Abstract
Rationale: Academic success involves the ability to use cognitive skills in a school environment. Poor academic performance has been linked to disrupted sleep associated with sleep-disordered breathing (SDB). In parallel, poor sleep is associated with increased risk for obesity, and weight management problems have been linked to executive dysfunction, suggesting that interactions may be operational between SDB and obesity to adversely affect neurocognitive outcomes.
Objectives: To test whether mediator relationships exist between body weight, SDB, and cognition.
Methods: Structural equation modeling was conducted on data from 351 children in a community-based cohort assessed with the core subtests of the Differential Abilities Scales after an overnight polysomnogram. Body mass index, apnea–hypopnea index, and cognitive abilities were modeled as latent constructs.
Measurements and Main Results: In a sample of predominantly white children 6 to 10 years of age, SDB amplified the adverse cognitive and weight outcomes by 0.55- to 0.46-fold, respectively. Weight amplified the risk by 0.39- to 0.40-fold for SDB and cognitive outcomes, respectively. Poor ability to perform complex mental processing functions increased the risk of adverse weight and SDB outcomes by 2.9- and 7.9-fold, respectively.
Conclusions: Cognitive functioning in children is adversely affected by frequent health-related problems, such as obesity and SDB. Furthermore, poorer integrative mental processing may place a child at a bigger risk for adverse health outcomes.
Keywords: sleep-disordered breathing, weight, BMI, cognition, verbal abilities
At a Glance Commentary
Scientific Knowledge on the Subject
The independent associations between obesity, sleep-disordered breathing, and cognitive dysfunction have been proposed in children but thus far have not been explored in terms of their mediation. In this study, we report on the multidirectional relationships when studying weight, sleep-disordered breathing, and cognitive processing in 351 community children.
What This Study Adds to the Field
The main findings include that sleep-disordered breathing, weight, and cognition showed mediator roles in their dependency. The mediator role of weight and sleep-disordered breathing is comparable and points toward increasingly adverse outcomes. In contrast, good cognitive abilities might be protective to some extent. Thus, public health campaigns aiming to reduce the risk of obesity and associated morbidities should emphasize the health and educational benefits in children.
Weight problems in children are a rapidly expanding worldwide health concern. Most studies have primarily focused on the health consequences of childhood obesity (i.e., hypertension, diabetes, or future risk for cardiovascular disease in adulthood), with major efforts being directed toward targeted interventions, such as exercise, diet, and school programs (1, 2). However, there is a paucity of studies aiming to elucidate the contribution of body weight to cognitive functioning (3–7).
In parallel with the emergence of the obesity epidemic in children, the potential contribution of sleep duration and regularity to the propensity for obesity has increasingly gained attention (8–10) because short and variable sleep duration appear to be adversely associated with weight and cardiometabolic risks in children (11–13). In addition, the presence of overweight-obesity is conclusively associated with an increased prevalence of sleep-disordered breathing (SDB) (14–18). Interactions between body weight and SDB have recently emerged, whereby the concurrent presence of these two health problems adversely affects dietary preferences and may be particularly detrimental to daily physical activity patterns (19, 20). Furthermore, increased ghrelin levels support the presence of increased appetite and caloric intake in obese patients with SDB, promoting a vicious cycle leading to incremental severity of these two underlying conditions (19, 20). As such, the association between body weight and sleep disorders is becoming increasingly recognized.
Cumulative evidence strongly supports a causal relationship between SDB and neurobehavioral deficits, and a model on executive dysfunction or disruption of prefrontal cortical processes by SDB has been proposed and is widely accepted (21). Inattention with collinear behavioral problems and learning problems has been found in children with SDB (21–26). Notwithstanding such highly concordant findings, the pathogenesis of cognitive and behavioral deficits in patients with SDB remains to be fully elucidated. Furthermore, sleep plays a fundamental role in learning by affecting memory and brain plasticity (27, 28). Similarly, learning processes exert influences on sleep (29), and sleep deprivation and chronic sleep restriction impair performance, health, and well-being (30–33).
Therefore, the interrelations between cognitive performance, obesity, and SDB are not only pertinent but possible, particularly considering the emerging studies on the neuropsychological profile of obese children, which are suggestive of poorer mental flexibility, reduced attention endurance or prolonged reaction time, and poorer school performance and intelligence (5–7). Lower math and reading test scores in obese children have been reported, and overweight girls exhibited socio-behavioral problems and lower self-esteem (3, 4). Because some of the cognitive dysfunctions tend to disappear after treatment of obesity, inattention or executive dysfunctions could precede, contribute, or worsen the prognosis of the eating disorder leading to obesity or may be a consequence of obesity. As such, potential associations between body weight and cognitive performance in children are emerging.
We have previously found that SDB is frequently present in poor academic performers and that treatment of SDB reverses this problem, at least in part (34).
Taken together, the findings reported herein raise the hypothesis that complex relationships derived from permutations between sleep, body weight, and cognition are present. Accordingly, body weight may affect sleep and result in cognitive problems, while sleep may affect body weight and result in similar cognitive problems, or cognitive problems may increase body weight and result in SDB, etc. In other words, the dependency of any given outcome, namely sleep, body weight, and cognition, may be mediated. This study was designed to test the hypothesis that mediator relationships of weight, SDB, and cognition occur. Our major aim was to quantify and characterize the previously discussed relationships between an independent variable (e.g., sleep disorder) and a dependent variable (e.g., cognitive function) via the inclusion of a third variable (e.g., weight) as a mediator to better clarify such relationships.
