Abstract
Rationale: The role of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) in the development or progression of interstitial lung disease (ILD) is controversial.
Objectives: To evaluate the association between statin use and ILD.
Methods: We used regression analyses to evaluate the association between statin use and interstitial lung abnormalities (ILA) in a large cohort of smokers from COPDGene. Next, we evaluated the effect of statin pretreatment on bleomycin-induced fibrosis in mice and explored the mechanism behind these observations in vitro.
Measurements and Main Results: In COPDGene, 38% of subjects with ILA were taking statins compared with 27% of subjects without ILA. Statin use was positively associated in ILA (odds ratio, 1.60; 95% confidence interval, 1.03–2.50; P = 0.04) after adjustment for covariates including a history of high cholesterol or coronary artery disease. This association was modified by the hydrophilicity of statin and the age of the subject. Next, we demonstrate that statin administration aggravates lung injury and fibrosis in bleomycin-treated mice. Statin pretreatment enhances caspase-1–mediated immune responses in vivo and in vitro; the latter responses were abolished in bone marrow–derived macrophages isolated from Nlrp3−/− and Casp1−/− mice. Finally, we provide further insights by demonstrating that statins enhance NLRP3-inflammasome activation by increasing mitochondrial reactive oxygen species generation in macrophages.
Conclusions: Statin use is associated with ILA among smokers in the COPDGene study and enhances bleomycin-induced lung inflammation and fibrosis in the mouse through a mechanism involving enhanced NLRP3-inflammasome activation. Our findings suggest that statins may influence the susceptibility to, or progression of, ILD.
Clinical trial registered with www.clinicaltrials.gov (NCT 00608764).
Keywords: statins, interstitial lung disease, pulmonary fibrosis, inflammasome, mitochondrial reactive oxygen species
At a Glance Commentary
Scientific Knowledge on the Subject
Although 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have immunomodulatory and antiinflammatory properties that in theory could be beneficial in the treatment of respiratory disease, they have also been implicated in the development of interstitial lung disease.
What This Study Adds to the Field
Our findings demonstrate that statin use is associated with interstitial lung abnormalities among current and former smokers in the COPDGene study. In addition, we found that statin pretreatment enhanced bleomycin-induced lung inflammation and fibrosis in vivo, augmented mitochondrial reactive oxygen species generation, and enhanced NLRP3 inflammasome activation.
Statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) are commonly prescribed medications whose major indications include the treatment of hypercholesterolemia in the primary (1, 2) and secondary (3, 4) prevention of cardiovascular disease–related morbidity and mortality. In addition to reducing cholesterol levels, statins may have immunomodulatory (5) and antiinflammatory (6) properties that in theory could be beneficial in the treatment of some respiratory diseases (7).
The role of statins in the development of interstitial lung disease (ILD), a group of respiratory diseases characterized by varying degrees of pulmonary interstitial fibrosis and inflammation (8), is controversial. Although some studies evaluating human lung fibroblasts (9) and mice (10) suggest that statins could be beneficial in the treatment of fibrotic lung disease, contrasting observations suggest that statins enhance monocyte secretion of inflammasome-regulated cytokines (e.g., IL-1β and IL-18) (11–13) that may play important roles in the progression of pulmonary fibrosis (14), and numerous case-reports suggest that statins could cause various types of ILD (15).
Recently, we characterized a group of current and former smokers from the COPDGene study who, although previously undiagnosed with ILD, demonstrated chest computed tomography (CT) patterns of increased lung density (which we have previously defined as interstitial lung abnormalities [ILA]) (16). We demonstrated that the subjects with ILA had reductions in TLC and increases in respiratory symptoms (16). Based on case reports of statin-associated ILD (15) and prior data suggesting that smoking is associated with ILA (16–18), we hypothesized that statins would increase the risk for ILA in populations of smokers. To test this hypothesis we first evaluated the association between statin use and ILA in a large cohort of current and former smokers from the COPDGene study. Next, to provide experimental evidence that statins could contribute fibrotic lung disease we demonstrated that statin administration aggravates lung injury and fibrosis in bleomycin-treated mice. To explore the mechanism behind these observations we demonstrate that statin pretreatment enhanced caspase-1–mediated immune responses in vivo and in vitro; the latter responses were abolished in bone marrow–derived macrophages (BMDMs) isolated from Nlrp3−/− and Casp1−/− mice. Finally, we provide further insights by demonstrating that statins enhance Nlrp3-inflammasome activation through mitochondrial reactive oxygen species (mtROS) generation in macrophages. Some of this work was previously presented in abstract form (19).
Methods
For additional details regarding methods, see the online supplement.
Clinical Data
Study design.
The protocols for subject recruitment in COPDGene have been previously described (16). In brief, volumetric chest CT scans were evaluated by three readers (two chest radiologists and one pulmonologist) using a sequential reading method (16). ILA were defined as nondependent changes affecting greater than 5% of any lung zone including nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis. Qualitative CT assessment was performed by readers masked to any additional information including current use of medications. Disease-related demographic parameters and information about use of current medications were designated by self-report. The COPDGene study was approved by the institutional review boards of all participating centers.
