Abstract
Rationale: Hypoglossal nerve stimulation (HGNS) recruits lingual muscles, reduces pharyngeal collapsibility, and treats sleep apnea.
Objectives: We hypothesized that graded increases in HGNS relieve pharyngeal obstruction progressively during sleep.
Methods: Responses were examined in 30 patients with sleep apnea who were implanted with an HGNS system. Current (milliampere) was increased stepwise during non-REM sleep. Frequency and pulse width were fixed. At each current level, stimulation was applied on alternating breaths, and responses in maximal inspiratory airflow (VImax) and inspiratory airflow limitation (IFL) were assessed. Pharyngeal responses to HGNS were characterized by the current levels at which VImax first increased and peaked (flow capture and peak flow thresholds), and by the VImax increase from flow capture to peak (ΔVImax).
Measurements and Main Results: HGNS produced linear increases in VImax from unstimulated levels at flow capture to peak flow thresholds (215 ± 21 to 509 ± 37 ml/s; mean ± SE; P < 0.001) with increasing current from 1.05 ± 0.09 to 1.46 ± 0.11 mA. VImax increased in all patients and IFL was abolished in 57% of patients (non-IFL subgroup). In the non-IFL compared with IFL subgroup, the flow response slope was greater (1241 ± 199 vs. 674 ± 166 ml/s/mA; P < 0.05) and the stimulation amplitude at peak flow was lower (1.23 ± 0.10 vs. 1.80 ± 0.20 mA; P < 0.05) without differences in peak flow.
Conclusions: HGNS produced marked dose-related increases in airflow without arousing patients from sleep. Increases in airflow were of sufficient magnitude to eliminate IFL in most patients and IFL and non-IFL subgroups achieved normal or near-normal levels of flow, suggesting potential HGNS efficacy across a broad range of sleep apnea severity.
Keywords: obstructive sleep apnea, electrical hypoglossal nerve stimulation, pharynx, upper airway, titration
At a Glance Commentary
Scientific Knowledge on the Subject
Hypoglossal nerve stimulation is a potential novel therapeutic approach for patients with obstructive sleep apnea, although its ability to relieve pharyngeal airflow obstruction has not been determined.
What This Study Adds to the Field
Hypoglossal nerve stimulation produced marked dose-related increases in airflow without arousing patients from sleep, suggesting potential therapeutic efficacy across a broad range of sleep apnea severity.
Obstructive sleep apnea is characterized by recurrent episodes of upper airway obstruction during sleep (1). Airflow obstruction is thought to result from decreases in pharyngeal neuromuscular activity at sleep onset (2). These episodes lead to intermittent hypoxemia and recurrent arousals from sleep, accounting for the long-term neurocognitive (3, 4), metabolic (5), and cardiovascular (6) sequelae of this disorder. Nasal continuous positive airway pressure remains the mainstay of treatment for moderate to severe obstructive sleep apnea (7). Difficulties adhering to therapy can limit its effectiveness in the home setting (8, 9), and alternatives have been generally less effective in relieving upper airway obstruction during sleep (10–12).
Hypoglossal nerve stimulation (HGNS) has been piloted as treatment for obstructive sleep apnea (13). Implantable HGNS systems have been developed to stimulate the hypoglossal nerve during inspiration, and recruit the lingual musculature, leading to decreases in pharyngeal collapsibility during sleep (14–17). Motor activity protrudes the tongue, and mitigates airflow obstruction during sleep (18, 19). Resulting increases in inspiratory airflow can account for observed reductions in sleep apnea severity (19), although residual airflow obstruction can persist in some patients (20). Nevertheless, airflow responses to graded increases in HGNS have not been described; nor has the magnitude of these responses been well characterized.
The primary purpose of the present study was to characterize airflow responses to HGNS in patients implanted with a novel HGNS system. It was hypothesized that graded increases in HGNS intensity would result in progressive relief of upper airway obstruction (improvements in maximal inspiratory airflow), and that these responses are of sufficient magnitude to abolish airflow obstruction without arousing patients from sleep. The findings have major implications for HGNS titration and predicting responses to HGNS therapy. Some of the results of the present study have been previously reported in abstract form (21, 22).
Methods
Patient Population
Thirty patients with obstructive sleep apnea who were implanted with a novel HGNS system (Apnex Medical, Inc., St. Paul, MN) were recruited (23). Written informed consent was obtained. Key eligibility criteria for implantation were an apnea–hypopnea index of greater than or equal to 20 episodes per hour (predominantly obstructive hypopneas), and a central apnea index of less than or equal to 5% on a screening sleep study.
Experimental Techniques
Baseline sleep study.