Methods
Subjects
Data collection for this study was approved by the University of Louisville Human Research Committee and the boards of the participating schools (Jefferson County Public Schools and Archdiocese of Louisville Catholic Schools) from which the community sample was recruited. To screen for eligibility of the participants, parents filled out a sleep questionnaire (34, 35). On the basis of the completed questionnaires, nonsnoring children and snoring children were randomly selected and invited to participate in the study. Exclusionary criteria for participation in the study included chronic medical conditions, genetic or craniofacial syndromes, developmental delays, a current Individual Education Plan at school indicative of significant learning or other difficulties, current use of psychotropic medications, and the presence of an acute infection. Children who did not meet exclusionary criteria according to the questionnaire were invited to the sleep laboratory for overnight polysomnography followed by neurocognitive testing the next morning. Children were tested by trained psychometricians in a quiet room without a parent present. Psychometricians were blind to the child's sleep study and questionnaire results. Protocols were double scored by the psychometricians to ensure accuracy.
Measurements
General cognitive abilities.
Part of the neurocognitive assessment included the core subtests of the Differential Abilities Scales (DAS) (36). The DAS is a battery of cognitive tests designed to measure reasoning and conceptual ability in children, or general cognitive ability (GCA). The DAS provides individual subtest scores and the following composite scores that were analyzed in the current study: verbal and nonverbal reasoning, spatial conceptual ability, and GCA. The Verbal Ability cluster (VAB) reflects knowledge of verbal concepts and level of vocabulary development and is indicative of word retrieval from long-term memory. The core subtests administered included Word Definitions, which measures knowledge of word meanings as demonstrated through spoken language or the ability to formulate definitions of words (verbal fluency). Similarities measures verbal reasoning and knowledge, where inductive reasoning ability or the ability to relate three words to superordinate categories is necessary to earn credit. The Nonverbal Ability cluster (NAB) measures the child's inductive and sequential reasoning abilities. The core subtests are Matrices, measuring nonverbal reasoning, which involves perception and application of relationships among abstract figures. Sequential and Quantitative Reasoning involves detection of sequential patterns in figures or numbers. The Spatial Ability cluster (SAB) measures visuospatial construction ability, spatial memory, and spatial reasoning. The core subtests are Pattern Construction, which measures nonverbal reasoning and spatial visualization in reproducing designs with colored blocks incorporating response time in the individual scoring, and Recall of Designs, which involves the short-term recall of visual and spatial relationships through reproduction of abstract figures. The ability score for each subtest is converted to a T score, with a mean of 50 and a SD of 10. The sum of the core subtests is converted to yield a total standard score for the Ability cluster, with a mean of 100 and a SD of 15. Although the raw scores were used in the modeling procedures, we express standardized composite scores for descriptive purposes. The DAS was normalized on a large stratified sample of children across the United States and has good validity and reliability (36). The cluster scores are indicative of the “psychometric g,” which is the general ability to perform complex mental processing that involves conceptualization and the transformation of information (36) and which has been considered as a structural correlate to executive function.
Nighttime polysomnography.
A standard overnight, multichannel polysomnographic evaluation was performed at the Pediatric Sleep Medicine Center. Children were studied for up to 12 hours in a quiet, darkened room with an ambient temperature of 24°C with a parent or guardian present. No drugs were used to induce sleep. The following parameters were measured: chest and abdominal wall movements assessed by inductance plethysmography; heart rate assessed by electrocardiography; and airflow monitored by sidestream end-tidal capnography, which also provided breath-by-breath assessments of end-tidal carbon dioxide levels (BCI SC-300; Pryon Corp., Menomonee Falls, WI), nasal pressure, and an oronasal thermistor. SpO2 was assessed by pulse oximetry (Nellcor N 100; Nellcor Inc., Hayward, CA), with simultaneous recording of the pulse waveform. Bilateral electro-oculograms, eight channels of the electroencephalogram, chin and anterior tibial electromyograms, and analog output from a body-position sensor (Braebon Medical Corp., Ogdensburg, NY) were also monitored. All measures were digitized with a commercially available polysomnographic system (Stellate, Montreal, PQ, Canada). Tracheal sounds were monitored with a microphone sensor (Sleepmate, Midlothian, VA), and a digital, time-synchronized video recording was obtained.
Sleep architecture was scored by standard techniques (37). The proportion of time spent in each sleep stage was expressed as percentage of total sleep time (%TST). Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least two breaths (38). Hypopneas were defined as a decrease in oronasal flow of greater than 50% on the thermistor or the nasal pressure transducer signal with a corresponding decrease in SpO2 of greater than 3% or arousal (38–40). The obstructive apnea/hypopnea index (AHI) was defined as the number of apnea and hypopneas per hour of TST. Arousals were defined according to the American Academy of Sleep Medicine Scoring Manual (39, 40).