Laboratory Data
Cell culture.
J774A.1 macrophages and BMDMs were prepared and maintained as described previously (20). Cells were pretreated with statins 24 hours before incubation with LPS (500 ng/ml) for 4 hours, followed by stimulation with adenosine triphosphate (ATP). Glyburide was added to the medium 15 minutes before ATP treatment (20). MitoTEMPO was added to the medium 1 hour before LPS priming as described previously (20).
Mice.
Male C57B/L6 mice (8 wk old; 18–22 g) were used for in vivo experiments. Bleomycin (Hospira Inc., Adelaide, Australia) at a dose of 0.1 U/mice in 50 μl normal saline was instilled into lung intratracheally to induce lung inflammation and fibrosis, as previously described (21, 22). Mice were treated with pravastatin (40 mg/kg/d) or phosphate-buffered saline intraperitoneally daily starting from 3 days before bleomycin instillation. All experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal care and use for all experiments was approved by the Harvard Medical Area Standing Committee on Animals of Harvard Medical School.
Fluorescence staining.
After treatment, cells were fixed with 4% paraformaldehyde, and followed with the immunofluorescence staining protocol as described previously (23). MitoTracker green, MitoSOX red, and DAPI were used to label total mitochondria, mtROS, and the nuclei, respectively. Stained samples were fixed onto the slides and viewed with Olympus (Tokyo, Japan) Fluoview-FV10i Confocal and Olympus FSX100 fluorescence microscopy. Fluorescence picture was simultaneously captured by standard confocal imaging techniques.
Statistical Analysis
In COPDGene, bivariate analyses were conducted with Fisher exact test (for categorical variables) and two-tailed t test or Wilcoxon rank-sum test (for continuous variables) where appropriate. Logistic regression models were used in multivariate analyses to study the relation between ILA and statin use. All of the adjusted models included age; sex; race; pack-years of smoking; current smoking status; chronic obstructive pulmonary disease (defined as ≥ Global Initiative for Chronic Obstructive Pulmonary Disease stage 2) (24); self-report of either high cholesterol or coronary artery disease; and additional covariates where indicated. We estimated the risk of ILA attributable to statin use in smokers from COPDGene (25). All analyses were performed using Statistical Analysis Software version 9.1 (SAS Institute, Cary, NC). Forest plots were generated using the rmeta package as implemented in R version 2.9 (26). For the laboratory data, means ± SD are reported. Student t test was used for statistical analysis. P values less than 0.05 were considered statistically significant.
Results
Of the 2,508 subjects from COPDGene, 2,207 (88%) provided information about current use (and type of) statin prescribed. Of these 2,207 subjects, 2,115 (96%) had a CT available and were included in these analyses. Cardiovascular disease characteristics and medications of subjects stratified by ILA status are presented in Table 1 (additional baseline characteristics have been published previously [16], and baseline characteristics stratified by statin use are included in Table E1 in the online supplement). In addition to increases in statin use, in univariate analyses subjects with ILA were more likely to have coronary artery disease, diabetes, high blood pressure, and to be taking β-blockers (Table 1).
TABLE 1.
CHARACTERISTICS OF SMOKERS FROM COPDGENE STRATIFIED BY THE PRESENCE OF RADIOGRAPHIC INTERSTITIAL LUNG ABNORMALITIES
| Number (%) |
|||
| Variable* | No ILA(n = 1184 [87%]) | ILA(n = 172 [13%]) | P Value† |
| Demographic parameters | |||
| High cholesterol | 504 (43%) | 82 (48%) | 0.22 |
| Coronary artery disease | 69 (6%) | 19 (11%) | 0.02 |
| Diabetes | 141 (12%) | 30 (17%) | 0.05 |
| High blood pressure | 481 (41%) | 90 (52%) | 0.005 |
| Previous myocardial infarction | 59 (5%) | 12 (7%) | 0.27 |
| Previous cerebrovascular accident | 29 (2%) | 8 (5%) | 0.12 |
| Previous thromboembolic disease | 42 (4%) | 9 (5%) | 0.28 |
| Medications | |||
| Aspirin | 186 (16%) | 37 (22%) | 0.06 |
| β-Blockers | 139 (12%) | 30 (17%) | 0.04 |
| Angiotensin-converting enzyme inhibitors | 155 (13%) | 18 (10%) | 0.39 |
| Gemfibrozil | 1 (<1%) | 0 (0%) | 1.0 |
| Niacain | 4 (<1%) | 2 (1%) | 0.17 |
| Fish oil | 27 (2%) | 6 (3%) | 0.30 |
| Statins | |||
| All statins | 315‡ (27%) | 66 (38%) | 0.002 |
| Lipophilic statins | 280 (24%) | 51 (32%) | 0.03 |
| Hydrophilic statins | 33 (4%) | 15 (12%) | <0.001 |
Definition of abbreviation: ILA = interstitial lung abnormalities.
