Abstract
Rationale: Cross-sectional studies demonstrate an association between metabolic syndrome and impaired lung function.
Objectives: To define if metabolic syndrome biomarkers are risk factors for loss of lung function after irritant exposure.
Methods: A nested case-control study of Fire Department of New York personnel with normal pre–September 11th FEV1 and who presented for subspecialty pulmonary evaluation before March 10, 2008. We correlated metabolic syndrome biomarkers obtained within 6 months of World Trade Center dust exposure with subsequent FEV1. FEV1 at subspecialty pulmonary evaluation within 6.5 years defined disease status; cases had FEV1 less than lower limit of normal, whereas control subjects had FEV1 greater than or equal to lower limit of normal.
Measurements and Main Results: Clinical data and serum sampled at the first monitoring examination within 6 months of September 11, 2001, assessed body mass index, heart rate, serum glucose, triglycerides and high-density lipoprotein (HDL), leptin, pancreatic polypeptide, and amylin. Cases and control subjects had significant differences in HDL less than 40 mg/dl with triglycerides greater than or equal to 150 mg/dl, heart rate greater than or equal to 66 bpm, and leptin greater than or equal to 10,300 pg/ml. Each increased the odds of abnormal FEV1 at pulmonary evaluation by more than twofold, whereas amylin greater than or equal to 116 pg/ml decreased the odds by 84%, in a multibiomarker model adjusting for age, race, body mass index, and World Trade Center arrival time. This model had a sensitivity of 41%, a specificity of 86%, and a receiver operating characteristic area under the curve of 0.77.
Conclusions: Abnormal triglycerides and HDL and elevated heart rate and leptin are independent risk factors of greater susceptibility to lung function impairment after September 11, 2001, whereas elevated amylin is protective. Metabolic biomarkers are predictors of lung disease, and may be useful for assessing risk of impaired lung function in response to particulate inhalation.
Keywords: metabolic syndrome X, September 11 terrorist attacks, biologic markers
At a Glance Commentary
Scientific Knowledge on the Subject
Development of ventilatory dysfunction after particulate exposure is a major health concern worldwide. The prevalence of metabolic syndrome is high in industrialized nations and rapidly increasing in developing nations with high ambient particulates. The interaction of these two disorders is a topic of considerable importance. Our work shows that dyslipidemia, elevated heart rate, and leptin are independent risk factors of greater susceptibility to lung function impairment after September 11, 2001, whereas elevated amylin is protective.
What This Study Adds to the Field
Metabolic syndrome biomarkers expressed soon after exposure to World Trade Center dust predict FEV1 decline. This suggests that the systemic inflammation produced by metabolic syndrome impacts the progression to abnormal lung function in a longitudinally followed cohort. These data provide a base to explore the mechanism of interaction and to better understand pathogenesis of airflow obstruction in patients with metabolic syndrome and particulate exposure.
Before September 11, 2001 (9/11), the Fire Department of New York City (FDNY) Bureau of Health Services routinely administered pulmonary function tests (PFTs) to firefighters. After the World Trade Center (WTC) collapse, exposed rescue workers, which included firefighters and emergency medical service personnel (n = 13,234; 95%), were intensively screened between September 12, 2001, and March 10, 2008, through the FDNY-WTC Medical Monitoring and Treatment Program (FDNY-WTC-MMTP). Respiratory compromise after WTC exposure has been documented in FDNY rescue workers (1–6), other exposed workers (7), and lower Manhattan residents (8, 9). Studies have found that the exposed rescue workers had decline of FEV1 during the first year after 9/11 at a rate 12 times greater than that found before 9/11 (1, 3).
As part of the first medical monitoring examination (MME) post-9/11, all participants received PFTs and fasting blood levels of high-density lipoprotein (HDL), glucose, and triglycerides; concurrent serum samples were collected and banked in a biorepository. Symptomatic firefighters were referred to subspecialty pulmonary evaluation (SPE) between September 12, 2001, and March 10, 2008 (n = 1,720), and obstruction was the predominant physiologic finding underlying the reduction in their lung function after 9/11 (10).
Inflammation is a key feature of metabolic syndrome (MetSyn) (11). MetSyn is a set of risk factors that includes abdominal obesity, insulin resistance, dyslipidemia, and hypertension (12). MetSyn affects more than 30% of adults, or more than 47 million Americans. Patients who have this syndrome have a 1.5-fold to threefold increased risk of developing atherosclerotic cardiovascular disease, and a threefold to fivefold increased risk of type II diabetes (13).
Previous cross-sectional studies have suggested associations of impaired lung function with insulin resistance, type II diabetes mellitus, and MetSyn (14–18). The temporal relationship of MetSyn biomarkers and ventilatory function decline is in dispute. One longitudinal study showed that baseline lower FEV1 was an independent predictor of development of MetSyn (19). Recent literature has implicated systemic inflammation in chronic airflow obstruction (20). Airflow obstruction from particulate matter (PM) and smoke-induced inflammation is poorly understood, but high ambient PM significantly decreases FEV1 (21–23). MetSyn coexisting with chronic obstructive pulmonary disease (COPD) is frequent. Nearly half of COPD patients demonstrate the presence of one or more components of MetSyn (13). Abdominal obesity compressing the lung is one of the causes of impaired lung function in MetSyn (18), but systemic inflammation caused by MetSyn also contributes to lung disease (24, 25). In air pollution studies, long-term PM exposure and MetSyn increase systemic inflammation (11). Similar interactions are seen between MetSyn and COPD, possibly explaining the increased cardiovascular mortality in COPD (24, 26–28). Our study goal was to investigate if MetSyn biomarkers can predict future loss of lung function in a longitudinal study of a well-defined cohort of FDNY rescue workers with exposure to WTC dust. Some of the results have been previously reported in the form of an abstract (29).