Each patient underwent a standard overnight sleep study to characterize sleep and breathing patterns. Airflow was assessed with a nasal pressure cannula and an oronasal thermistor. Hypopneas were defined by a greater than 50% fall in airflow amplitude, or a discernable reduction in airflow that was associated with either a greater than or equal to 4% oxyhemoglobin desaturation or an arousal from sleep. Apneas were defined by a greater than 90% reduction in airflow for greater than or equal to 10 seconds.
HGNS device and implantation procedure.
Patients were implanted with a stimulating lead using a guarded bipolar electrode array within an insulating cuff to prevent current spread and focus stimulation on the nerve itself (see online supplement). The neurostimulator, respiration sensing, and stimulation leads were surgically implanted under general anesthesia. Briefly, the cuff of the stimulating lead was placed on the hypoglossal nerve distal to branches innervating the styloglossus and hyoglossus muscles, and placement was verified intraoperatively with fluoroscopic assessment of pharyngeal opening during stimulation. The stimulation lead body was connected to the neurostimulator, which was implanted in the ipsilateral infraclavicular space subcutaneously. Two respiratory impedance sensing leads were tunneled subcutaneously toward the midline and then bilaterally along each costal margin. Adverse events related to device implantation are described in the Methods section of the online supplement (Table E1).
Awake titration study.
Approximately 1 month after HGNS implantation, the twitch and tongue movement thresholds were determined by the lowest current level at which lingual muscle activation and bulk movement occurred, respectively.
Titration sleep study.
Each patient then returned for another overnight sleep study to determine the effect of stimulation intensity (current) on tidal airflow during sleep (discussed later). A mask and pneumotachograph (n = 26) or nasal cannula (n = 4) was used to quantify airflow responses to stimulation.
Experimental Protocol
The HGNS system was designed to stimulate during inspiration. In this titration protocol, alternating breaths were stimulated so that responses in inspiratory airflow could be compared with adjacent unstimulated breaths during sleep. HGNS was applied with increasing current amplitudes from 0–4 mA, whereas frequency and pulse width were fixed at 40 Hz and either 90 μs (n = 26) or 60 μs (n = 4). Flow responses in patients stimulated with 40 Hz and 60 μs did not differ, leading us to combine results from all patients.
During sleep, stimulation current was titrated upward in 0.1- to 0.3-mA steps until the airflow response plateaued or the patient aroused. Arousal was defined by a visible shift in EEG rhythm (24), increase in heart rate, or increase in maximal inspiratory airflow from baseline levels at stimulation offset (18).
Data Analysis
Maximal inspiratory airflow (VImax) was measured during stimulated and adjacent unstimulated breaths during stable non-REM sleep, as defined by stability in VImax immediately before and after stimulated breaths (18). At each stimulation level, breaths were assessed for the presence or absence of inspiratory flow limitation (IFL). Airflow responses at increasing current were characterized by the flow capture threshold at which airflow began to increase, and the peak flow threshold at which VImax peaked or plateaued with or without the elimination of IFL. The stimulus response slope was calculated as the quotient of differences in VImax and current between the peak and capture flow thresholds. Unstimulated baseline levels of airflow were measured to assess for stability in the state of pharyngeal patency during sleep over the range of current applied.
Statistical Analysis
Student t tests were used to compare airflow on and off stimulation (peak vs. baseline), and least squares linear regression was used to characterize airflow responses to graded levels of stimulation. The Pearson product moment correlation coefficient was calculated to examine the association between baseline and peak flow across the entire group. Least squares linear regression was also used to assess for drift in unstimulated airflow levels as current was varied. The sensitivity of the flow response to stimulation current was examined in 25 patients in whom flow capture thresholds was determined. Groups were stratified by the presence or absence of IFL at the peak flow threshold to compare flow responses. Results were expressed at means ± SEM, except in Table 1 where values are represented as mean ± SD. Statistical significance was inferred at a P less than 0.05 level.
TABLE 1.