Anthropometric measurements
Height was measured (to 0.1 cm) with a stadiometer (Holtain, Crymych, UK), and children were weighed (to 0.1 kg) with a calibrated scale. Body mass index (BMI) was calculated, and the z score was generated using the online BMI z score calculator provided by the Centers for Disease Control and Prevention 200 growth standards and software (http://www.cdc.gov/epiinfo/) (Centers for Disease Control and Prevention, Atlanta, GA). BMI z scores of 1.65 or greater were considered to fulfill the criterion for obesity, BMI z scores of 1.04 or greater were defined as overweight, and BMI z scores of less than −1.28 were considered as underweight. A BMI z score falling between the latter boundaries was considered normal body weight.
Statistical Analysis
Descriptive and correlation analysis were conducted using the STATISTICA data analysis software system (version 10; StatSoft, Inc., Tulsa, OK). Post hoc analyses (unequal N honestly significant difference) were conducted for significant group differences. A zero-order correlation matrix was calculated to test the hypothesis that BMI, AHI, and cognition were significantly associated.
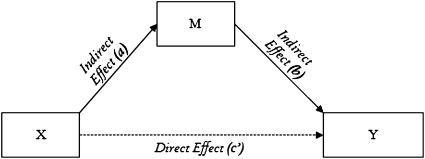
Structural equation modeling (SEM) was conducted using Amos 18 (Amos Development Corp., Meadville, PA). SEM is a very powerful multivariate analysis technique that includes specialized versions of a number of other analysis methods as special cases (e.g., causal modeling, confirmatory factor analysis, regression models, covariance structure models, and correlation structure models). SEM offers unique advantages in the assessment of complex, interrelated, dependent relationships while taking measurement errors into account. As a result, one of the strengths of SEM is that the technique models measured and latent (i.e., not measured directly but estimated from several measured variables) variables. Technically, we modeled a mediator variable (M) as being a third variable that links the independent variable (X) to the dependent variable (Y) and thus conveys the effects of X on Y. This recursive mediation model provides two paths feeding into a single dependent variable (Y); the independent (X) and the mediating (M) variable affect the dependent variable (Y), and the independent variable (X) affects the mediator (M) (Figure 1). All models were fitted through maximum likelihood estimation, bootstrapping (i.e., critical ratios for differences between parameters that fall within ±1.96 are not significant at P < 0.05) and 95% bias-corrected confidence intervals (i.e., being less likely to lead to Type I error, having high statistical power, and having no requirement of the assumption of normal distribution) (41, 42). Good model fit to the data was evaluated using the CMIN/DF (the relative chi-square) less than 1.5, goodness of fit index greater than 0.9, root mean square error of approximation less than 0.01 (excellent) or 0.05 (good), and Akaike information criterion (a comparative measure of fit) being the lower the better by convention. For more details, see the SEM handbooks (43).
Figure 1.
Scheme of a mediation model. Conventionally the path X to Y (without M) is the total effect, with (c) being the direct effect and (c′) being the indirect effect (ab). M = the mediating variable; X = the independent variable; Y = the dependent variable.
Results
Covariates
During the first stages of the study, the Spatial Abilities cluster subtests were not administered to all children. As a result, only the complete datasets of 351 subjects were used for analyses (55.5% boys and 44.5% girls, 62.6% non-Hispanic White ethnicity, 28.9% African American ethnicity, and 8.6% other ethnicity). This group did not differ (P > 0.05) from the group without the Spatial Abilities cluster on any of the measures of interest.
The sample had a mean age of 7.9 ± 0.8 years and a median obstructive AHI of 0.8/hrTST (quartile [Q]1: 0.3/hrTST and Q3: 1.7/hrTST; AHI ≤ 1/hrTST: 57.4% and AHI > 1/hrTST: 42.6%); median BMI was 17.5 (Q1: 15.6 and Q3: 20.8; underweight: 4.3%; normal weight: 45.7; overweight: 16.1%; obese: 33.9%). AHI and BMI were associated such that significantly more obese children had AHI > 1hr/TST (18.10% of the total sample) and more normal weight children had AHI ≤ 1/hrTST (29.02% of the total sample) [χ2(3)8.6; P = 0.034].
Children's GCA was 100.8 ± 13.5 (95% confidence interval [(CI], 99.4–102.3), with cluster scores of VAB: 99.4 ± 15.9 (95% CI, 97.8–101.1), NAB: 100.8 ± 13.4 (95% CI, 99.4–102.2), and SAB: 101.9 ± 12.6 (95% CI, 100.6–103.3). No sex differences were found in the cognitive abilities [F(4,346) = 1.5; P = 0.21]. African-American children exhibited lower abilities (GCA: 95.3 ± 11.5, VAB: 93.8 ± 13.8, NAB: 97.07 ± 12.3, SAB: 97.3 ± 10.6) when compared with the other children (non-Hispanic White: 103.4 ± 13.6, VAB: 102.3 ± 16.2, NAB: 102.3 ± 13.5, SAB: 104 ± 12.8, and other ethnicity: GCA: 100.6 ± 14.3, VAB: 97.1 ± 15.2, NAB: 102 ± 14.9, SAB: 102.6 ± 13.5 [F(8,690) = 4.6; P = 0.00002]).
Table 1 shows that AHI and weight were independently associated with cognitive problems.
TABLE 1.