Methods
Study Design and Participants
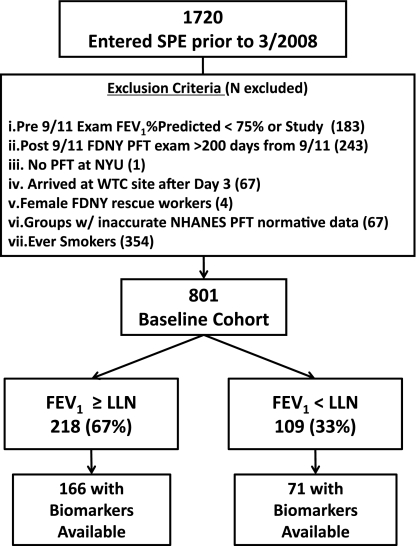
Symptomatic participants (n = 1,720) referred to SPE between October 1, 2001, and March 10, 2008, entered the study cohort and had a median time between MME and SPE of 28 months, (interquartile range, 12–49 mo). Baseline cohort (n = 801 and 1,720) was defined after inclusion and exclusion criteria were applied.
The baseline cohort was stratified by tertiles of body mass index (BMI) FEV1%Pred at SPE and the cohort control was randomly selected (n = 171). The most and least affected subgroups in the bottom and top octiles of FEV1%Pred at SPE (n = 100 for each) were oversampled. When all subgroups were combined, the study subcohort represented 327 and 801 (n = 237 with biomarkers) (Figure 1). Cases were defined as having FEV1%Pred less than lower limit of normal (LLN) as defined by National Health and Nutrition Examination Survey III and control subjects as FEV1%Pred greater than or equal to LLN at SPE. Subjects signed informed consents approved by the institutional review boards of Montefiore Medical Center (#07–09–320) and New York University (#11–00439).
Figure 1.
Study design. FDNY = Fire Department of New York; LLN = lower limit of normal as defined by NHANES III; NHANES = National Health and Nutrition Examination Survey; NYU = New York University; PFT = pulmonary function test; SPE = subspecialty pulmonary examination; WTC = World Trade Center.
Demographics
Demographic data were obtained from the FDNY-WTC-MMTP (Table 1). Arrival time was defined at the first MME based on FDNY-WTC Exposure Intensity Index (10). Those arriving after September 12, 2001, were excluded from analysis because of low numbers and significant demographic differences as previously described (10). Never-smokers reported having never smoked at all follow-up MMTP visits.
TABLE 1.
DEMOGRAPHICS AND BMI OF SUBSPECIALTY PULMONARY EVALUATION COHORT
| Study Subcohort |
Subcohort Biomarkers Available* |
||||||
| Baseline Never-smokers | Cases FEV1 Less Than LLN | Control Subjects FEV1 Greater Than or Equal to LLN | Cases Less Than LLN | Control Subjects Greater Than or Equal to LLN | P Value† | ||
| N | 801 | 109 | 218 | 71 | 166 | ||
| WTC arrival time, n (%)‡ | Morning of 9/11 | 197 (25) | 30 (28) | 49 (22) | 15 (21) | 32 (19) | 0.108 |
| Afternoon of 9/11 | 498 (62) | 57 (52) | 148 (68) | 42 (59) | 117 (71) | ||
| Anytime on 9/12 | 106 (13) | 22 (10) | 21 (10) | 14 (20) | 17 (10) | ||
| 9/11 to evaluation, mo§ | MME | 2.7 (2–4) | 2.6 (2.1–4) | 2.7 (2.1–3.8) | 2.7 (2.3–3.8) | 2.7 (2.1–3.3) | 0.51 |
| SPE | 33.8 (25–57) | 32.5 (23–55) | 33.5 (25–54) | 34.3 (23.5–56.4) | 33.1 (26.2–54.1) | 0.81 | |
| BMI, kg/m2§ | MME | 28 (26–30) | 29 (27–31) | 27.6 (26–30) | 29 (26.6–31.2) | 27.6 (25.8–30.1) | 0.04 |
| SPE | 28.9 (27–31) | 30 (28–33) | 28.4 (26–31) | 29.4 (27.5–32.8) | 28.3 (26.3–30.7) | 0.005 | |
| Years of service at 9/11§ | 18.4 (11–23) | 18 (14–22) | 18.7 (11–24) | 18.3 (13.3–22.2) | 16.7 (10.5–22.5) | 0.60 | |
| Age at 9/11§ | 40 (36–45) | 40 (35–45) | 42 (37–46) | 39 (35 –45) | 42 (37–46) | 0.15 | |
| Race‡ | White | 782 (98) | 105 (96) | 210 (96) | 67 (94) | 161 (97) | 0.46 |
| African American | 19 (2) | 4 (4) | 8 (4) | 4 (6) | 5 (3) | ||
Definition of abbreviations: BMI = body mass index; LLN = lower limit of normal as defined by NHANES III; NHANES = National Health and Nutrition Examination Survey; MME = medical monitoring entry; SPE = subspecialty pulmonary examination; WTC = World Trade Center.
Available serum biomarkers and all measures of metabolic syndrome.
Data compared between cases and control subjects in subcohort with biomarkers available; P values determined by Mann-Whitney and Pearson chi-square as appropriate.
Values expressed as n (%).
Values expressed as medians (interquartile range).
Serum Sampling and Analysis
Blood drawn at the MME was kept for 1 hour at room temperature and centrifuged at 1,800 g for 10 minutes. Serum was stored at −80°C (Bio-Reference Laboratories, NJ); thawed once at 4°C; assayed by 13-Plex Metabolic Hormone Panel (Millipore, Billerica, MA) on a Luminex 200IS (Luminex Corporation, Austin, TX); and with MasterPlex TM QT software (Ver.1.2; MiraiBio, South San Francisco, CA). The dynamic range of the assay was defined by the manufacturer. Each batch of samples processed contained control subjects and cases in approximately 16:7 ratio. Cut-off values were defined as the study cohort's 25th and 75th percentile of biomarker expression (138 and 171 with serum available).
MetSyn Criteria
MetSyn diagnosis requires three out of five World Health Organization modified National Heart Lung and Blood Institute/American Heart Association criteria: (1) triglycerides greater than or equal to 150 mg/dl; (2) HDL cholesterol less than 40 mg/dl in men; (3) systolic blood pressure greater than or equal to 130 mm Hg or diastolic blood pressure greater than or equal to 85 mm Hg; (4) fasting glucose greater than or equal to 100 mg/dl (12, 30); and (5) BMI greater than 30 was substituted for increased waist circumference as per World Health Organization (31).