DEMOGRAPHIC, ANTHROPOMETRIC, AND BASELINE SLEEP STUDY CHARACTERISTICS
| Characteristic | Mean | SD | |
| Anthropometry and demographics | |||
| Age, yr | 53.1 | ± | 9.7 |
| Body mass index, kg/m2 | 32.5 | ± | 3.8 |
| Waist circumference, cm | 106.6 | ± | 10.7 |
| Neck circumference, cm | 41.9 | ± | 4.1 |
| Sex, M/F | 20/10 | ||
| Sleep-disordered breathing | |||
| Apnea–hypopnea index, events/h | 45.4 | ± | 7.8 |
| Non-REM apnea–hypopnea index, events/h | 44.3 | ± | 19.7 |
| REM apnea–hypopnea index, events/h | 47.8 | ± | 23.8 |
| Apnea index, events/h | 4.8 | ± | 6.0 |
| Hypopnea index, events/h | 40.6 | ± | 15.5 |
| ODI 4%, events/h | 21.4 | ± | 17.4 |
| Respiratory arousal index, events/h | 31.0 | ± | 18.6 |
| Arousal index, events/h | 43.6 | ± | 17.7 |
| % Obstructive events | 96.2 | ||
| % Mixed events | 1.8 | ||
| % Central events | 2.0 | ||
| Sleep architecture | |||
| Sleep latency, min | 15.4 | ± | 17.0 |
| TST, min | 349.5 | ± | 70.7 |
| Sleep efficiency, % | 77.5 | ± | 12.6 |
| N1 as % of TST | 29.2 | ± | 11.4 |
| N2 as % of TST | 48.9 | ± | 8.1 |
| N3 as % of TST | 9.1 | ± | 7.7 |
| REM as % of TST | 12.8 | ± | 6.4 |
Definition of abbreviations: N1 = stage 1 non-REM sleep; N2 = stage 2 non-REM sleep; N3 = stage 3 non-REM sleep; ODI 4% = oxyhemoglobin desaturation index including events with more than 4% desaturation; TST = total sleep time.
Results
Patient Characteristics
The patients’ demographic and anthropometric characteristics and baseline sleep study results are described in Table 1. The patients were middle-aged and moderately obese men and women with moderate and severe obstructive sleep apnea. By design, these patients had predominantly obstructive hypopneas rather than apneas. Sleep architecture was characterized by elevated N1 and reduced REM sleep.
Single Breath Stimulation Airflow Responses
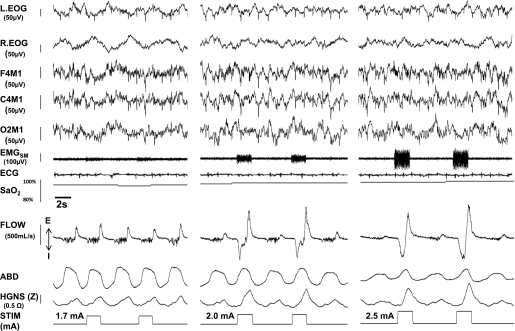
Stimulation responses are illustrated for one representative patient in non-REM sleep at three stimulation current levels (Figure 1). In each panel, two stimulated breaths are shown (stimulation marker signal at bottom and stimulus artifact in submental EMG (EMGSM), and are bracketed by adjacent unstimulated breaths during stable non-REM sleep. Unstimulated breaths displayed evidence of severe inspiratory airflow limitation as characterized by an early plateau in inspiratory flow at a low level and high frequency mid-inspiratory oscillations in airflow (consistent with snoring). During unstimulated breaths, maximal inspiratory airflow remained stable, regardless of the stimulation amplitude applied on the stimulated breaths. The return of flow to the baseline levels on the intervening unstimulated breaths provided evidence that stimulation was not causing arousal from sleep. In contrast, a graded response in maximal inspiratory airflow (downward direction) was observed with increasing levels of maximal inspiratory airflow as current was increased. Inspiratory airflow limitation persisted at low (left panel) and mid-levels (middle panel) of stimulation, but was abolished at still higher current level applied (right panel). When a low level of current was applied (1.7 mA) (Figure 1, left panel), maximal inspiratory airflow (VImax) did not increase relative to adjacent breaths before and after stimulation, indicating that the applied current remained below the flow capture threshold. As current was increased to 2 mA (middle panel) maximal inspiratory airflow (VImax) increased during the stimulated compared with unstimulated breaths. Nevertheless, inspiration remained flow-limited, as evidenced by an early peak in inspiratory airflow followed by a roll-off and plateauing of inspiratory flow later in inspiration (indicative of “negative effort dependence,” a recognized phenomenon in collapsible biologic conduits) (25, 26). When the stimulus intensity was increased to 2.5 mA, VImax increased further, and inspiratory airflow no longer plateaued, indicating the flow limitation had been abolished. Of note, stimulation was not associated with any shift in EEG frequency, change in heart rate, or increase in maximal inspiratory airflow during unstimulated breaths, indicating that arousal had not occurred.
Figure 1.