Data on disease-related demographic and medication variables were determined by self-report. Data missing for high blood pressure, previous cerebrovascular accident, and previous thromboembolic disease (n = 1).
P values compare those with ILA with those without ILA using Fisher exact tests.
Three subjects reporting statin medication use did not provide specific drug information and are included in this group.
Statins and ILA
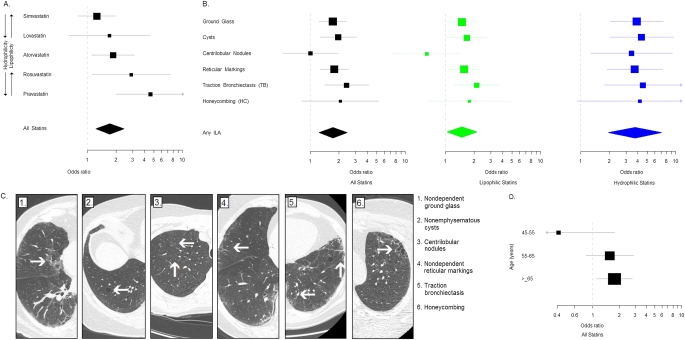
In COPDGene, 38% of subjects with ILA were taking statins compared with 27% of subjects without ILA (Table 1, Figure 1A). Compared with those not taking statins, statin users had a 60% increase in their odds to have ILA after adjustment for relevant covariates including a history of high cholesterol or coronary artery disease (Table 2). There was no significant decrement in the association between statins and ILA in models adjusting for additional cardiovascular medications and diseases (Table 2). With the exception of statin use, in multivariate models no additional cardiovascular medication or disorder was positively associated with ILA. In contrast, current use of angiotensin-converting enzyme inhibitors was inversely associated with ILA (odds ratio [OR], 0.56; 95% confidence interval [CI], 0.32–0.98; P = 0.04). In COPDGene, the risk of ILA attributable to statin use was 14% (95% CI, 1–22%).
Figure 1.
Statin use is associated with interstitial lung abnormalities (ILA). (A) Odds ratios (OR) for the association between individual statins (arranged in order of increasing hydrophilicity as measured by decreasing logD) and ILA. ORs and 95% confidence intervals (CIs) are represented by boxes (with their size proportional to the sample size) and bars, respectively. The overall association between statin use and ILA is represented by a diamond. The upper limit of the 95% CI for the association between pravastatin and ILA is greater than 10 (95% CI, 1.99–10.70). (B) OR for the association between statins and specific radiologic features. Black boxes (with their size proportional to the sample size) and bars represent ORs and 95% CIs for the association between statins overall and specific radiologic features. Green boxes and bars represent ORs and 95% CIs for the association between lipophilic statins and specific radiologic features. Blue boxes and bars represent ORs and 95% CIs for the association between hydrophilic statins and specific radiologic features. The association between statin use (including all statins, lipophilic, and hydrophilic statins) and ILA in general is represented by diamonds. The upper limits of the 95% CIs for the association between hydrophilic statins and bronchiectasis and honeycombing are greater than 10 (95% CI, 1.78–11.40, and 95% CI, 0.89–19.70, respectively). (C) Axial volumetric chest computed tomographic (CT) images of the hemithorax representing specific radiologic findings of ILA present in COPDGene subjects on statin medications. 1: Nondependent ground-glass present in a subject taking simvastatin. 2: Nonemphysematous cysts present in a subject taking lovastatin. 3: Centrilobular nodules present in a subject taking rosuvastatin. 4: Nondependent reticular markings present in a subject taking atorvastatin. 5: Traction bronchiectasis present in a subject taking rosuvastatin. 6: Honeycombing present in a subject taking pravastatin. (D) OR for the association between statins and ILA stratified by age (including subjects aged 45–55 yr [n = 415], subjects aged 55–65 yr [n = 451], and in subjects >65 yr old [n = 490]). Black boxes (with their size proportional to the sample size) and bars represent ORs and 95% CIs for the association between statins overall and ILA stratified by age.
TABLE 2.
UNIVARIATE AND MULTIVARIATE ANALYSES OF ASSOCIATION BETWEEN STATINS AND INTERSTITIAL LUNG ABNORMALITIES
| Odds Ratio (95% Confidence Interval) P Value |
|||
| Unadjusted | Adjusted Model 1* | Adjusted Model 2† | |
| All statins | 1.72 (1.23–2.40) | 1.60 (1.03–2.50) | 1.62 (1.02–2.58) |
| 0.002 | 0.04 | 0.04 | |
| Lipophilic statins | 1.49 (1.04–2.14) | 1.36 (0.85–2.18) | 1.37 (0.83–2.26) |
| 0.03 | 0.20 | 0.22 | |
| Hydrophilic statins | 3.73 (1.96–7.09) | 3.39 (1.64–7.02) | 4.00 (1.86–8.59) |
| <0.001 | 0.001 | <0.001 | |
Model 1: A multivariate model evaluating the association between statin use and interstitial lung abnormalities including adjustment for age, sex, race, body mass index, pack-years of smoking, current smoking status, chronic obstructive pulmonary disease (≥Global Initiative for Chronic Obstructive Pulmonary Disease Stage 2), and having either high cholesterol or coronary artery disease.