Spirometry
Spirometry was performed at MME (Portascreen Spirometry; S&M Instruments, Doylestown, PA) and at SPE (Jaeger Masterscreen; Viasys Healthcare, San Diego, CA) (7, 32). Spirometry was done according to American Thoracic Society/European Respiratory Society guidelines and FEV1%Pred was determined using National Health and Nutrition Examination Survey III normative data (Table 2). Change in FEV1%Pred was calculated for each individual by linear regression between the earliest and latest available PFT from 1997 to September 10, 2001 (Table 2).
TABLE 2.
LUNG FUNCTION OF COHORT
| Study Subcohort |
Subcohort Biomarkers Available* |
|||||||
| Baseline Never-smokers | Cases FEV1 Less Than LLN | Control Subjects FEV1 Greater Than or Equal to LLN | Cases Less Than LLN | Control Subjects Greater Than or Equal to LLN | P Value† | |||
| N | 801 | 109 | 218 | 71 | 166 | |||
| FEV1‡ | Pre 9/11 | % | 100 (91–109) | 89 (82–96) | 108 (98–118) | 88 (82–96) | 108 (98–117) | <0.0001 |
| L | 4.3 (3.8–4.8) | 3.8 (3.5–4) | 4.5 (4–5) | 3.8 (3.5–4.2) | 4.5 (4–5.1) | <0.0001 | ||
| MME | % | 90 (82–100) | 78 (72–88) | 97 (89–107) | 77 (71– 88) | 96 (89–106) | <0.0001 | |
| L | 3.8 (3.4–4.3) | 3.4 (3.4) | 4 (3.6–4.6) | 3.3 (3.1–3.7) | 4.1 (3.6–4.5) | <0.0001 | ||
| SPE | % | 94 (84–102) | 73 (67–76) | 106 (93–112) | 73 (68–76) | 104 (93–112) | <0.0001 | |
| L | 3.9 (3.5–4.3) | 3 (2.7–3) | 4.2 (3.7–4.7) | 3.1 (2.8–3.3) | 4.2 (3.7–4.6) | <0.0001 | ||
| FEV1/FVC | SPE | 78 (74–82) | 73 (65–77) | 79 (75–83) | 72 (65–77) | 79 (75–82) | <0.0001 | |
Definition of abbreviations: LLN = lower limit of normal as defined by NHANES III; NHANES = National Health and Nutrition Examination Survey; MME = medical monitoring entry; SPE = subspecialty pulmonary examination.
Available serum biomarkers and all measures of metabolic syndrome.
P values determined by Mann-Whitney except exposure P value determined by Pearson chi-square; between cases and control subjects of subcohort biomarkers available.
Values expressed as medians (interquartile range).
Statistical Analysis
Database management and statistics were performed using SPSS 19 (IBM, Armonk, NY) and GraphPad Prism Version 5.02 (GraphPad Software Inc., La Jolla, CA). Analyte levels were compared by Mann-Whitney U test. Demographics and the proportion of individuals that met each criteria of MetSyn were compared in cases and control subjects by Pearson chi-square. The odds ratio (OR) of case and control status, while adjusting for confounding, was determined using multivariate binary logistic regression. The goodness-of-fit of the regression model was assessed by Hosmer-Lemeshow. Statistical significance was assessed by P less than 0.05. Multiple tests were controlled for by calculating the false discovery rate; P less than or equal to 0.05 was considered significant.
Results
Participants
Derivation of baseline cohort, cases, and control subjects from those that received SPE is described in Figure 1. Controls had similar FEV1%Pred compared with the baseline cohort (n = 801) and the study cohort (n = 327). Cases and control subjects had similar WTC arrival time, duration from 9/11 to MME, time from 9/11 to SPE, years of service, and age at 9/11. Serum drawn at the first monitoring examination was available for 166 (76%) of 218 of the control subjects and 71 (65%) of 109 of lung impairment cases. The demographics of the total groups were similar to control subjects and cases with serum available (Table 1). BMI was elevated in cases compared with control subjects at the first MME and at SPE (Table 1).
Longitudinal BMI and Lung Function
Cases had lower FEV1%Pred than control subjects: pre-9/11 (88% vs. 108%), MME (77% vs. 96%), and SPE (73% vs. 104%); P less than 0.0001 for all evaluations. Lung function in cases, as measured by FEV1%Pred, continued to decline from MME to SPE (77–73%), whereas lung function for control subjects increased (96–104%) (Table 2). To confirm that the median FEV1 represented individual deterioration in cases and improvement in control subjects, patients were used as their own control subjects. The mean ratio of FEV1%Pred measured at these two time points (SPE and MME) was 0.88 in cases and 1.04 in control subjects (P < 0.0001).
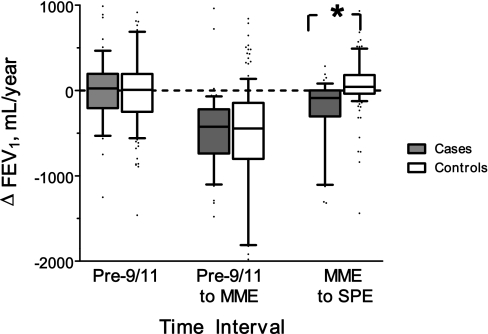
The difference in FEV1%Pred between cases and control subjects pre-9/11 FEV1 could be a manifestation of differential susceptibility to lung injury before WTC exposure. Because this cohort has had spirometries starting in 1997, we were able to calculate the change in FEV1 in milliliter per year in cases and control subjects (Figure 2). This allowed us to assess if cases manifested accelerated decline of lung function before WTC exposure; there was no significant difference in FEV1 decline between cases and control subjects before 9/11. Additionally, there was no significant difference between cases and control subjects in the decline in FEV1 in the interval around WTC exposure. A markedly different pattern occurred in the interval between MME and SPE, where cases lost 89 ml/yr FEV1 post-9/11, whereas control subjects gained 42 ml/yr (P < 0.0001) (Figure 2).
Figure 2.