Representative polysomnographic recording examples of hypoglossal nerve stimulation (HGNS) response at low (1.7 mA, left panel), moderate (2 mA, middle panel), and high (2.5 mA, right panel) levels of stimulation in one patient. In each panel, two stimulated breaths are shown (stimulation marker signal at bottom and stimulus artifact in EMGSM), and are bracketed by adjacent unstimulated breaths during stable non-REM sleep. Unstimulated breaths displayed evidence of severe inspiratory airflow limitation as characterized by an early plateau in inspiratory flow at a low level and high frequency mid-inspiratory oscillations in airflow, consistent with snoring. During unstimulated breaths, maximal inspiratory airflow did not change across all stimulation levels, indicating that severe inspiratory flow limitation persisted across stimulation levels. In contrast, a graded response in maximal inspiratory airflow (downward direction) was observed with increasing levels of maximal inspiratory airflow as current was increased. Inspiratory airflow limitation persisted at low (left panel) and mid-levels (middle panel) of stimulation, but was abolished at the highest stimulation level applied (right panel). Note time lags of respiratory impedance signal (HGNS [Z]) and stimulus current marker signal (STIM) of approximately 400 ms and approximately 250 ms, respectively, relative to the airflow and ABD signals caused by signal processing and transmission from the implanted neurostimulation device. ABD = abdominal piezoelectric gauge; EMGSM = submental electromyogram; F4M1, C4M1, and O2M1 = electroencephalogram leads; FLOW = tidal airflow; HGNS (Z) = implanted respiratory impedance sensor; L. EOG = left electrooculogram; R. EOG = right electrooculogram; STIM = stimulation current marker signal.
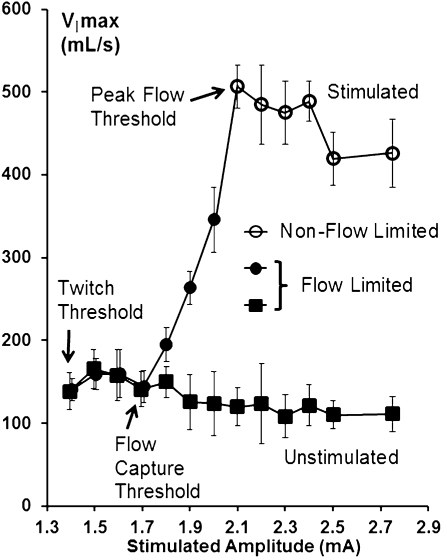
The flow responses observed in the representative patient in Figure 1 were used to generate an illustrative flow-response curve in Figure 2. As increasing stimulation was applied, VImax increased linearly from the flow capture threshold to the peak flow threshold, but inspiratory airflow remained flow limited over this current range. Once current exceeded the peak flow threshold, increases in stimulation amplitude no longer generated any further increases in inspiratory airflow and IFL was abolished.
Figure 2.
Inspiratory airflow (VImax) response to increasing hypoglossal nerve stimulation current amplitude during non-REM sleep for stimulated and unstimulated breaths in the patient illustrated in Figure 1. As stimulation current increased beyond the flow capture threshold, VImax increased linearly until the peak flow threshold was attained, at which point VImax plateaued as increasing stimulus current was applied. Note that inspiratory flow limitation persisted at intermediate current levels (closed circles). Further increases in current abolished inspiratory flow limitation (open circles).
Flow Responses Characteristics
Airflow response to stimulation.
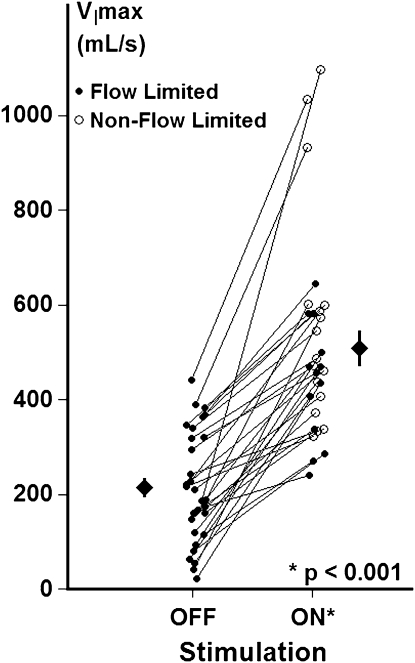
Maximal airflow responses to stimulation are illustrated for the entire group in Figure 3 (n = 30). During non-REM sleep, patients exhibited a mean VImax off stimulation of 215 ± 21 ml/s and on stimulation of 509 ± 37 ml/s, making for a mean increase in VImax of 294 ± 33 ml/s at the peak flow threshold. VImax increased in all 30 patients on compared with off stimulation, and the level of stimulated peak flow correlated with the unstimulated flow (r = 0.50; P = 0.005), suggesting that the degree of airway opening depended on the severity of upper airway obstruction at baseline.
Figure 3.