ZERO-ORDER CORRELATION MATRIX OF THE VARIABLES OF INTEREST
| Age | AHI | BMI | GCA | VAB | NAB | SAB | |
| Age | 1 | ||||||
| AHI | 0.00 | 1 | |||||
| BMI | 0.22* | 0.22* | 1 | ||||
| GCA | 0.07 | −0.16* | −0.11* | 1 | |||
| VAB | 0.10 | −0.13* | −0.12* | 0.82* | 1 | ||
| NAB | 0.12* | −0.18* | −0.08 | 0.83* | 0.50* | 1 | |
| SAB | −0.07 | −0.07 | −0.07 | 0.77* | 0.43* | 0.54* | 1 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; GCA = general cognitive abilities; NAB = nonverbal abilities; SAB = spatial abilities; VAB = verbal abilities.
Significant results at P < 0.05.
Recursive Mediation Models
For clarification purposes, each of the models used the same set of variables (i.e., age, sex, ethnicity, and cognition) represented by the raw scores on the VAB, NAB, and SAB (denoted as COGN), body weight (denoted as BMI), and SDB (denoted as AHI). Based on the assumption of imprecision in the measurements, COGN, BMI, and AHI were entered in the model as latent constructs.
Model 1: the mediator “sleep-disordered breathing”.
Model 1A identified the mediator role of AHI in the dependence on BMI of COGN outcomes, or how cognition is influenced by weight, with a χ2(12) = 11.2 (P = 0.512) (Table 2). Other significant paths were the indicators of COGN (i.e., at P < 0.001) for VAB (β = 0.67), NAB (β = 0.79), and SAB (β = 0.52). Age was a significant indicator of BMI (β = 3.34; P = 0.006), AHI (β = 14.09; P = 0.008), and COGN (β = 14.96; P = 0.007). The error variances between age and SAB (r = −0.59; P < 0.001), sex and ethnicity (r = 0.15; P = 0.011), and ethnicity and NAB (r = 0.23; P = 0.008) were correlated. The standardized indirect effect between BMI and COGN was −0.11 ± 0.05 (P = 0.009), supportive of a mediation effect. The model fit was very good. This model is supportive of a substantive mediator role of SDB when having BMI problems toward poorer cognitive performance. Namely, one third of the total impact of BMI on COGN can be ascribed to AHI or, in other words, the indirect effect increases the risk 0.55-fold.
TABLE 2.
MEDIATION MODELS FOR WEIGHT, SLEEP-DISORDERED BREATHING, AND COGNITION
| a* | b |
c′ |
Critical Ratios |
Fit |
||||||||||
| Model | X | M | Y | β ± SE (95% CI) | P Value | β ± SE (95% CI) | P Value | β ± SE (95% CI) | P Value | a–b | a–c′ | b–′’ | CMIN/DF; GFI | RMSEA; AIC |
| 1A | BMI | AHI | COGN | 0.12 ± 0.05 (0.03 to –0.22) | 0.012 | −0.96 ± 0.02 (−0.99 to –0.93) | <0.001 | −0.20 ± 0.04 (−0.28 to −0.11) | <0.001 | −7.72 | −8.07 | 2.62 | 0.9; 0.99 | 0.00; 59.2 |
| 1B | COGN | AHI | BMI | −0.77 ± 0.08 (−0.88 to −0.60) | <0.001 | −0.88 ± 0.17 (−1.23 to −0.58) | <0.001 | −1.49 ± 0.16 (−1.85 to −1.220) | <0.001 | −18.07 | 21.10 | −0.35 | 1.5; 0.99 | 0.038; 67.6 |
| 2A | COGN | BMI | AHI | −0.73 ± 0.05 (−0.80 to −0.60) | <0.001 | −0.74 ± 0.15 (−1.09 to −0.56) | <0.001 | −1.39 ± 0.10 (−1.62 to −1.25) | <0.001 | −15.78 | −19.71 | 4.72 | 1.3; 0.99 | 0.032; 66.2 |
| 2B | AHI | BMI | COGN | 0.41 ± 0.16 (0.26 to 0.54) | 0.036 | −0.58 ± 0.07 (−0.65 to −0.53) | <0.001 | −0.60 ± 0.08 (−0.66 to −0.52) | <0.001 | 6.52 | 6.34 | 2.68 | 1.5; 0.99 | 0.038; 67.5 |
| 3A | AHI | COGN | BMI | −0.88 ± 0.11 (−0.98 to −0.57) | <0.001 | −1.31 ± 0.27 (−1.85 to −1.09) | <0.001 | −0.39 ± 0.26 (−0.89 to −0.18) | <0.001 | 3.41 | −3.44 | 1.78 | 1.5; 0.99 | 0.041; 67.5 |
| 3B | BMI | COGN | AHI | −0.74 ± 0.14 (−0.93 to −0.44) | <0.001 | −1.07 ± 0.07 (−1.19 to −0.88) | <0.001 | −0.10 ± 0.13 (−0.27 to 0.18) | 0.055 | −3.45 | −3.69 | 4.18 | 1.6; 0.98 | 0.042; 68.2 |
Definition of abbreviations: AHI = apnea–hypopnea index (or sleep disordered breathing); AIC = Akaike information criterion; BMI = body mass index (or weight); CMIN/DF = minimum value of the discrepancy function divided by degrees of freedom; COGN = cognition predicted by raw scores of the Verbal Abilities cluster; GFI = goodness of fit index; M = the mediating variable; RMSEA = root mean square error of approximation; X = the independent variable; Y = the dependent variable.
a, b, and c are standardized regression weights with SE.