Change in FEV1 (milliliter per year) significantly different between cases and control subjects only at medical monitoring examination (MME) to subspecialty pulmonary examination (SPE) time period. n = 237; * P < 0.0001. Slope was not statistically significant between cases and control subjects at pre–September 11th (n = 169) and between pre–September 11th to MME (n = 237). Results represented as box (median and interquartile range) and whiskers (10th–90th percentile). Significance determined by Mann-Whitney U test.
Clinical Biomarkers of MetSyn at FDNY-WTC MME in Cases and Control Subjects
Cases had significantly higher glucose (median, P value) (93 vs. 90 mg/dl; P = 0.03) and heart rate (71 vs. 66 bpm; P = 0.02) than control subjects. Other parameters of MetSyn measured in cases and control subjects were not significant: triglycerides (158 vs. 135 mg/dl; P = 0.09); systolic blood pressure (120 vs. 114; P = 0.09); and HDL (43 vs. 47 mg/dl; P = 0.15). Serum levels of 13 metabolic protein biomarkers showed that cases compared with control subjects had higher levels of leptin (8,035 vs. 5,370 pg/ml; P < 0.001); lower levels of amylin (54.8 vs. 59.2 pg/ml; P < 0.01); and lower levels in pancreatic polypeptide (108.5 vs. 151.5 pg/ml; P = 0.05) (Table 3).
TABLE 3.
CLINICAL PARAMETERS AND BIOMARKERS OF METABOLIC SYNDROME AT MEDICAL MONITORING ENTRY
| Serum Analytes | Cases FEV1 Less Than LLN* | Control Subjects FEV1 Greater Than or Equal to LLN† | P Value† |
| Triglycerides, mg/dl | 158 (108–272) | 135 (95–219) | 0.09 |
| HDL, mg/dl | 43 (37–54) | 47 (41–55) | 0.15 |
| Glucose, mg/dl | 93 (87–102) | 90 (84–95) | 0.03 |
| Heart rate, bpm | 71 (64–78) | 66 (59–75) | 0.02 |
| Systolic BP, mm Hg | 120 (110–130) | 114 (110–122) | 0.09 |
| Diastolic BP, mm Hg | 72 (70–80) | 72 (70–80) | 0.32 |
| Amylin, pg/ml | 54.8 (41–64) | 59.2 (46.4–148) | 0.009 |
| C-peptide, pg/ml | 632.7 (317–1,699) | 794.8 (345–1,489) | 0.94 |
| Ghrelin, pg/ml | 21.1 (16–28) | 21.4 (13.7–36) | 0.79 |
| GIP, pg/ml | 79.5 (27–194) | 77.1 (32–185) | 0.70 |
| GLP-1, pg/ml | 49.1 (24–90) | 67.4 (33.3–99) | 0.08 |
| Glucagon, pg/ml | <13.7 (<13.7–37) | 13.7 (<13.7–37) | 0.12 |
| IL-6, pg/ml | <13.7 (<13.7–14) | <13.7 (<13.7–13.7) | 0.85 |
| Insulin, pg/ml | 708.7 (348–1,695) | 651 (266–1,039) | 0.17 |
| Leptin, pg/ml | 8,035 (4,696–13,085) | 5,370 (3,487–9,496) | 0.001 |
| MCP-1, pg/ml | 358.2 (242–457) | 351 (247–425) | 0.53 |
| PP, pg/ml | 108.5 (63–215) | 151.5 (90–234) | 0.05 |
| PYY, pg/ml | 89.7 (64–162) | 124.4 (62–173) | 0.34 |
| TNF-α, pg/ml | 4.4 (3–6) | 4.2 (3–6) | 0.58 |
Definition of abbreviations: BP = blood pressure; GIP = gastric inhibitory polypeptide; GLP-1 = glucagon-like peptide-1; HDL = high-density lipoprotein; LLN = lower limit of normal as defined by NHANES III; MCP-1 = monocyte chemotactic protein-1; NHANES = National Health and Nutrition Examination Survey; PP = pancreatic polypeptide; PYY = pancreatic peptide YY; TNF = tumor necrosis factor.
All values represented as median (interquartile range).
P value by Mann-Whitney U test.
Association between MetSyn Biomarkers and Lung Function
We assessed the proportion of cases and control subjects that met each MetSyn criteria and exceeded biomarker cut-off values at MME (Table 4). There was a trend toward an increased percentage of individuals with MetSyn in cases compared with control subjects (27% vs. 16%; P = 0.07). Cases had a larger proportion of individuals with higher glucose (28% vs. 16%; P = 0.03) and lower HDL (32% vs. 20%; P = 0.05). The differences in lipid profiles between cases and control subjects were accentuated when individuals had combined abnormalities of triglycerides and HDL (dyslipidemia, defined as triglycerides ≥150 mg/dl and HDL<40 mg/dl; 28% vs. 14%; P = 0.01). In addition, we defined a 66-bpm cutoff value based on the median of the nonsmoking cohort (n = 801). Cases compared with control subjects had a larger proportion with elevated heart rate (65% vs. 48%; P = 0.02). We then defined cutoff values for metabolic analytes using the top quartile for leptin (≥10,300 pg/ml) and amylin (≥116 pg/ml), and the bottom quartile for pancreatic polypeptide (≤89 pg/ml). Cases had a higher proportion of individuals with elevated leptin (42% vs. 21%; P < 0.001) and reduced pancreatic polypeptide (38% vs. 24%; P = 0.04). Elevated amylin was markedly underrepresented in cases (7% vs. 31%; P < 0.0001) (Table 4).
TABLE 4.