Baseline (unstimulated) and peak (stimulated) maximal inspiratory airflow (VImax) during non-REM sleep. Maximal inspiratory airflow (VImax) with stimulation OFF (mean baseline unstimulated breaths) and ON (at peak flow threshold) is represented for each patient and for the group as a whole (means ± SEM). A significant increase in VImax was observed for the group as a whole (P < 0.001). At the peak flow threshold, flow limitation was eliminated in 17 of 30 patients (open circles, stimulation ON), and persisted in the remaining 13 patients (solid circles, stimulation ON).
Moreover, inspiratory airflow limitation was abolished altogether in 17 patients (Figure 3, open circles, stimulation on) and improved markedly in the remaining 13 patients (Figure 3, closed circles, stimulation on). A similar increase in airflow was achieved in the IFL and non-IFL groups (256 ± 31 vs. 323 ± 52 ml/s; P = 0.15), indicating substantial improvements in pharyngeal patency during stimulation in both groups. These groups did not differ in unstimulated (241 ± 25 vs. 182 ± 36 ml/s) or peak flow levels (564 ± 58 vs. 438 ± 35 ml/s). Nevertheless, the IFL subgroup required greater current to achieve peak flow (1.80 ± 0.20 vs. 1.23 ± 0.10 mA; P < 0.05).
Sensitivity of the flow response to stimulation.
Stimulation generated progressive increases in airflow from a flow capture threshold (216 ± 24) ml/s at 1.05 ± 0.09 mA) to a peak flow threshold of 538 ± 41 ml/s at 1.46 ± 0.11 mA. This 0.41 ± 0.06 mA (P < 0.001) increase in stimulation current was associated with increases in airflow of 321 ± 36 ml/s (P < 0.001), indicating marked sensitivity in the flow response to stimulation.
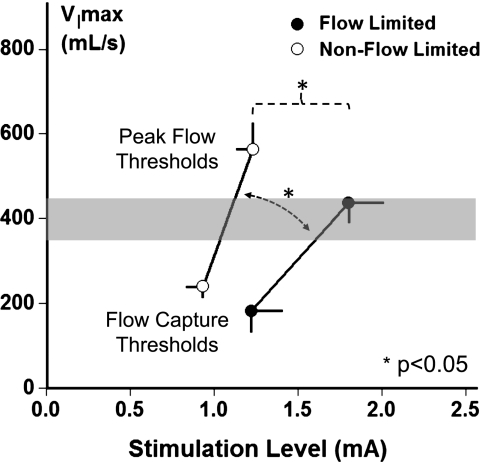
Further insight into stimulus response mechanisms can be gained by comparing the sensitivity of the flow response in groups with and without flow limitation from the flow capture to peak flow thresholds (Figure 4). These groups did not differ significantly in age, body mass index, or sex; nor did the flow-limited group differ significantly from the non–flow-limited group in the baseline unstimulated level of flow (174 ± 43 vs. 244 ± 28 ml/s), the flow capture threshold (1.23 ± 0.18 vs. 0.93 ± 0.10 mA), or the twitch motor threshold level (0.78 ± 0.12 vs. 0.67 ± 0.04 mA), respectively. Compared with the flow-limited group (closed circles), the flow response in the non–flow-limited group (open circles) was greater (steeper slope, 674 ± 166 vs. 1241 ± 199 ml/s/mA; P < 0.05), indicating greater sensitivity in the response to stimulation in this group. Peak airflow did not differ in the flow-limited compared with non–flow-limited group (438 ± 35 vs. 564 ± 58 ml/s), although the flow-limited group required a greater increase in stimulation current to achieve peak flow from the flow capture threshold (0.57 ± 0.12 vs. 0.30 ± 0.03 mA; P < 0.05). Of note, both groups attained normal or near normal levels of maximal inspiratory airflow during sleep of approximately 400 ml/s or greater (shaded region).
Figure 4.
Maximal inspiratory airflow (VImax) versus stimulation current (milliamperes) in groups with (solid circles) and without (open circles) inspiratory flow limitation at the peak flow threshold. The flow response slope in the non–flow-limited group was greater than that in the flow-limited subgroup (1241 ± 199 vs. 674 ± 167 ml/s/mA; n = 25; P < 0.05). Lower levels of stimulation current were required to achieve peak airflow in the non–flow-limited compared with flow-limited subgroup (1.23 ± 0.10 vs. 1.80 ± 0.20 mA; n = 25; P < 0.05), although peak inspiratory airflow did not differ between non–flow-limited and flow-limited subgroups (564 ± 58 vs. 438 ± 35 ml/s). Both groups attained normal or near normal levels during sleep of approximately 400 ml/s or greater (shaded region).
