamin D supplementation reduces asthma morbidity through beneficial effects on weight control.
Vitamin D has been positively correlated with lung function measures, as shown in a cross-sectional study of 14,000 U.S. adults (88). In a murine model, the offspring of two groups of female BALB/c mice (one group was fed a vitamin D–sufficient diet, and the other was fed a vitamin D–insufficient diet) and vitamin D–sufficient male mice were compared regarding lung volume and function, as assessed by plethysmography and the forced oscillation technique (89). In that model, mice born to mothers with vitamin D deficiency were shown to have decreased lung function (primarily by reduced lung volume) without changes in somatic growth. Although there was suggestive evidence of altered lung structure, the nature of the observed structural difference was not conclusive. These data are consistent with an earlier study that showed decreased lung compliance in pups born to vitamin D–deficient rats (90). Indeed, polymorphisms in the VDR gene (VDR) have been linked to increased airway resistance in mice (91). Vitamin D has also been shown to stimulate alveolar type II cell DNA synthesis (92) and surfactant production (93, 94) and may regulate alveolarization (95, 96). Thus, vitamin D deficiency may predispose to asthma or increase asthma morbidity by altering lung development in early life.
Finally, vitamin D may influence asthma by regulating the expression of disease-susceptibility genes. Recent studies have demonstrated in vitro binding of VDR in approximately 2,500 to 3,500 genes in lymphoblastoid and preosteoblastic cell lines; a fraction (∼200–1,000) of these genes are differentially expressed after calcitriol stimulation, and some are in autoimmune pathways (14, 97). Expression of one of these genes (IL receptor B [IL17RB]) has been positively correlated with total IgE in children with asthma (98), and a second gene (tumor necrosis factor ligand superfamily, member 4 [TNFS4]) has been implicated in mediation of allergic responses in conjunction with thymic stromal lymphopoietin (TSLP) (99, 100).
Summary and Future Directions
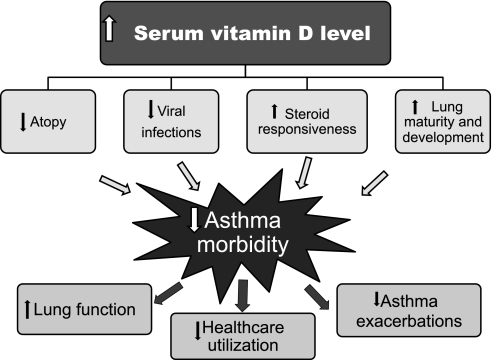
Findings from experimental and human studies suggest beneficial effects of vitamin D on asthma and asthma morbidity (Figure 1). Given the known limitations of observational studies, however, a recommendation for or against vitamin D supplementation as preventive or adjuvant therapy for asthma cannot be made until ongoing and future clinical trials are completed. Should these clinical trials yield positive results, others studies will be needed to assess the optimal delivery, dosing, and safety of vitamin D supplementation to prevent and treat asthma.
Figure 1.
Potential protective effects of vitamin D against asthma morbidity.
If a beneficial effect of vitamin D on asthma is confirmed, protective mechanisms should be explored. Current evidence most consistently favors a beneficial effect of vitamin D on asthma morbidity by prevention of viral infections and enhanced steroid responsiveness, which can, alone or together, explain the observed inverse associations between vitamin D status and severe asthma exacerbations in childhood. Although down-regulation of Th2 immune responses may ultimately explain a primary preventive effect of vitamin D against asthma, there is weak and inconsistent evidence for a link between vitamin D and atopic responses. For example, antiviral properties may explain the observed inverse association between maternal vitamin D intake or status during pregnancy and wheeze before 5 years of age. Much work needs to be done to confirm or refute the beneficial effects of vitamin D on asthma through promoting normal lung development or prevention of obesity.
A question often asked by clinicians is whether patients with asthma should be screened for vitamin D deficiency or insufficiency. There is no evidence to support such screening for the purpose of asthma management. However, it would be advisable to measure a serum vitamin D level in children and adults who belong to groups at high risk for vitamin D deficiency, namely African Americans, Mexican Americans, and individuals who are obese or have limited sun exposure (e.g., those who are institutionalized) (101). Vitamin D supplementation is only recommended for patients who have a serum vitamin D (25[OH]D) level less than 20 ng/ml because this could compromise their musculoskeletal health.