PROPORTION OF INDIVIDUALS THAT MEET EACH OF THE CRITERIA AT MEDICAL MONITORING ENTRY
| Biomarker | Cases* FEV1 Less Than LLN | Control Subjects* FEV1 Greater Than or Equal to LLN | P Value† |
| Metabolic syndrome‡ | 0.27 (19/71) | 0.16 (26/166) | 0.07 |
| Triglycerides ≥150 mg/dl | 0.51 (36/71) | 0.46 (76/166) | 0.57 |
| HDL<40 mg/dl | 0.32 (23/71) | 0.20 (33/166) | 0.05 |
| HDL<40 and triglycerides ≥150 mg/dl | 0.28 (20/71) | 0.14 (23/166) | 0.006 |
| Glucose ≥100 mg/dl | 0.28 (20/71) | 0.16 (26/166) | 0.03 |
| Heart rate ≥66 bpm | 0.65 (46/71) | 0.48 (79/166) | 0.02 |
| BMI ≥30 kg/m2 | 0.32 (23/71) | 0.26 (43/166) | 0.34 |
| Leptin ≥10,300 pg/ml§ | 0.42 (30/71) | 0.21 (34/166) | 0.001 |
| Pancreatic polypeptide ≤89 pg/ml‖ | 0.38 (27/71) | 0.24 (40/166) | 0.04 |
| Amylin ≥116 pg/ml§ | 0.07 (5/71) | 0.31 (51/166) | ≤0.0001 |
Definition of abbreviations: BMI = body mass index; HDL = high-density lipoprotein; LLN = lower limit of normal as defined by NHANES III; NHANES = National Health and Nutrition Examination Survey.
Proportions in group (n in group/total).
P value determined by Pearson chi-square.
National Heart Lung and Blood Institute/American Heart Association Criteria.
Values were represented as top quartiles.
Values were represented as bottom quartiles.
We then assessed if any of the biomarkers with significantly different prevalence altered the OR of being a case using logistic regression models adjusted for BMI, age on 9/11, race, and WTC arrival time (Table 5). When biomarkers and clinical parameters were included in the final model, dyslipidemia, elevated heart rate, and elevated leptin significantly increased the odds of being a case: dyslipidemia (OR, 3.03; 95% confidence interval [CI], 1.39–6.16); heart rate greater than or equal to 66 bpm (OR, 2.20; 95% CI, 1.14–4.24); and leptin greater than or equal to 10,300 pg/ml (OR, 3.00; 95% CI, 1.35–6.66). Elevated amylin was strongly protective, decreasing the odds of being a case by 84% (OR, 0.16; 95% CI, 0.06–0.43). Models using these biomarkers as continuous variables also showed significant association with case status (data not shown). Elevated glucose and elevated pancreatic polypeptide became nonsignificant, and consequently were not included in the final multibiomarker model (data not shown).
TABLE 5.
FACTORS AT MME PREDICTING FEV1 LESS THAN LLN AT SPE
| Variable | OR* | P Value | 95% CI | |
| HDL<40 | 1.86 | 0.06 | 0.97–3.57 | |
| HDL<40 and triglycerides ≥150 | 2.67 | 0.01 | 1.32–5.41 | |
| Glucose ≥100 mg/dl | 2.46 | 0.01 | 1.23–4.92 | |
| Heart rate ≥66 bpm | 1.86 | 0.05 | 1.01–3.40 | |
| Leptin ≥10,300 pg/ml | 2.72 | 0.01 | 1.32–5.61 | |
| Amylin ≥116 pg/ml | 0.17 | <0.0001 | 0.06–0.45 | |
| HDL <40 and triglycerides ≥150 | 3.03 | 0.005 | 1.39–6.16 | |
| Final model† | Heart rate ≥66 bpm | 2.20 | 0.02 | 1.14–4.24 |
| Leptin ≥10,300 pg/ml | 3.00 | 0.007 | 1.35–6.66 | |
| Amylin ≥116 pg/ml | 0.16 | <0.0001 | 0.06–0.43 |
Definition of abbreviations: CI = confidence interval; HDL = high-density lipoprotein; LLN = lower limit of normal; MME = medical monitoring entry; OR = odds ratio; SPE = subspecialty pulmonary examination.
Logistic regression adjusted for age at 9/11, body mass index at SPE, race, and World Trade Center arrival time.
Model parameters: −2 log likelihood, 239.163; Cox and Snell R Square, 189; Hosmer-Lemeshow goodness-of-fit chi-square, 7.540; df = 8; P = 0.48.
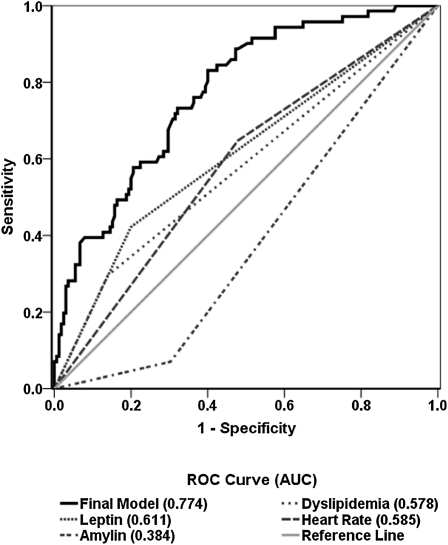
We assessed the ability of the final logistic regression model to predict case status using receiver operating characteristic analysis. The final model had an area under the curve of 0.774 after adjusting for aforementioned confounders (Figure 3). The area under the curve for dyslipidemia, heart rate, leptin, and amylin as single analytes was 0.578, 0.585, 0.611, and 0.384, respectively. This indicates that the final model was more accurate in predicting the abnormal lung function compared with individual biomarkers. The final model was robust, with likelihood ratio of 239.16 and Hosmer-Lemeshow chi-square value of 7.54 (P = 0.48) (Table 5).
Figure 3.
Receiver operator characteristic (ROC) reduced and final models. Final model ROC curve (solid black line) includes leptin greater than or equal to 10,300 pg/ml, amylin greater than or equal to 116 pg/ml, high-density lipoprotein less than 40, triglycerides greater than or equal to 150, and heart rate greater than or equal to 66 bpm, adjusted for age at September 11th, body mass index at subspecialty pulmonary examination, race, and World Trade Center arrival time. Individual components (dotted lines) are crude models with the single predictor indicated, adjusted for age at September 11th, body mass index at subspecialty pulmonary examination, race, and World Trade Center arrival time. AUC = area under the curve.
We assessed the impact of multiple comparisons of cases and control subjects by Mann-Whitney U test for the 13 analytes. The false discovery rate was 0.01 for leptin and 0.05 for amylin in the final model, and was found to be significant (P ≤ 0.05). The interaction terms between leptin and amylin were not significant (data not shown). Applying the model with protective and risk factors to the study group with a case prevalence of 30.1% (95% CI, 24.4–36.4) the following parameters were found: sensitivity 40.8% (95% CI, 29.5–53.2); specificity 86.1% (95% CI, 79.6–90.8); positive predictive value 55.8% (95% CI, 41.4–69.3); and negative predictive value 77.2% (95% CI, 70.3–82.9).