Model 1B identified the mediating role of AHI in the dependence on COGN of BMI outcomes with a χ2(9) = 13.6 (P = 0.139) (Table 2). COGN was significantly predicted by VAB (β = 0.38; P < 0.001) and NAB (β = 0.19; P < 0.001). Age was a significant indicator of BMI (β = 5.62; P = 0.001), AHI (β = 4.87; P < 0.001), and COGN (β = 8.25; P = 0.001). Each of the clusters’ error variances was moderately correlated (i.e., VAB-NAB: r = 0.48; P < 0.001, NAB-SAB: r = 0.42; P < 0.001, and VAB-SAB: r = 0.32; P < 0.001). The error variances between COGN and VAB were weakly inversely related (r = −0.19; P = 0.001). The error variance of VAB was negatively related with ethnicity (r = −0.14, P = 0.003) and with COGN (r = −0.19; P = 0.001). Sex and ethnicity error variances were weakly correlated (r = 0.13; P = 0.01). SAB error variance was negatively correlated with ethnicity (r = −0.11; P = 0.03) and age (r = −0.41; P = 0.002). The standardized indirect effect between COGN and BMI was 0.68 ± 0.19 (P < 0.0001) and underscored mediation. The model fit was good and indicated that the presence of AHI is detrimental to the impact of COGN on BMI (i.e., increases the risk about 0.46–fold).
Model 2: the mediator “weight”.
Model 2A, where BMI mediates the COGN to AHI dependency, has a χ2(9) = 12.2 (P = 0.200) (Table 2). COGN was significantly predicted by VAB (β = 0.38; P < 0.001) and NAB (β = 0.21; P < 0.001). Age was a significant indicator of BMI (β = 4.69; P = 0.001), AHI (β = 5.71; P < 0.001), and COGN (β = 8.21; P < 0.001). Each of the clusters’ error variances was moderately correlated (i.e., VAB-NAB: r = 0.48; P < 0.001, NAB-SAB: r = 0.42; P < 0.001, and VAB-SAB: r = 0.33; P < 0.001). The error variances of VAB with COGN (r = −0.20; P < 0.001) and with ethnicity (r = −0.14; P = 0.003) were weakly inversely related. SAB error variance was negatively correlated with ethnicity (r = −0.11; P = 0.03) and age (r = −0.41; P = 0.002). Sex and ethnicity error variances were weakly correlated (r = 0.13; P = 0.02). The standardized indirect effect between COGN and AHI was 0.54 ± 0.14 (P = 0.001); as a result, the mediation effect increases the adverse outcome 0.39-fold. The model fit was good. This model shows that BMI plays an increased adverse role.
Model 2B, where BMI mediates the AHI to COGN dependency, had a χ2(9) = 13.5 (P = 0.143) (Table 2). COGN was significantly predicted by VAB (β = 0.30; P < 0.001) and NAB (β = 0.28; P < 0.001). Age was a significant indicator of BMI (β = 4.18; P = 0.001), AHI (β = 3.79; P = 0.001), and COGN (β = 6.61; P = 0.001). Each of the clusters’ error variances was moderately correlated (i.e., VAB-NAB: r = 0.48; P < 0.001, NAB-SAB: r = 0.42; P < 0.001, and VAB-SAB: r = 0.33; P < 0.001). The error variances of SAB with COGN (r = −0.17; P = 0.001) and with ethnicity (r = −0.11; P = 0.020) were weakly inversely related. Sex and ethnicity error variances were weakly correlated (r = 0.14; P = 0.013). VAB error variance was weakly correlated with ethnicity (r = −0.13; P = 0.007). The standardized indirect effect between AHI and COGN was −0.24 ± 0·09 (P = 0.001). The model fit was good. This model shows that BMI mediates the impact of SDB on adverse cognitive performance (i.e., a 0.40-fold increased risk).
Model 3: the mediator “cognition”.
Model 3A, where COGN mediates the AHI to BMI dependency, had a χ2(11) = 17.5 (P = 0.095) (Table 2). COGN was significantly predicted by VAB (β = 0.30; P < 0.001) and NAB (β = 0.13; P = 0.009). Ethnicity was predictive of AHI (β = 2.44; P = 0.005) and COGN (β = 3.93; P = 0.014). Age was a significant indicator of BMI (β = 5.59; P = 0.001), AHI (β = 1.38; P = 0.001), and COGN (β = 6.61; P = 0.001). Each of the clusters’ error variances was moderately correlated (i.e., VAB-NAB: r = 0.48; P < 0.001, NAB-SAB: r = 0.42; P < 0.001, and VAB-SAB: r = 0.32; P < 0.001). The error variances between COGN and VAB were weakly inversely related (r = −0.19; P = 0.003). SAB error variance was negatively correlated with BMI (r = −0.19; P < 0.001). AHI and NAB error variances were inversely weakly correlated (r = −0.11; P = 0.01). Mediation by the standardized indirect effect between AHI and BMI (1.15 ± 0.35; P < 0.001) is possible. The model fit was adequate. Therefore, cognition will likely adversely mediate the BMI outcome in children with SDB. Namely, it mediates a 2.9-fold increased risk on problematic weight outcome.