Model 2: A multivariate model evaluating the association between statin use and interstitial lung abnormalities including adjustment with the same covariates as Model 1 and additional adjustments for high blood pressure, diabetes, histories of myocardial infarction, cerebrovascular accident, venous thromboembolism, and additional cardiac medications (including aspirin, β-blockers, angiotensin-converting enzyme inhibitors, gemfibrozil, niacin, and fish oil).
Hydrophilic versus Lipophilic Statins
The association between statin use and ILA varied by statin type (Table 2, Figure 1A). The prevalence of ILA varied from 8% in subjects on simvastatin to 23% for subjects on pravastatin (see Table E2). There was evidence that statins with increased hydrophilicity (as measured by decreasing logD) (27, 28) (Figures 1A and 1B) were associated with increases in the odds for ILA (P < 0.001 for trend). Pravastatin (a hydrophilic statin) was the statin drug most strongly associated with ILA (OR, 4.61; 95% CI, 1.99–10.70; P < 0.001). There was no evidence for increased coronary artery disease among subjects taking hydrophilic statins (OR, 0.90; 95% CI, 0.49–1.66; P = 0.73).
Statins, Specific Radiologic Features, and Age
Most CT radiologic features of ILA were associated with statin use (Figures 1B and 1C, see Table E3). In addition to radiologic features that can be identified in inflammatory lung diseases (e.g., ground-glass), statin use was also associated with radiologic features more typical of pulmonary fibrosis (e.g., statin users had a 125% increase in their odds of having traction bronchiectasis; OR, 2.25; 95% CI, 1.33–3.82; P = 0.003). Although there was no evidence for an interaction between statin use and many relevant covariates (including current use and pack-year history of tobacco smoke exposure), there was significant evidence that age modified the association between statin use and ILA (P = 0.04 for the interaction) (Figure 1D; see online supplement).
Statins Exacerbate Bleomycin-induced Fibrosis in the Mouse
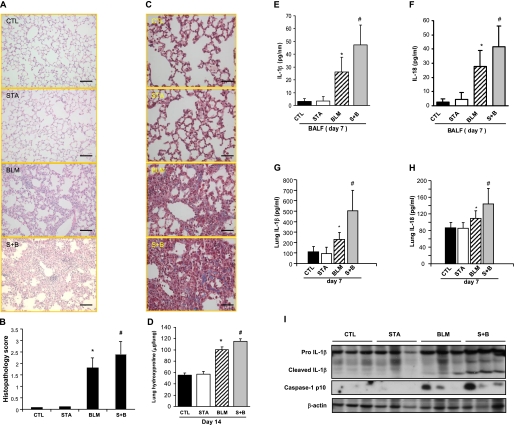
To investigate the effect of statins on lung injury and fibrogenesis in an experimental model, mice were pretreated with pravastatin (based on our clinical findings) before intratracheal bleomycin administration. Lungs from mice treated with pravastatin and bleomycin showed increased lung fibrosis (Figures 2A and 2B), HT15 trichrome staining (Figure 2C), and collagen deposition (Figure 2D) at Day 14 compared with mice exposed to bleomycin alone (similar findings were noted in the mice at Day 7) (see Figures E1A and E1B). Comparably, pretreatment with pravastatin enhanced weight loss and inflammatory cell recruitment in mice (see Figures E1C and E1D). The pravastatin and bleomycin experimental group also had significant increases in IL-1β and IL-18 in the bronchoalveolar lavage fluid and in the lung homogenate (Figures 2E–2H) compared with mice treated with bleomycin alone; this correlated with increased caspase-1 activation and cleaved IL-1β expression (Figure 2I). Pravastatin alone had no impact on histologic changes and collagen deposition in the absence of bleomycin instillation (Figures 2A–2D).
Figure 2.
Statin increases bleomycin-induced lung inflammatory response and fibrotic changes in mice. (A) Sections of paraffin-embedded lung tissue from mice with different treatments were stained with hematoxylin and eosin (original magnification ×200). Bar, 20 μm, Day 14; BLM = bleomycin; CTL = control; S+B = pravastatin + bleomycin; STA = pravastatin. (B) Semiquantitative histopathology score was shown. (C) Sections of paraffin-embedded lung tissue from mice treated with different treatments were stained with Masson trichrome (original magnification ×400). Bar, 40 μm, Day 14; BLM = bleomycin; CTL = control; S+B = pravastatin + bleomycin; STA = pravastatin. (D) Pulmonary collagen deposition was quantified and expressed as micrograms of hydroxyproline per left lung. (E and F) Concentration of IL-1β and IL-18 in bronchoalveolar lavage fluid (BALF) at Day 7 was measured by ELISA. (G and H) Concentration of IL-1β and IL-18 in lung homogenates was measured by ELISA. (I) Lung homogenates were analyzed by immunoblotting for IL-1β and caspase-1. BLM = mice received with intraperitoneal injection of phosphate-buffered saline (PBS) and intratracheal instillation of bleomycin; CTL = mice received with intraperitoneal injection of PBS and intratracheal instillation of PBS; S+B = mice received with intraperitoneal injection of pravastatin and intratracheal instillation of bleomycin; STA = mice received with intraperitoneal injection of pravastatin and intratracheal instillation of PBS. Day 7: CTL, n = 5; STA, n = 5; BLM, n = 8; S+B, n = 9. Day 14: CTL, n = 5; STA, n = 5; BLM, n = 11; S+B, n = 11. *P < 0.05 compared with CTL group. #P < 0.05 compared with BLM group.