Discussion
Nonsmokers with normal pre-9/11 lung function, dyslipidemia, elevated heart rate, and leptin greater than or equal to 10,300 pg/ml within 6 months of 9/11 had significantly increased risk of developing abnormal lung function over the subsequent 6 years. In contrast, patients with elevated amylin were significantly protected from post-9/11 decline in lung function. Dyslipidemia, elevated heart rate, and leptin were independent risk factors, whereas amylin is a protective factor for subsequent decline in lung function and significantly altered the odds of being a case in a confounder-adjusted multibiomarker model. Elevated glucose and reduced pancreatic polypeptide predicted impaired lung function in single biomarker model, but their effect became nonsignificant in the final model. In this limited cohort of 237 patients, we did not observe a significant impact of arrival time on lung function previously observed (1). The logistic regression model also showed that BMI at SPE was not significantly associated with case status (data not shown). The biomarker models were adjusted for age, BMI at SPE, race, and WTC arrival time, suggesting these potential confounders did not account for the impact of the biomarkers on the observed post-9/11 lung impairment. These novel findings suggest biomarkers of MetSyn may promote lung function impairment.
Cases and control subjects were defined by FEV1 at SPE. Control subjects maintained normal lung function, whereas cases developed abnormal FEV1 by SPE. Using FEV1 as single measure of lung function could lead to misclassification because FEV1 is reduced in restriction and obstruction. In prior investigation, however, it was observed that obstruction caused most abnormal FEV1. Cases in the pilot study have low FEV1/FVC ratio (median, 72; interquartile range, 65–77), confirming that FEV1 less than LLN is a surrogate for obstruction in this population. We therefore believe that FEV1 less than LLN is the single best outcome measure to define lung injury in the FDNY cohort at each point of interest. Although misclassification may occur when using FEV1 less than LLN as a single measure of abnormal lung function, it does not prevent detecting strong associations between biomarkers and lung injury. Cases and control subjects were similar in two important characteristics. First, cases and control subjects arrived at WTC before September 13th, were intensely exposed to dust and smoke, and experienced the same drop in FEV1 within 6 months post-9/11. This suggests a similar response to acute irritant exposure. Second, in the 3 years pre-9/11, there was no significant difference in FEV1 decline, demonstrating that cases do not have accelerated decline of lung function normally. Cases and control subjects differed in two significant ways. Cases had significantly lower, but normal FEV1 in their pre-9/11 PFT with median FEV1 of 88% predicted compared with 108% in control subjects. One explanation of this difference is that their susceptibility to intense irritant exposure produced clinically significant divergence in the groups over time. Another explanation is that subjects with lower baseline pre-9/11 FEV1 were closer to LLN, and therefore needed a smaller relative decline to drop below LLN post-9/11. The second difference is that cases had a median rate of decline of 89 ml/yr, whereas control subjects increased 42 ml/yr between MME and SPE. This demonstrates a disease process in cases that produced progressive loss of lung function for years after the insult. In contrast, control subjects had a small but measurable increase in lung function after the initial insult, indicating some restorative capacity in this group.
This case-control study was nested within a larger, intensively evaluated, longitudinally followed cohort. The differences of biomarkers between cases and control subjects appear before clinical disease presentation, making reverse causation less likely. The appearance of these risk factors before subjects presenting for SPE may indicate that biomarkers of MetSyn are involved in the development of lung function impairment. The study cohort was narrowly defined to eliminate potential confounders, such as prior lung disease, sex, and tobacco use. Cases that developed abnormal lung function and control subjects that preserved lung function after 9/11 came from the same restricted study cohort. Both groups had similar representation of individuals with high BMI and early arrival time. The logistic models effectively adjusted for these confounders. Thus, we were able to observe three significant and independent risk factors that interacted with WTC exposure to increase the risk of developing lung disease in this study. We also found one protective factor, which improved the ability of the model to predict abnormal lung function.
In the subjects, there is a trend for an association between classically defined MetSyn and eventual FEV1 loss. Large cross-sectional studies demonstrate MetSyn and reduced lung function are associated with the obesity component (as defined by BMI) of MetSyn. Obesity is the strongest predictor of abnormal pulmonary function (18, 33). The findings are consistent with other WTC-exposed cohorts in that symptomatic cases had higher BMI than asymptomatic control subjects (34). Individual MetSyn components were stronger predictors of lung disease than the aggregate case definition. This is not surprising because MetSyn diagnostic criteria have been optimized to predict eventual cardiovascular disease and stroke (12). The biomarkers of MetSyn (i.e., amylin and leptin) play a key role in metabolism (35).
The standard clinical definition of MetSyn includes measures of glucose, HDL, and triglycerides. In this study, cases of lung function impairment had increased prevalence of higher glucose and dyslipidemia compared with control subjects. The longitudinal observations show that glucose intolerance and lipid abnormalities predict abnormal lung function and are consistent with results from large cross-sectional studies demonstrating an association between elevated glucose and abnormal pulmonary function (35–39). Mechanisms underlying this association are unclear, but animal models demonstrate that hyperlipidemia is associated with inflammatory lung injury (36, 40). The significant effect of glucose was lost in the final model, suggesting the impact of glucose on lung function is either indirect or weaker than dyslipidemia.
The elevated heart rate observed in cases suggests altered autonomic balance in patients who progressed to abnormal lung function (41–43). Elevated heart rate has been linked to lower levels of pancreatic polypeptide and subsequent elevation in serum glucose (44). These associations are consistent with our observations. The trend toward elevated systolic blood pressure in cases is also consistent with either higher sympathetic tone in cases or higher parasympathetic tone in control subjects.
Elevated leptin is another MetSyn biomarker that is significantly associated with reduced lung function in large cross-sectional studies (45, 46). We show that elevated leptin increases the odds of abnormal FEV1 by more than twofold after adjustment for BMI. A role for this adipocyte-derived cytokine in impaired lung function is plausible because leptin has receptors in the lung and is increased in inflammation (41, 47, 48). The triglycerides and HDL mechanism underlying the association of leptin with lung function is poorly understood.