Model 3B, where COGN mediates the BMI to AHI dependency, had a χ2(10) = 16.22 (P = 0.093) (Table 2). COGN was significantly predicted by VAB (β = 0.33; P < 0.001) and NAB (β = 0.18; P < 0.001). Age was a significant indicator of BMI (β = 1.87; P = 0.007), AHI (β = 5.76; P < 0.001), and COGN (β = 7.09; P < 0.001). Each of the clusters’ error variances was moderately correlated (i.e., VAB-NAB: r = 0.48; P < 0.001, NAB-SAB: r = 0.43; P < 0.001 and VAB-SAB: r = 0.31; P < 0.001). VAB error variance was inversely related to COGN (r = −0.28; P < 0.001) and to age (r = 0.63; P = 0.006). SAB error variance was negatively correlated with age (r = −0.41; P = 0.002). The standardized indirect effect between BMI and AHI was 0.79 ± 0.16 (P = 0.001). The model fit was adequate. Cognition completely mediates the detrimental impact of BMI on SDB with a 7.9-fold increased risk. The standardized regression weights can be compared among each other, and the best fitting model can be compared on the Akaike information criterion in combination with evaluation of the other fit indices.
Discussion
SDB, weight, and cognition showed mediator roles in their dependency. The mediator role of weight and SDB is comparable and points toward increasingly adverse outcomes. In contrast, good cognitive abilities might be protective to some extent.
Several limitations to this study need to be addressed. Children with individual learning programs and those with mental or medical problems were excluded, such that generalization of our findings is restricted to otherwise normally developing children. Therefore, poor cognitive performance reflects a normal to borderline range, which may dampen some of the mediation relationships studied herein. Similarly, based upon the AHI cut-off, only 35 children had an AHI > 5/hrTST. In other words, more severe SDB cases may alter the magnitude of the mediation effects reported here. All children were assessed the morning after their sleep study or after 7.9 ± 0.7 hours of sleep. This is important because sleep deprivation in the nights preceding cognitive testing may adversely affect performance. In addition, even though a full discussion on the operationalization of the applied cognitive test is not within the scope of this study, the cognitive battery of tests effectively assesses conceptual and reasoning abilities, thereby reflecting a variety of separate and distinct areas of cognitive functioning. To simplify the modeling procedures and to verify our hypothesis, we used the raw cluster scores, not the individual test scores. Finally, some relaxation in the a priori assumptions was needed as part of the modeling procedures.
A child's progress in school is closely related to the child's cognitive strengths and weaknesses. As a result, measures of general cognitive abilities are often the reference point toward academic achievement and success in life. Because sleep is correlated to daytime performance such as learning, its role has thus far been vastly underestimated. In the current study, we focused on SDB and increased BMI as two highly prevalent and potentially interdependent health problems (44). Likewise, compromised health may affect learning abilities in children. In fact, cognitive processing might precede, contribute to, or worsen health conditions, and vice versa. However, our findings were restricted to general cognitive abilities and integrative processing, and therefore future studies on more specific neuropsychological functioning may shed light on the impact of compromised health in specific cognitive domains. Significant neurocognitive and neurobehavioral deficits have been associated with SDB throughout the recent decades, with inattention and executive dysfunction being repeatedly reported (21, 25, 26, 45). In general, the current obesity epidemic could further amplify the association between cognition and SDB in children. The role of obesity has not been specifically addressed despite being a common and highly prevalent condition in the pediatric population, with a median incidence of obesity in the 10 to 12% range (40).
Our findings concur with emerging reports of diminished cognitive performance with increasing BMI (6, 7, 46). In fact, there is a 0.55-fold increased risk of poorer abilities that is mediated by SDB (model 1A). Thus, the presence of SDB in overweight and obese children increases their risk for decreased cognitive performance. Therefore, these findings would justify appropriate screening for this adverse outcome. Conversely, the structural and functional differences in the brain of children with SDB have yet to be examined. However, in children with congenital central hypoventilation syndrome, a genetic disorder characterized by SDB, evidence of localized injury in hippocampal areas has emerged (47). In adults, preliminary neuroimaging-derived neural correlates in patients with SDB suggest that atrophy of the hippocampus and white matter lesions in the frontal lobes are present, along with widespread neural differences in the motor, sensory, and autonomic brain regions (48). Thus, imaging studies in obese and nonobese children with and without SDB are needed to gauge the impact of these conditions on the CNS, particularly considering our findings, whereby a 0.46-fold increased impact of cognitive processing on weight was mediated by SDB (model 1B). This finding would therefore promote screening for SDB in poorly performing children, and evidence to this effect was reported among academically failing young children (34). Thus, based on our findings, implementation of screening for SDB in children who are overweight and who have scholastic difficulties is advocated in the clinical setting.