Discussion
Acute unilateral stimulation of the hypoglossal nerve during sleep in patients with obstructive sleep apnea resulted in progressive increases in inspiratory airflow with increasing stimulation intensity. Stimulation increased airflow markedly and abolished IFL in most patients. Moreover, inspiratory airflow returned to baseline unstimulated levels before and immediately after the stimulated breath, suggesting that HGNS exerted a direct effect on lingual muscles and airway patency without arousing patients from sleep. Airflow increased in all patients, and rose progressively with stimulus amplitude. Such consistent, progressive flow responses suggest a direct relationship of tongue position to pharyngeal patency during sleep. Finally, the increases in airflow were of sufficient magnitude to suggest potential therapeutic efficacy of HGNS across a broad range of sleep apnea severity.
Mechanism for Increased Airflow during Stimulation
In previous studies, investigators have demonstrated that electrical stimulation of the genioglossus increases upper airway flow during sleep in patients with sleep apnea (18, 19). These increases have been attributed to decreases in pharyngeal collapsibility (14, 16, 17), which decreases the back pressure to inspiratory airflow (27). In previous studies, stimulating the genioglossus muscle and hypoglossal nerve led to an approximately 3- to 5-cm H2O decrease in critical pressure, which can account for an approximately 150- to 250-ml/s increase in maximal inspiratory airflow (18, 19). In the present study, these observations were extended by characterizing flow responses over a range of stimulus amplitudes and demonstrate even greater increases in airflow approximating 300 ml/s, possibly because of differences in cuff placement, nerve anatomy, stimulation intensity, or increased sensitivity of the flow response. Peak flow responses were likely underestimated because inspiratory airflow limitation was eliminated in most patients and maximal inspiratory airflow can no longer increase after flow limitation resolves (27). These findings suggest that the current HGNS approach can produce substantial relief of upper airway obstruction during sleep.
Flow-response Curve Characteristics
Responses in airway patency were characterized by delineating flow increases from baseline over a range of current amplitude. In analyzing flow-response curve characteristics, it was found that the slope of the flow response to stimulation was greater in the patients whose airflow obstruction (IFL) abated than in those whose IFL persisted. Differences in the slope of the flow- response curve could be related to variability in the degree of neuromechanical coupling or cuff placement between the flow-limited and non–flow-limited groups. These slope differences were not associated with differences in the flow capture threshold during sleep or twitch threshold during wakefulness, suggesting that cuff placement and nerve impedance were similar between groups. Rather, peak flow was achieved at lower stimulation amplitudes in the non–flow-limited group, suggesting enhanced mechanical effects of lingual muscle contraction on the pharynx. This effect could reflect greater linkage between the tongue and other pharyngeal structures, because of differences in lingual muscle fiber orientation, lingual-palatine linkage, or pharyngeal site of collapse (14, 28, 29). Alternatively, lingual muscle recruitment patterns could differ among patients, as suggested by observed decreases in inspiratory airflow in one patient at stimulation amplitudes well above the peak flow threshold, which were also associated with tongue retraction during wakefulness and sedation. These decreases suggest current spread to lingual retractor muscles (18), which could have resulted from a more proximal cuff electrode placement (19). Finally, the enhanced flow peak flow response correlated with baseline unstimulated flow, suggesting that baseline differences in the severity of airflow obstruction can also account for observed differences in peak flow responses to a given stimulus amplitude. Thus, augmented flow responses may result from increased mechanical linkage between lingual and pharyngeal structures, a predominance of lingual protrusor muscle recruitment, or lesser degrees of airflow obstruction at baseline.
Arousals
HGNS evolved from initial studies examining the effects of transcutaneous submental stimulation on upper airway patency (30). Investigators demonstrated improvements in airway patency that were later thought likely related to arousals rather than selective stimulation of the lingual muscles during sleep. Subsequently, investigators documented arousal thresholds during submental stimulation, which confounded assessment of airflow responses and limited clinical applicability of this technique during sleep (31, 32). Investigators further refined the stimulation technique by inserting temporary fine-wire electrodes into lingual muscles, and demonstrated that protrusor muscle stimulation mitigated and retractor muscle stimulation worsened pharyngeal patency during sleep (13, 16, 18, 19, 33). In these prior studies, investigators scrutinized EEG and ECG signals to exclude responses associated with cortical or autonomic activation (13, 18, 19). Additional studies with implantable nerve cuff (13) and fine-wire electrodes have demonstrated responses to selective lingual muscle stimulation during sleep and anesthesia (13–17). The present study also screened for evidence of cortical and autonomic activation, and further required strict temporal synchrony between the stimulus burst and airflow response to exclude arousal responses from the analysis. The following provide further evidence that arousal did not confound the assessment of flow responses during sleep. First, flow returned to baseline levels on alternating unstimulated breaths, as shown in Figure 1. Second, the graded, linear response in flow to increasing stimulation intensity up to the peak flow threshold as shown in Figure 2 is consistent with a dose-dependent mechanical opening of the airway rather than an arousal mechanism. Third, the standard protocol of the sleep titration study was to increase stimulation intensity until arousals were actually observed to occur. Fourth, flow limitation persisted in selected patients over a broad range of stimulation currents, which could only occur during sleep rather than wakefulness. These analyses reinforce the conclusion that unilateral HGNS produces progressive relief of upper airway obstruction without arousing patients from sleep.