This report is the first to use human data that describe an association between amylin and lung function. In animal models, there are amylin receptors in the lung (42). Amylin administration produces reduced pulmonary vascular resistance; increased tracheal mucus; and improved sensitivity to butylcholine, an acetylcholine analog (43, 44). There are also data in humans that amylin reduces leptin resistance (35). The reduced risk of developing abnormal lung function in patients with elevated amylin may be mediated by its impact on parasympathetic tone or leptin sensitivity.
This study has several limitations. It uses a single cohort of FDNY rescue workers. In addition, early serum samples from other WTC-exposed cohorts are unavailable, making these findings unique to this cohort. The 13 metabolic analytes combined with five MetSyn clinical parameters had a sensitivity of 41%, suggesting that other pathways to lung disease remain unidentified in this analysis. Serum inflammatory biomarkers are also elevated in COPD exacerbations (49–51). We have recently reported that low α1-antitrypsin, elevated granulocyte-macrophage colony-stimulating factor, and macrophage-derived chemokine are additional risk factors for the development of WTC-related lung injury (52–54). The contribution of the serum glucose became nonsignificant in the final model. It is possible that elevated glucose would be significantly associated with impairment of lung function in a study with more participants. IL-6, a known biomarker of MetSyn, was not significantly different between cases and control subjects. However, levels were below the limits of detection, limiting the ability to interpret this finding. The MetSyn parameters measured are not biomarkers of exposure because all individuals with serum stored during the monitoring arrived at the WTC site within 3 days of its collapse. Because there is no unexposed control group, one cannot determine if WTC exposure is necessary for the observed effect, but it is essential to define the study cohort. For these reasons, replication of these findings in other longitudinally followed populations with and without PM exposure is important.
The results of this study emphasize the importance of rapidly mobilizing resources to conduct medical monitoring and sample banking after a disaster. By identifying individuals at greater risk, early intervention may reduce lung function impairment. Dyslipidemia and elevated leptin and heart rate were observed to be risk factors for development of abnormal lung function. Elevations of serum amylin were found to be protective. MetSyn biomarkers in individuals susceptible to irritant-induced impaired lung function identify pathways that could be responsible for this susceptibility. This insight on protein expression may guide future genetic polymorphism studies in leptin, pancreatic polypeptide, or other regulatory elements. Candidate susceptibility polymorphisms from this cohort could then be assayed in other WTC cohorts to assess their generalizability as risk factors for impaired lung function.
Supplementary Material
Footnotes
Supported by K23HL084191 (A.N.), K24A1080298 (M.D.W.), RO1HL057879 (M.D.W.), HL090316, Al080298A, TL1RR029892; T32 ES007267 (B.N., N.F.); U01CA008617, RO1HL090316 (W.N.R.), NIOSH (U10-OH008243, U10-OH008242) and 1 UL1RR029893.
Author Contributions: conception and design, A.N., M.D.W., and D.J.P.; analysis and interpretation, A.N., M.D.W., S.K., B.N., A.L.C., N.F., K.J.K., and E.J.G.; drafting the manuscript for important intellectual content, A.N., M.D.W., B.N., S.K., H.W.C., W.N.R., K.K., D.J.P., and T.K.A.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1672OC on November 17, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, Kelly KJ. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med 2002;347:806–815 [DOI] [PubMed] [Google Scholar]
- 2.Banauch GI, Dhala A, Alleyne D, Alva R, Santhyadka G, Krasko A, Weiden M, Kelly KJ, Prezant DJ. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med 2005;33:S102–106 [DOI] [PubMed] [Google Scholar]
- 3.Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, Christodoulou V, Arcentales N, Kelly KJ, Prezant DJ. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med 2006;174:312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman DM, Baron SL, Bernard BP, Lushniak BD, Banauch G, Arcentales N, Kelly KJ, Prezant DJ. Symptoms, respirator use, and pulmonary function changes among New York City firefighters responding to the World Trade Center disaster. Chest 2004;125:1256–1264 [DOI] [PubMed] [Google Scholar]
- 5.Banauch GI, Alleyne D, Sanchez R, Olender K, Cohen HW, Weiden M, Kelly KJ, Prezant DJ. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med 2003;168:54–62 [DOI] [PubMed] [Google Scholar]
- 6.Banauch GI, Dhala A, Prezant DJ. Pulmonary disease in rescue workers at the World Trade Center site. Curr Opin Pulm Med 2005;11:160–168 [DOI] [PubMed] [Google Scholar]
- 7.Herbert R, Moline J, Skloot G, Metzger K, Baron S, Luft B, Markowitz S, Udasin I, Harrison D, Stein D, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect 2006;114:1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reibman J, Lin S, Hwang SA, Gulati M, Bowers JA, Rogers L, Berger KI, Hoerning A, Gomez M, Fitzgerald EF. The World Trade Center residents' respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect 2005;113:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagan J, Galea S, Ahern J, Bonner S, Vlahov D, CDC Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center—Manhattan, New York, 2001. JAMA 2002;51:781–784 [PubMed] [Google Scholar]
- 10.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, Gustave J, Zeig-Owens R, Zheng S, Goldring RM, Berger KI, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest 2010;137:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JC, Schwartz J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect 2008;116:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 14.Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J 1998;12:641–645 [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and type 2 diabetes: findings from the British Women's Heart and Health Study. Diabetologia 2004;47:195–203 [DOI] [PubMed] [Google Scholar]
- 16.Lin WY, Yao CA, Wang HC, Huang KC. Impaired lung function is associated with obesity and metabolic syndrome in adults. Obesity (Silver Spring) 2006;14:1654–1661 [DOI] [PubMed] [Google Scholar]
- 17.Fimognari FL, Pasqualetti P, Moro L, Franco A, Piccirillo G, Pastorelli R, Rossini PM, Incalzi RA. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol A Biol Sci Med Sci 2007;62:760–765 [DOI] [PubMed] [Google Scholar]