Statins Enhance Activation of the NLRP3 Inflammasome
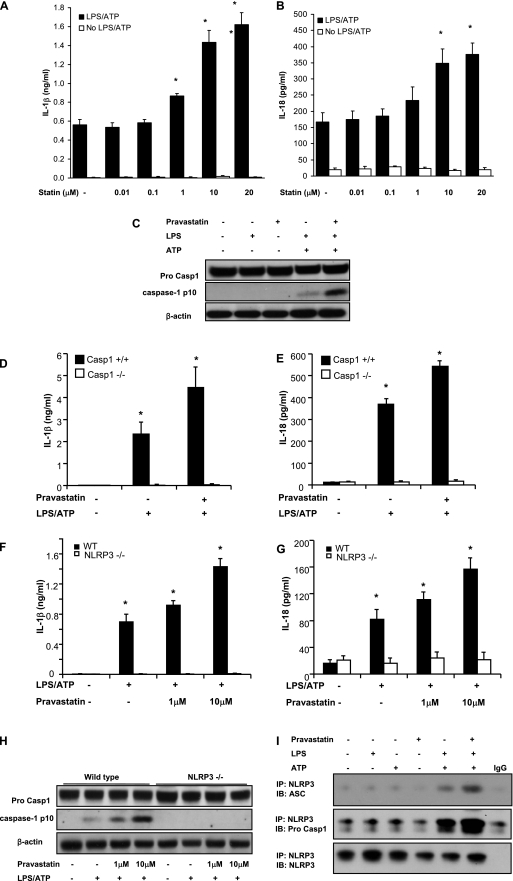
To further explore the mechanism behind these observations, J774A.1 macrophages were pretreated with pravastatin for 24 hours followed by stimulation with LPS and ATP (20). Pretreatment with pravastatin increased IL-1β and IL-18 secretion in a dose-dependent manner (Figures 3A and 3B) (similar findings were noted with atorvastatin [see Figures E2A and E2B]) and resulted in accumulation of the cleaved form of caspase-1 (p10) in stimulated cells (Figure 3C, see Figure E2C). No effect of statin was noted in the absence of LPS and ATP stimulation (Figures 3A–3C, see Figures E2A and E2B). The effect of pravastatin on IL-1β and IL-18 secretion was dependent on caspase-1, as demonstrated by the loss of this effect on BMDMs from Casp-1−/− mice (Figures 3D and 3E). To determine which inflammasome pathway was involved in the promotion of caspase-1 activation by pravastatin, BMDM from Nlrp3−/− mice were subjected to LPS and ATP stimulation. The stimulatory effect of pravastatin pretreatment on secretion of IL-1β and IL-18 and caspase-1 activation was abolished in Nlrp3−/− BMDM (Figures 3F–3H). Similarly, glyburide, a NLRP3 inflammasome inhibitor, prevented the enhancement of IL-1β and IL-18 secretion by pravastatin (see Figures E2D and E2E). Pravastatin pretreatment also enhanced the molecular interactions between NLRP3 and apoptosis-associated speck-like protein containing a CARD (ASC; an NLRP3 inflammasome cofactor necessary to activate caspase-1), and between NLRP3 and caspase-1, further supporting a role for NLRP3 in pravastatin-mediated inflammasome activation (Figure 3I). Pravastatin had no effect on the protein expression of P2X7 receptor, apoptosis-associated speck-like protein containing a CARD (ASC), and LPS-induced pro–IL-1β (see Figure E2F).
Figure 3.