Limitations
Several limitations should be considered in interpreting our findings. First, the protocol stimulated every other breath to establish a quasi steady-state baseline level of airflow obstruction. Unstimulated airflow levels in our apneic patients were often insufficient to prevent recurrent apneas or hypopneas, and breathing patterns could not be completely stabilized until higher levels of HGNS were applied, when flow and tidal volumes increased. Although airflow and ventilation increased progressively, unstimulated flows remained constant on adjacent breaths, and provided a stable baseline from which to gauge airflow responses across stimulation current levels. Second, esophageal manometry was not used to monitor inspiratory effort and assess for the presence of IFL. We could nevertheless rely on the inspiratory flow contour to distinguish flow-limited from non–flow-limited breaths, because the inspiratory plateau remains a well-validated index of inspiratory airflow limitation (34, 35) during natural sleep. Third, we recognized that arousal could confound the assessment of flow responses and designed the experimental protocol and analytic approaches to minimize this possibility. Fourth, the study assessed acute airflow responses to HGNS, and did not address factors associated with the chronic use of HGNS therapy. Fifth, flow-response curves were not delineated in all body positions and sleep stages because of time constraints and titration protocol development. Nevertheless, streamlined methods allowed us to enlarge the patient sample across sites, thereby increasing the generalizability of the findings.
Implications for Therapy
Our findings have several implications for HGNS therapy. First, the airflow response to peak stimulation was of sufficient magnitude to relieve upper airway obstruction during sleep and reduce sleep apnea severity. In patients with baseline levels of maximal inspiratory airflow during sleep-disordered breathing episodes of 50–250 ml/s, a mean increase of 294 ml/s would likely yield relatively normal levels of peak inspiratory airflow found in asymptomatic snoring and normal nonsnoring individuals during sleep (36, 37). Second, flow changed instantaneously with stimulation and increased progressively with stimulus intensity, suggesting that increases in flow are a direct effect of stimulation rather than a result of arousals from sleep. Third, inspiratory airflow was exquisitely sensitive to small changes in stimulus amplitude, suggesting that peak flow responses, as discerned from the flow-response curve, should be targeted for HGNS therapy and that further increases in stimulation intensity will not yield further benefit. Fourth, a brisk flow response was associated with complete elimination of upper airway obstruction in most patients. The stimulus intensity required to generate normal levels of airflow was greater in those with persistent flow limitation, implying that other pharyngeal or lingual muscles must still be recruited to completely abolish IFL during sleep. Further studies in additional patients, sleep stages, and body positions are required to determine the clinical and physiologic predictors of this response.
Supplementary Material
Footnotes
Author Contributions: All authors meet established criteria for authorship in that each has made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data, and has participated in drafting the article or revising it critically for important intellectual content. In addition, each author has approved this version of the manuscript to be published.
Supported by Apnex Medical, Inc., and by NIH grant HL50381.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1614OC on December 1, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
Investigators and Institutions Participating in the Trial: Maree Barnes, M.D., Fergal O'Donoghue, M.D., Christopher Worsnop, M.D., and Matthew Campbell, M.D., Austin Hospital, Melbourne, Australia; Jon Freeman, Ph.D. and Steven Park, M.D., Clinilabs, New York, New York; Doug McEvoy, M.D., Nick Antic, M.D., Peter Catcheside, Ph.D., and Sam Robinson, M.D., Repatriation General Hospital/Flinders, Adelaide, Australia; Tod Huntley, M.D., Michel Levine, M.D., and Caryn Vogel, M.D., St. Vincent Hospital, Indianapolis, Indiana; David Hillman, M.D., Peter Eastwood, Ph.D., and Geoffrey Hee, M.D., Sir Charles Gairdner Hospital, Perth, Australia; Eric Kezirian, M.D. and David Claman, M.D., University of California San Francisco, San Francisco, California; Con Iber, M.D., Mike Howell, M.D., and George S. Goding, Jr., M.D., University of Minnesota, Minneapolis, Minnesota; and John Wheatley, M.D., Tracey Robinson, M.D., Kristina Kairaities, M.D., Peter Middleton, M.D., Jimmy Chien, M.D., Jin-Gun Cho, M.D., Peter Wu, M.D., and Carsten Palme, M.D., Westmead Hospital, Sydney, Australia.