- 18.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509–516 [DOI] [PubMed] [Google Scholar]
- 19.Hsiao FC, Wu CZ, Su SC, Sun MT, Hsieh CH, Hung YJ, He CT, Pei D. Baseline forced expiratory volume in the first second as an independent predictor of development of the metabolic syndrome. Metabolism 2010;59:848–853 [DOI] [PubMed] [Google Scholar]
- 20.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G, Ostro BD. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health 2006;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, Gerbase MW, Keller R, Kunzli N, Leuenberger P, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med 2007;357:2338–2347 [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med 2010;7:e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquis K, Mattais F, Duguay V, Beazeau A, LeBlanc P, Jobin J, Poirier P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005;25:226–232 [DOI] [PubMed] [Google Scholar]
- 25.Tiengo A, Fadini GP, Avogaro A. The metabolic syndrome, diabetes and lung dysfunction. Diabetes Metab 2008;34:447–454 [DOI] [PubMed] [Google Scholar]
- 26.Hunninghake GM, Kanarek DJ. Pulmonary sequestration supplied by a coronary artery. Thorax 2005;60:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunninghake DB. Cardiovascular disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:44–49 [DOI] [PubMed] [Google Scholar]
- 28.Watz H, Waschki B, Kirsten A, Muller KC, Kretschmar G, Meyer T, Holz O, Magnussen H. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009;136:1039–1046 [DOI] [PubMed] [Google Scholar]
- 29.Naveed B, Comfort A, Ferrier N, Kasturiarachchi K, Rom W, Prezant D, Weiden M, Nolan A. Biomarkers of metabolic syndrome predict accelerated decline of lung function in NYC firefighters that were exposed to WTC particulates. Am J Respir Crit Care Med 2011;183:A4795 [Google Scholar]
- 30.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469–480 [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 2005;13:322–327 [PubMed] [Google Scholar]
- 32.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511–522 [DOI] [PubMed] [Google Scholar]
- 33.Lim SY, Rhee EJ, Sung KC. Metabolic syndrome, insulin resistance and systemic inflammation as risk factors for reduced lung function in Korean nonsmoking males. J Korean Med Sci 2010;25:1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman SM, Maslow CB, Reibman J, Pillai PS, Goldring RM, Farfel MR, Stellman SD, Berger KI. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med 2011;184:582–589 [DOI] [PubMed] [Google Scholar]
- 35.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab 2010;21:473–479 [DOI] [PubMed] [Google Scholar]
- 36.Duan S, Zhang Y, Wu SJ, Jiang LZ, Zhang J, Gan Y, Chen P, Zhao SP, Li GY. Atorvastatin attenuates inflammatory infiltration and vascular remodeling in lung of hypercholesterolemia rabbits. Exp Lung Res 2010;36:573–592 [DOI] [PubMed] [Google Scholar]
- 37.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2002;155:842–848 [DOI] [PubMed] [Google Scholar]
- 38.Klein OL, Meltzer D, Carnethon M, Krishnan JA. Type II diabetes mellitus is associated with decreased measures of lung function in a clinical setting. Respir Med 2011;105:1095–1098 [DOI] [PubMed] [Google Scholar]
- 39.van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest 2010;138:393–406 [DOI] [PubMed] [Google Scholar]
- 40.Duxbury JA, Wright K, Bradley D, Barnes P. Administration of medication in the acute mental health ward: perspective of nurses and patients. Int J Ment Health Nurs 2010;19:53–61 [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol 1999;365:273–279 [DOI] [PubMed] [Google Scholar]
- 42.Wang MW, Young AA, Rink TJ, Cooper GJ. 8-37h-CGRP antagonizes actions of amylin on carbohydrate metabolism in vitro and in vivo. FEBS Lett 1991;291:195–198 [DOI] [PubMed] [Google Scholar]
- 43.Brain SD, Wimalawansa S, MacIntyre I, Williams TJ. The demonstration of vasodilator activity of pancreatic amylin amide in the rabbit. Am J Pathol 1990;136:487–490 [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner U, Fehmann HC, Bredenboker D, Yu F, Barth PJ, von Wichert P, Goke B. The stimulatory effect of amylin on mucus secretion in isolated rat trachea. Res Exp Med (Berl) 1993;193:347–352 [DOI] [PubMed] [Google Scholar]
- 45.Sin DD, Man SF. Impaired lung function and serum leptin in men and women with normal body weight: a population based study. Thorax 2003;58:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickson DA, Burchfiel CM, Petrini MF, Liu J, Campbell-Jenkins BW, Bhagat R, Marshall GD. Leptin is inversely associated with lung function in African Americans, independent of adiposity: the Jackson Heart Study. Obesity 2011;19:1054–1061 [DOI] [PubMed] [Google Scholar]
- 47.Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M. Leptin and leptin receptor expression in asthma. J Allergy Clin Immunol 2009;124:230–237, e231–234 [DOI] [PubMed] [Google Scholar]
- 48.Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of leptin in the respiratory system: an overview. Respir Res 2010;11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, et al. Acute exacerbations of COPD: identification of biological clusters and their biomarkers. Am J Respir Crit Care Med 2011;184:662–671 [DOI] [PubMed] [Google Scholar]
- 50.Sin DD, Miller BE, Duvoix A, Man SF, Zhang X, Silverman EK, Connett JE, Anthonisen NA, Wise RA, Tashkin D, et al. Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verrills NM, Irwin JA, He XY, Wood LG, Powell H, Simpson JL, McDonald VM, Sim A, Gibson PG. Identification of novel diagnostic biomarkers for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:1633–1643 [DOI] [PubMed] [Google Scholar]
- 52.Banauch GI, Brantly M, Izbicki G, Hall C, Shanske A, Chavko R, Santhyadka G, Christodoulou V, Weiden MD, Prezant DJ. Accelerated spirometric decline in New York City firefighters with alpha1-antitrypsin deficiency. Chest 2010;138:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naveed B, Comfort A, Ferrier N, Segal L, Kasturiarachchi K, Kwon S, Chen L, Gordon T, Cohen M, Prophete C, et al. WTC dust induces GM-CSF in serum of FDNY rescue workers with accelerated decline of lung function and in cultured alveolar macrophages. Am J Respir Crit Care Med 2011;183:A4770 [Google Scholar]
- 54.Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, Kwon S, Kasturiarachchi KJ, Cohen HW, Zeig-Owens R, Glaser MS, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.