Weight plays a comparable mediator role. Alterations in brain morphology, especially in the frontal lobe areas, have been reported in clinically overweight to obese young adults (49). Reduced focal gray matter volume and enlarged orbitofrontal white matter found in these adults with elevated BMI would suggest that potential structural changes in a developing brain may not only be present but may be further accentuated. In adults, diffusion tension imaging indicated that increasing BMI was independently associated with lower fractional anisotropy in the genu, splenium, and fornix, and a BMI–age interaction emerged in the splenium and body of the corpus callosum (50). These brain regions are critically important pathways for integrative processing and undergo myelination through childhood and adolescence. The observation in our models that chronological age was significantly predictive toward each of the three variables (i.e., AHI, BMI, and cognition) may underscore the importance of development as a particularly vulnerable stage. Further, specific craniofacial features that appear to influence brain development (51) may also predispose to SDB. In other words, neurological aspects of intrinsic brain functioning may contribute to the pathophysiology of SDB (52), in which BMI likely increases risk. In fact, the intriguing part regarding model 1B as described here is that, assuming neurological problems precede SDB, the impact of SDB on weight is not significantly different from the neurological (or cognitive) impact on weight. However, BMI and neurological function have independent and significant impacts on SDB (models 1B and 2A/3A especially). It would be speculation to contemplate specific pathophysiological mechanisms based on the present study. However, we can point out that SDB affects cognitive outcomes (as shown by other studies) and that the presence of BMI problems adversely affects such relationships, particularly during a period where our brain is rapidly developing. Our models are indeed suggestive for intricate interdependencies in the mediation between BMI, SDB, and cognition.
During childhood, developmental stage can be expressed as chronological age, developmental age, and physical maturation. These stages do not necessarily develop in synchrony and are considered to reflect the interplay of nature and nurture. Chronological age played a substantial role toward each of the measures of interest in all models, as well as an intriguing residual variance association of spatial abilities and age, suggesting that the nature of the tests used (53), or lower socioeconomic status (54), unfamiliarity (55), and other factors may account for this finding. Namely, spatial processing skills are an important component in learning and thus in cognitive development. Cognition was comparably predicted by verbal and nonverbal abilities except when cognitive outcome depended on weight when mediated by SDB. In the latter, a more widespread dysfunctional pattern was found. Considering that a 0.48-point decrease in grade point average was found for children in grades 7 through 9 when becoming overweight (not found for children in grades 3–6) and a 2-fold likelihood of low grades in overweight children (7), the impact of the developmental stage at which SDB and weight and cognition problems occur warrants further exploration. In future studies, similar modeling incorporating systemic inflammatory markers may improve the performance of the models presented herein (56).
In summary, multidirectional relationships were found when studying SDB, BMI, and cognitive processing in community children, with either of these elements serving as a precursor, mediator, or outcome. Because only rough indicators of these conditions were modeled (i.e., AHI, BMI, and COGN), we opted for unobserved latent constructs allowing residual variances, and accordingly less stringent interpretations are possible. Notwithstanding, careful consideration of the mediation findings reported should serve as indicators of the unique importance of sleep integrity and normal somatic function to foster brain plasticity.
Supplementary Material
Acknowledgments
The authors the postdoctoral fellows and staff involved in the several phases of this project and the children and their families for their participation.
Footnotes
Supported by National Institutes of Health grant HL65270 and a Comer Children's Hospital Research Award.
Author Contributions: Concept, design, analysis, and interpretation, K.S.; editing and review, D.G.
Originally Published in Press as DOI: 10.1164/rccm.201104-0721OC on November 3, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Flynn MA, McNeil DA, Maloff B, Mutasingwa D, Wu M, Ford C, Tough SC. Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with 'best practice' recommendations. Obes Rev 2006;7:7–66 [DOI] [PubMed] [Google Scholar]
- 2.Cromley T, Neumark-Sztainer D, Story M, Boutelle KN. Parent and family associations with weight-related behaviors and cognitions among overweight adolescents. J Adolesc Health 2010;47:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judge S, Jahns L. Association of overweight with academic performance and social and behavioral problems: an update from the early childhood longitudinal study. J Sch Health 2007;77:672–678 [DOI] [PubMed] [Google Scholar]
- 4.Datar A, Sturm R, Magnabosco JL. Childhood overweight and academic performance: national study of kindergartners and first-graders. Obes Res 2004;12:58–68 [DOI] [PubMed] [Google Scholar]
- 5.Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite 2007;49:675–678 [DOI] [PubMed] [Google Scholar]
- 6.Li X. A study of intelligence and personality in children with simple obesity. Int J Obes Relat Metab Disord 1995;19:355–357 [PubMed] [Google Scholar]
- 7.Mo-suwan L, Lebel L, Puetpaiboon A, Junjana C. School performance and weight status of children and young adolescents in a transitional society in thailand. Int J Obes Relat Metab Disord 1999;23:272–277 [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XBM, Wang Y. Is sleep duration associated with childhood obesity? a systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274 [DOI] [PubMed] [Google Scholar]
- 10.Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol 2008;77:266–276 [DOI] [PubMed] [Google Scholar]