References
- 1.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931–938 [DOI] [PubMed] [Google Scholar]
- 2.Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D, Pierce RJ. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 2005;564:549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM, O'Hearn DJ, Neubauer DN, Nieto FJ, Schwartz AR, Smith PL, Bandeen-Roche K. Modeling hypersomnolence in sleep-disordered breathing: a novel approach using survival analysis. Am J Respir Crit Care Med 1999;159:1703–1709 [DOI] [PubMed] [Google Scholar]
- 4.Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, Maislin G, Dinges DF. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:1162–1168 [DOI] [PubMed] [Google Scholar]
- 5.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005;99:1998–2007 [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053 [DOI] [PubMed] [Google Scholar]
- 7.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med 2007;356:1751–1758 [DOI] [PubMed] [Google Scholar]
- 8.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887–895 [DOI] [PubMed] [Google Scholar]
- 9.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep 1997;20:278–283 [DOI] [PubMed] [Google Scholar]
- 10. Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med 2003;168:238–241. [DOI] [PubMed]
- 11.Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, Nadeau J, Permutt L, Gleadhill I, Smith PL. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1992;145:527–532 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144:494–498 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de HP, Ejnell H, Hochban W, Knaack L, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001;127:1216–1223 [DOI] [PubMed] [Google Scholar]
- 14.Oliven A, Odeh M, Geitini L, Oliven R, Steinfeld U, Schwartz AR, Tov N. Effect of co-activation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol 2007;103:1662–1668 [DOI] [PubMed] [Google Scholar]
- 15.Oliven A, Tov N, Geitini L, Steinfeld U, Oliven R, Schwartz AR, Odeh M. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J 2007;30:748–758 [DOI] [PubMed] [Google Scholar]
- 16.Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De BW, Van de HP, Smith PL, Eisele DW, Allan L, Schneider H, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 2003;95:2023–2029 [DOI] [PubMed] [Google Scholar]
- 17.Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 2009;106:1668–1673 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 1996;81:643–652 [DOI] [PubMed] [Google Scholar]
- 19.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 1997;123:57–61 [DOI] [PubMed] [Google Scholar]
- 20.Kezirian EJ, Boudewyns A, Eisele DW, Schwartz AR, Smith PL, Van de Heyning PH, De Backer WA. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev 2010;14:299–305 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AR, Barnes M, Hillman DR, Kezirian EJ, Smith PL, Hoegh T, Parrish D, Eastwood PR. Acute effects of hypoglossal nerve stimulation on airflow and arousal. Am J Respir Crit Care Med 2011;183:A2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AR, Barnes M, Hillman DR, Kezirian EJ, Malhotra A, Smith PL, Hoegh T, Parrish D. Upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Sleep 2011;34:A118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastwood PR, Barnes M, Walsh JH, Maddison KJ, Hee G, Schwartz AR, Smith PL, Malhotra A, McEvoy RD, Wheatley JR, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 2011;34:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007.
- 25.Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol 1989;66:1626–1634 [DOI] [PubMed] [Google Scholar]
- 26.Dawson SV, Elliott EA. Wave-speed limitation on expiratory flow: a unifying concept. J Appl Physiol 1977;43:498–515 [DOI] [PubMed] [Google Scholar]
- 27.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 1996;110:1077–1088 [DOI] [PubMed] [Google Scholar]
- 28.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 1998;507:265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller D, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol (Lond) 1999;519(Pt 2):601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miki H, Hida W, Chonan T, Kikuchi Y, Takishima T. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis 1989;140:1285–1289 [DOI] [PubMed] [Google Scholar]
- 31.Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol 1993;75:1053–1061 [DOI] [PubMed] [Google Scholar]
- 32.Edmonds LC, Daniels BK, Stanson AW, Sheedy PF, Shepard JWJ. The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am Rev Respir Dis 1992;146:1030–1036 [DOI] [PubMed] [Google Scholar]
- 33.Oliven A, Schnall RP, Pillar G, Gavriely N, Odeh M. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol 2001;127:217–226 [DOI] [PubMed] [Google Scholar]
- 34.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med 1994;150:475–480 [DOI] [PubMed] [Google Scholar]
- 35.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med 1998;157:1461–1467 [DOI] [PubMed] [Google Scholar]
- 36.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 2008;105:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 2007;102:547–556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.