Statin enhances NLRP3 inflammasome activation in macrophages. (A and B) Pravastatin pretreatment enhances the secretion of IL-1β and IL-18 in macrophages stimulated with LPS and adenosine triphosphate (ATP). J774A.1 macrophages were pretreated with pravastatin or vehicle (phosphate-buffered saline) for 24 hours and then incubated with LPS (500 ng/ml) for 4 hours, followed by stimulation with ATP (5 mM) for 1 hour. Secretion of IL-1β and IL-18 into the media was measured by ELISA. *P < 0.01 versus cells treated with LPS and ATP. (C) Pravastatin pretreatment increases caspase-1 activation. J774A.1 macrophages were pretreated with pravastatin (10 μM) for 24 hours and then incubated with LPS (500 ng/ml) for 4 hours, followed by stimulation with ATP (5 mM) for 15 minutes. Cell lysates were analyzed by immunoblotting for caspase-1. (D and E) Bone marrow–derived macrophages from caspase-1−/− mice were pretreated with pravastatin (10 μM) for 24 hours and then incubated with LPS (200 ng/ml) for 4 hours, followed by stimulation with ATP (5 mM) for 1 hour. Secretion of IL-1β and IL-18 was analyzed by ELISA. *P < 0.01 versus caspase-1−/− cells treated with LPS and ATP. (F–H) NLRP3 inflammasome is involved in the role of pravastatin on caspase-1 activation. Bone marrow–derived macrophages from NLRP3−/− mice were pretreated with pravastatin (10 μM) for 24 hours and then incubated with LPS (200 ng/ml) for 4 hours, followed by stimulation with ATP (5 mM) for 15 minutes (H) or 1 hour (F and G). Secretion of IL-1β and IL-18 was analyzed by ELISA. *P < 0.01 versus NLRP3−/− cells treated with LPS and ATP. Cell lysates were analyzed by immunoblotting for caspase-1. (I) Pravastatin increases interaction of NLRP3 inflammasome-associated molecules. J774A.1 macrophages were pretreated with pravastatin (10 μM) for 24 hours and then incubated with LPS (500 ng/ml) for 4 hours, followed by stimulation with ATP (5 mM) for 15 minutes. Cell lysates were analyzed for interaction of NLRP3 and apoptosis-associated speck-like protein containing a CARD (ASC) or NLRP3 and pro–caspase-1 by immunoprecipitation. WT = wild type.
Activation of NLRP3 by Statins Is Dependent on Up-regulation of mtROS
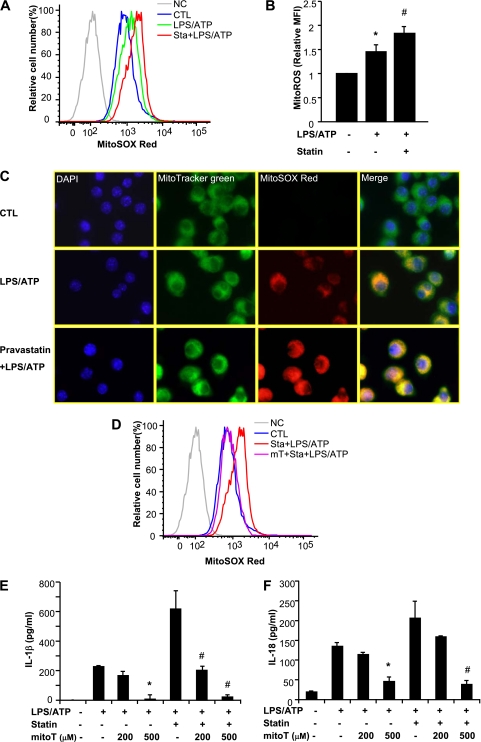
MtROS is a critical factor for NLRP3 inflammasome activation (20, 23). To determine the effect of pravastatin on mtROS, MitoSOX red (a membrane-permeable fluorogenic dye for the selective detection of mitochondrial O2−) was used to detect mtROS. Pravastatin enhanced mtROS generation in stimulated cells (Figures 4A and 4B). Confocal fluorescence microscopy (using MitoTracker green to label total mitochondria) confirmed that mtROS colocalized to mitochondria in stimulated cells (Figure 4C). Scavenging of mtROS by MitoTEMPO (a derivative of the antioxidant TEMPO that concentrates in the mitochondrial matrix) resulted in reduced fluorescence intensity of MitoSOX (Figure 4D, see Figures E3A and E3B) and inhibited IL-1β and IL-18 secretion in a dose-dependent manner (Figures 4E and 4F), implying a direct role for mtROS in inflammasome activation by pravastatin. In contrast, mitoTEMPO did not affect tumor necrosis factor secretion, a cytokine not influenced by caspase-1 activation (see Figure E3C).
Figure 4.
Statin pretreatment increases mitochondrial reactive oxygen species (mtROS) generation. (A) LPS-primed J774A.1 macrophages were stained with MitoSOX for 15 minutes before stimulation with adenosine triphosphate (ATP) in the absence or presence of pravastatin, and then analyzed by flow cytometric analyses. Representative histograms are shown. CTL = control; NC = negative control—no staining. (B) Relative mean fluorescence intensity (MFI) of MitoSOX was represented. *P < 0.01 versus untreated cells. #P < 0.05 versus cells stimulated with LPS and ATP. (C) MitoTracker was used to show mitochondria, MitoSOX red was used to show ROS, and DAPI was used to show the nuclei of the cells. MitoSOX red labeled ROS was shown in mitochondria and was increased by pravastatin pretreatment, compared with LPS-ATP treatment only. (D) J774A.1 macrophages were pretreated with MitoTEMPO (500 μM) 1 hour before LPS treatment in the absence or presence of pravastatin. Level of mtROS in cells was analyzed by MitoSOX labeling. (E and F) Macrophages were pretreated with MitoTEMPO 1 hour before LPS treatment in the absence or presence of statin, followed by ATP stimulation for 1 hour. Cytokine secretion was analyzed by ELISA. *P < 0.01 versus cells treated with LPS and ATP. #P < 0.01 versus statin-pretreated cells stimulated with LPS and ATP.