- 11.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 2011;127:e345–e352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaput JP, Klingenberg L, Sjodin A. Do all sedentary activities lead to weight gain: sleep does not. Curr Opin Clin Nutr Metab Care 2010;13:601–607 [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spruyt K, Gozal D. Mr. Pickwick and his child went on a field trip and returned almost empty handed…what we do not know and imperatively need to learn about obesity and breathing during sleep in children! Sleep Med Rev 2008;12:335–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin 2007;2:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunetti L, Tesse R, Miniello VL, Colella I, Delvecchio M, Logrillo VP, Francavilla R, Armenio L. Sleep-disordered breathing in obese children: the Southern Italy experience. Chest 2010;137:1085–1090 [DOI] [PubMed] [Google Scholar]
- 17.Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol 2010;108:436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab 2010;95:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spruyt K, Sans Capdevila O, Serpero LD, Kheirandish-Gozal L, Gozal D. Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr 2010;156:724–730, 730.e721–730.e723 [DOI] [PubMed] [Google Scholar]
- 20.Nakra N, Bhargava S, Dzuira J, Caprio S, Bazzy-Asaad A. Sleep-disordered breathing in children with metabolic syndrome: the role of leptin and sympathetic nervous system activity and the effect of continuous positive airway pressure. Pediatrics 2008;122:e634–e642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 2002;11:1–16 [DOI] [PubMed] [Google Scholar]
- 22.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep 2010;33:1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery-Downs HE, Gozal D. Sleep-associated respiratory disorders and their psychobehavioral consequences in children. : Montagna P, Chokroverty S, Handbook of clinical neurology, Vol. 98. Sleep disorders. Amsterdam, The Netherlands: Elsevier B.V.; 2011. pp. 489–499 [DOI] [PubMed] [Google Scholar]
- 24.Hodges EK, Bloomfield E, Coulas T, Giordani B. Cognitive and behavioral change after adenotonsillectomy in children with sleep-disordered breathing: a review. Minerva Psichiatr 2008;49:307–320 [Google Scholar]
- 25.Key APF, Molfese DL, O'Brien L, Gozal D. Sleep-disordered breathing affects auditory processing in 5–7-year-old children: evidence from brain recordings. Dev Neuropsychol 2009;34:615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol 2009;44:414–422 [DOI] [PubMed] [Google Scholar]
- 27.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med Rev 2006;10:307–321 [DOI] [PubMed] [Google Scholar]
- 28.Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med 2009;5:S20–S26 [PMC free article] [PubMed] [Google Scholar]
- 29.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature 2004;430:78–81 [DOI] [PubMed] [Google Scholar]
- 30.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10–14. Sleep 1998;21:861–868 [PubMed] [Google Scholar]
- 31.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev 2003;74:444–455 [DOI] [PubMed] [Google Scholar]
- 32.Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep 1981;4:299–312 [DOI] [PubMed] [Google Scholar]
- 33.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol 2006;57:139–166 [DOI] [PubMed] [Google Scholar]
- 34.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616–620 [DOI] [PubMed] [Google Scholar]
- 35.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87–94 [DOI] [PubMed] [Google Scholar]
- 36.Elliott CD. Differential abilities scale: handbook. San Antonio, TX: The Psychological Corporation; 1990 [Google Scholar]
- 37.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. Bethesda, MD: National Institutes of Health; 1968 [Google Scholar]
- 38.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–753 [DOI] [PubMed] [Google Scholar]
- 39.American Sleep Disorders Association Task Force Eeg arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184 [PubMed] [Google Scholar]
- 40.American Thoracic Society Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878 [DOI] [PubMed] [Google Scholar]
- 41.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 42.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 2002;7:83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 1998 [Google Scholar]
- 44.Kheirandish-Gozal L. Fat and lymphadenoid tissues: a mutually obstructive combination. Am J Respir Crit Care Med 2011;183:694–695 [DOI] [PubMed] [Google Scholar]
- 45.Kohler MJ, Lushington K, van den Heuvel CJ, Martin J, Pamula Y, Kennedy D. Adenotonsillectomy and neurocognitive deficits in children with sleep disordered breathing. PLoS ONE 2009;4:e7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krukowski RA, Smith West D, Philyaw Perez A, Bursac Z, Phillips MM, Raczynski JM. Overweight children, weight-based teasing and academic performance. Int J Pediatr Obes 2009;4:274–280 [DOI] [PubMed] [Google Scholar]
- 47.Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, Thompson PM, Harper RM. Hippocampal volume reduction in congenital central hypoventilation syndrome. PLoS ONE 2009;4:e6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmemnan ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med 2006;2:461–471 [PubMed] [Google Scholar]
- 49.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 2006;31:1419–1425 [DOI] [PubMed] [Google Scholar]
- 50.Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 2011;19:500–504 [DOI] [PubMed] [Google Scholar]
- 51.Broderick M, Guilleminault C. Neurological aspects of obstructive sleep apnea. Ann N Y Acad Sci 2008;1142:44–57 [DOI] [PubMed] [Google Scholar]
- 52.Gozal D. The brain in sleep-disordered breathing: is it the chicken or is it the egg? Am J Respir Crit Care Med 2002;166:1305–1306 [DOI] [PubMed] [Google Scholar]
- 53.Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage 2010;51:817–827 [DOI] [PubMed] [Google Scholar]
- 54.Jensen AR, Reynolds CR. Race, social class and ability patterns on the wisc-r. Pers Individ Dif 1982;3:423–438 [Google Scholar]
- 55.Bullens J, Szekely E, Vedder A, Postma A. The effect of experience on children's ability to show response and place learning. Br J Dev Psychol 2010;28:909–920 [DOI] [PubMed] [Google Scholar]
- 56.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med 2007;176:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.