Ongoing clinical trials (Table 3) should yield valuable insights into the role of vitamin D supplementation in preventing the development of childhood asthma and reducing asthma morbidity. Questions to be addressed in future clinical trials include (1) whether vitamin D supplementation protects against viral illnesses or prevents childhood asthma when given in infancy (with or without supplementation during pregnancy), (2) whether vitamin D reduces severe asthma exacerbations or improves asthma control (as adjuvant to ICS) in children of school age, and (3) whether vitamin D is more effective in members of ethnic minority groups at risk for vitamin D deficiency.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201108-1502CI on October 20, 2011
Supported by grant HL079966 from the US National Institutes of Health and an endowment from the Heinz Foundation.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011;32:1–14 [PubMed] [Google Scholar]
- 2.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368:733–743 [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009;123:S131–S145 [DOI] [PubMed] [Google Scholar]
- 4.Ginde AA, Sutherland ER. Vitamin D in asthma: panacea or true promise? J Allergy Clin Immunol 2010;126:59–60 [DOI] [PubMed] [Google Scholar]
- 5.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 2001;167:1945–1953 [DOI] [PubMed] [Google Scholar]
- 6.Manicourt DH, Devogelaer JP. Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J Clin Endocrinol Metab 2008;93:3893–3899 [DOI] [PubMed] [Google Scholar]
- 7.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004;59:469–478 [DOI] [PubMed] [Google Scholar]
- 8.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedon JC, Shore SA. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010;7:325–335 [DOI] [PubMed] [Google Scholar]
- 9.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–693 [DOI] [PubMed] [Google Scholar]
- 10.Celedon JC, Sredl D, Weiss ST, Pisarski M, Wakefield D, Cloutier M. Ethnicity and skin test reactivity to aeroallergens among asthmatic children in Connecticut. Chest 2004;125:85–92 [DOI] [PubMed] [Google Scholar]
- 11.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila) 2005;44:683–692 [DOI] [PubMed] [Google Scholar]
- 12.Hollis BW. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Endocrinol Metab Clin North Am 2010;39:271–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008;4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol 2009;5:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 18.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011;26:455–457 [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. The IOM D-lemma. Public Health Nutr 2011;14:939–941 [DOI] [PubMed] [Google Scholar]
- 20.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 2009;124:e362–e370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983;221:1181–1183 [DOI] [PubMed] [Google Scholar]
- 24.Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by vitamin D receptor ligands. J Steroid Biochem Mol Biol 2004;89–90:437–441 [DOI] [PubMed] [Google Scholar]
- 25.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 26.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007;8:285–293 [DOI] [PubMed] [Google Scholar]
- 27.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 2003;89:922–932 [DOI] [PubMed] [Google Scholar]
- 28.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 2003;23:117–145 [DOI] [PubMed] [Google Scholar]
- 29.Iho S, Kura F, Sugiyama H, Takahashi T, Hoshino T. The role of monocytes in the suppression of PHA-induced proliferation and IL 2 production of human mononuclear cells by 1,25-dihydroxyvitamin D3. Immunol Lett 1985;11:331–336 [DOI] [PubMed] [Google Scholar]
- 30.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci USA 1987;84:3385–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 2008;324:23–33 [DOI] [PubMed] [Google Scholar]
- 32.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, Su SB, Chan CC, Adorini L, Caspi RR. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol 2009;182:4624–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol 2003;112:585–592 [DOI] [PubMed] [Google Scholar]
- 34.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1Alpha,25-dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 2001;167:4974–4980 [DOI] [PubMed] [Google Scholar]
- 35.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol 2002;168:1181–1189 [DOI] [PubMed] [Google Scholar]
- 36.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol 2004;34:1068–1076 [DOI] [PubMed] [Google Scholar]
- 37.McGlade JP, Gorman S, Zosky GR, Larcombe AN, Sly PD, Finlay-Jones JJ, Turner DJ, Hart PH. Suppression of the asthmatic phenotype by ultraviolet B-induced, antigen-specific regulatory cells. Clin Exp Allergy 2007;37:1267–1276 [DOI] [PubMed] [Google Scholar]
- 38.Wittke A, Chang A, Froicu M, Harandi OF, Weaver V, August A, Paulson RF, Cantorna MT. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch Biochem Biophys 2007;460:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 2005;5:271–283 [DOI] [PubMed] [Google Scholar]
- 40.Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, Corrigan CJ, Hickman E, Brown Z, Hawrylowicz CM. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest 2009;119:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 2002;195:603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 2003;134:128–132 [DOI] [PubMed] [Google Scholar]
- 43.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol 2008;180:5211–5221 [DOI] [PubMed] [Google Scholar]
- 44.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest 2010;120:3242–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med 2004;170:967–973 [DOI] [PubMed] [Google Scholar]
- 46.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med 2004;170:1057–1065 [DOI] [PubMed] [Google Scholar]
- 47.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci 2004;1037:84–95 [DOI] [PubMed] [Google Scholar]
- 48.Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin D status and asthma in adults. Allergy 2010;65:666–667 [DOI] [PubMed] [Google Scholar]
- 49.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr 2010;156:948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008;62:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 2007;85:853–859 [DOI] [PubMed] [Google Scholar]