Discussion
Our findings in a large cohort of smokers demonstrate that statin use may increase the risk of developing radiographic evidence of ILD including findings characteristic of pulmonary fibrosis. This risk may be modified by the hydrophilicity of statin and the age of the subject. In support of our clinical findings, we demonstrate that statin use exacerbates bleomycin-induced lung fibrosis in mice. Further, our study demonstrates that statin pretreatment increases mtROS in stimulated cells, resulting in increased NLPR3 inflammasome-mediated immune responses.
Our data provide support to evidence from numerous case reports (15) suggesting that statins may cause ILD. Although prior case-control studies limited to the association between statin use and idiopathic pulmonary fibrosis alone (29, 30) have not demonstrated significant associations, these studies are limited by small sample size (29) and the potential for selection bias in control subjects (29), and include cases selected by diagnostic codes alone (30). In contrast, our study includes the CT characterization of a large cohort (>2,100 subjects), and presents associations between statins and ILA scored by multiple readers masked to information about current medication use.
Although our data, and those of others (30), support an association between cardiovascular disease the development of fibrotic lung disease, several lines of evidence suggest that cardiovascular disease alone is unlikely to entirely explain our findings: (1) the association between statin use and ILA is independent of presence of cardiovascular disease and additional medications commonly prescribed for cardiovascular disease; (2) although hydrophilic statin users in COPDGene were at greater risk for ILA, this was not coupled with an increased report of coronary artery disease (in fact, users of hydrophilic statin were slightly less likely to report coronary artery disease compared with users of lipophilic statin); and (3) we demonstrate experimentally that statin use can exacerbate lung fibrosis in mice.
Comparable with our clinical findings, our results in mice indicate that statin administration enhances bleomycin-induced caspase-1–mediated immune response in the lung. Moreover, we show that enhanced activation of caspase-1 correlates with an increase in fibrotic change in the lung treated with bleomycin and pravastatin. The effect of statin administration on cytokine secretion was exerted on upstream steps of NLRP3 based on the following observations: (1) NLRP3 deficiency completely impaired the effect of statin pretreatment on IL-1β and IL-18 secretion; (2) formation of NLRP3 inflammasome induced by LPS and ATP was increased by statin pretreatment; and (3) the effect of statin on the cytokine secretion was inhibited by glyburide, which suppresses the activation pathway upstream of the NLRP3 inflammasome but downstream of P2X7 receptor. These results suggest that statins target the activation pathway upstream of NLRP3 inflammasome, and further implicate activation of the NLRP3 inflammasome in fibrotic lung disease (31–33).
Although mtROS are important for various mitochondrial functions including biosynthesis of many molecules and catabolic pathways, it has been shown that excess mtROS generation hyperactivates immune responses (20, 23). Our data show that statin administration increases immune responses in our inflammasome-activating models; however, it is still unclear how statins enhance mtROS in the stimulated macrophages. Of note, in blocking the synthesis of cholesterol, statins block the synthesis of ubiquinone, which is essential in mitochondrial electron transport (34). Although not all studies demonstrate that statins increase mtROS (35), some of these discrepancies may be explained by different stimuli and differences in tissue specificity (35, 36).
Our findings contrast with two previous reports that suggest that statins could ameliorate bleomycin-induced lung injury (10, 37). These studies differ in the type of statin and the dose of bleomycin used (Ou and coworkers used simvastatin and instilled bleomycin 15- to 20-fold higher [0.3 U/10 g] than standard dosing for such experiments) (37), and in the timing of statin administration (Kim and coworkers administered pravastatin coincident with bleomycin instillation) (10). Importantly, it should be noted that pravastatin alone did not enhance NLRP3 inflammasome activation in vitro or aggravate the lung injury in vivo, in the absence of proinflammatory challenge. Moreover, it may be relevant that all subjects from COPDGene were current or former smokers, because cigarette smoke alone may result in pulmonary inflammation through NLRP3 inflammasome-mediated pathways in humans (38) and in mouse models (39).
One limitation of this study is the lack of correlative data allowing us to relate in human samples the mechanisms described in the mouse. Many prior studies have demonstrated that statins contribute to the release of inflammasome-related cytokines including IL-1β and IL-18 in human peripheral blood monocytes through a caspase-1–dependent mechanism. Up-regulation of the inflammasome in response to statins is blocked by the reintroduction of downstream products of the cholesterol synthesis pathway including mevalonate and geranylgeraniol (40–43). In these studies human peripheral blood monocytes frequently require a stimulus, such as Toll-like receptor ligands (e.g., LPS) to activate the inflammasome. However, in contrast to peripheral blood monocytes (or monocytic cell lines), recent evidence suggests that human alveolar macrophages may require ATP as an additional stimulus to activate the inflammasome (44). This may have relevance to our findings because studies have demonstrated that, although smoking up-regulates extracellular ATP (45), the regulation of intracellular ATP by statins is drug dependent (e.g., simvastatin, lovastatin, and fluvastatin result in decreases in cellular ATP levels, whereas atorvastatin, rosuvastatin, and pravastatin do not) (46).