- 52.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J 2010;35:1228–1234 [DOI] [PubMed] [Google Scholar]
- 54.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy 2009;39:875–882 [DOI] [PubMed] [Google Scholar]
- 55.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011;127:e180–e187 [DOI] [PubMed] [Google Scholar]
- 56.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, Zhang G, Sly PD, Holt PG. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J (In press) [DOI] [PubMed] [Google Scholar]
- 57.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126:52–58e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol 2011;127:1294–1296 [DOI] [PubMed] [Google Scholar]
- 59.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr 2011;158:437–441 [DOI] [PubMed] [Google Scholar]
- 60.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, Boner AL. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J 2011;37:1366–1370 [DOI] [PubMed] [Google Scholar]
- 61.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010;184:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther 2007;7:1449–1461 [DOI] [PubMed] [Google Scholar]
- 64.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res 2007;33:537–542 [DOI] [PubMed] [Google Scholar]
- 65.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007;179:2060–2063 [DOI] [PubMed] [Google Scholar]
- 66.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect 2006;134:1129–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol J 2008;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grant WB. Hypothesis–ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochem Photobiol 2008;84:356–365 [DOI] [PubMed] [Google Scholar]
- 69.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr 2009;63:473–477 [DOI] [PubMed] [Google Scholar]
- 71.Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamaki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis 2010;202:809–814 [DOI] [PubMed] [Google Scholar]
- 72.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE 2010;5:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 2004;58:563–567 [DOI] [PubMed] [Google Scholar]
- 74.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 2009;137:1396–1404 [DOI] [PubMed] [Google Scholar]
- 75.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010;91:1255–1260 [DOI] [PubMed] [Google Scholar]
- 76.Jartti T, Ruuskanen O, Mansbach JM, Vuorinen T, Camargo CA., Jr Low serum 25-hydroxyvitamin D levels are associated with increased risk of viral coinfections in wheezing children. J Allergy Clin Immunol 2010;126:1074–1076, 6 e1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 2006;116:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010;125:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med 2010;181:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy 2007;62:1085–1086 [DOI] [PubMed] [Google Scholar]
- 81.Nwaru BI, Ahonen S, Kaila M, Erkkola M, Haapala AM, Kronberg-Kippila C, Veijola R, Ilonen J, Simell O, Knip M, et al. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: a prospective cohort study. Pediatr Allergy Immunol 2010;21:29–37 [DOI] [PubMed] [Google Scholar]
- 82.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE: a significant but nonlinear relationship. Allergy 2009;64:613–620 [DOI] [PubMed] [Google Scholar]
- 83.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol 2009;297:L496–L505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasco JA, Wark JD, Carlin JB, Ponsonby AL, Vuillermin PJ, Morley R. Maternal vitamin D in pregnancy may influence not only offspring bone mass but other aspects of musculoskeletal health and adiposity. Med Hypotheses 2008;71:266–269 [DOI] [PubMed] [Google Scholar]
- 85.Bodnar LM, Catov JM, Roberts JM, Simhan HN. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J Nutr 2007;137:2437–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wattie N, Ardern CI, Baker J. Season of birth and prevalence of overweight and obesity in Canada. Early Hum Dev 2008;84:539–547 [DOI] [PubMed] [Google Scholar]
- 87.Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, Willett WC, Villamor E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr 2010;92:1446–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest 2005;128:3792–3798 [DOI] [PubMed] [Google Scholar]
- 89.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 2011;183:1336–1343 [DOI] [PubMed] [Google Scholar]
- 90.Gaultier C, Harf A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Lung mechanics in rachitic rats. Am Rev Respir Dis 1984;130:1108–1110 [DOI] [PubMed] [Google Scholar]
- 91.Berndt A, Savage HS, Stearns TM, Paigen B. Genetic analysis of lung function in inbred mice suggests vitamin D receptor as a candidate gene. Mol Genet Genomics 2011;286:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edelson JD, Chan S, Jassal D, Post M, Tanswell AK. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta 1994;1221:159–166 [DOI] [PubMed] [Google Scholar]
- 93.Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, Rao DS, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab 2002;76:46–56 [DOI] [PubMed] [Google Scholar]
- 94.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol 2005;289:L617–L626 [DOI] [PubMed] [Google Scholar]
- 95.Nadeau K, Montermini L, Mandeville I, Xu M, Weiss ST, Sweezey NB, Kaplan F. Modulation of Lgl1 by steroid, retinoic acid, and vitamin D models complex transcriptional regulation during alveolarization. Pediatr Res 2010;67:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol 1996;271:L392–L399 [DOI] [PubMed] [Google Scholar]
- 97.Pike JW, Meyer MB, Martowicz ML, Bishop KA, Lee SM, Nerenz RD, Goetsch PD. Emerging regulatory paradigms for control of gene expression by 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 2010;121:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hunninghake GM, Chu JH, Sharma SS, Cho MH, Himes BE, Rogers AJ, Murphy A, Carey VJ, Raby BA. The CD4+ T-cell transcriptome and serum IgE in asthma: IL17RB and the role of sex. BMC Pulm Med 2011;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X, Howard TD, Moore WC, Ampleford EJ, Li H, Busse WW, Calhoun WJ, Castro M, Chung KF, Erzurum SC, et al. Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol 2011;127:1457–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol 2007;120:238–244, quiz 45–46 [DOI] [PubMed] [Google Scholar]
- 101.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.