Our study has several additional limitations. First, although biopsies were not obtained in this cohort, it is important to note that biopsies on similarly ascertained cohorts of patients with ILA (47–49) have demonstrated histopathologic evidence of ILD (idiopathic interstitial pneumonias in particular). Second, although we did not find evidence that the association of statin use and ILA was modified by either current use of tobacco or the intensity of tobacco smoke exposure, it is worth noting that all subjects in COPDGene have a history of at least 10 pack-years of smoking. Our group (16, 18) and others (17) have previously demonstrated that smoking is associated with ILA. Therefore, it is possible that the association between statin use and ILA is limited to current and former smokers. Third, we do not have information on the duration of therapy or drug dosage in most of the patients on statins. However, it is unlikely that the variability in effective drug dosage alone explains the increased odds for ILA we demonstrate among subjects taking hydrophilic statins (at commonly prescribed doses there is a large difference in the expected cholesterol reduction between pravastatin and rosuvastatin) (27). Fourth, although we provide experimental evidence in support of our clinical findings, further experimental work in mice exposed to tobacco smoke would be helpful to more precisely define the combined effect of smoking and statin use. Finally, although prior studies have implicated a role for the NLRP3 inflammasome in fibrotic lung disease (31–33), further studies in people are required to determine the extent to which NLRP3 inflammasome activation plays a role in statin-induced ILD and ILD in general.
We urge caution in extrapolating our findings to the care of patients. Although increases in the risk of ILA, and radiologic features of pulmonary fibrosis, are causes for concern, these risks do not likely outweigh the substantial benefits of statin therapy in patients with cardiovascular disease. In addition, our findings do not rule out the possibility that statin use could benefit some patients with respiratory disease. Instead, we believe that clinicians should be aware that radiographic evidence of ILD, much like myopathy (50), can occur in some patients on statins.
Conclusions
In summary, our study demonstrates that statin use is associated with ILA among current and former smokers in the COPDGene study. We found that statin pretreatment enhanced bleomycin-induced lung inflammation and fibrosis in vivo, augmented mtROS generation, and enhanced NLRP3-inflammasome activation. Although our study raises concerns about the potential role for statins in the development or progression of ILD in settings of enhanced NLRP3 inflammasome activation, these risks likely do not outweigh the substantial benefits of statin therapy in patients with cardiovascular disease. Instead, our findings suggest that the use of statins in patients with ILD should be reevaluated.
Supplementary Material
Acknowledgments
The authors thank Emeka Ifedigbo, Chang Hyeok An, Seon Jin Lee, Mark A. Perrella, Bonna Ith, Manuela Cernadas, Elizabeth Henske, and Steven E. Seltzer for technical support and critical discussion.
Footnotes
COPDGene is supported by NIH grants U01 HL089897 and U01 HL089856. J.F.X. is supported by a National Nature Science Foundation of China grant NSF81170003. G.R.W. is supported by NIH grants K23 HL089353 and R01 HL107246, and an award from the Parker B. Francis Foundation. H.H. is support by NIH grant 5R21CA116271-2. B.E.H. is supported by 2T15LM007092-16 from the National Library of Medicine. I.O.R. is supported by NIH grant K23 HL087030. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Novartis, and Sunovion. The laboratory work was supported by R01-HL60234, R01-HL55330, R01-HL079904, and a FAMRI clinical innovator award to A.M.K.C. G.M.H. is supported by NIH grant K08 HL092222.
Author Contributions: J.-F.X., G.R.W., K.N., H.H., S.W.R., I.O.R., A.M.K.C., and G.M.H. conceived of the study with assistance from D.M., H.-P.L., J.-M.Q., D.A.L., J.D.C., and E.K.S.; J.-F.X. and K.N. did the in vitro experiments; J.-F.X., K.N., and A.S.P. did the in vivo experiments; acquisition of the clinical data, G.R.W., H.H., I.E.F., M.N., Y.O., T.Y., J.C.R., R.S.J.E., A.A.D., B.E.H., C.E.C., F.J.M., M.K.H., D.A.L., J.D.C., E.K.S., I.O.R., and G.M.H.; statistical analysis and interpretation of the clinical data, G.R.W., H.H., F.J.M., M.K.H., D.A.L., J.D.C., E.K.S., I.O.R., and G.M.H.; administrative, technical, or material support, I.E.F., R.S.J.E., A.A.D., B.E.H., C.E.C., and K.D.; and drafting the manuscript, J.-F.X., G.R.W., K.N., S.W.R., A.M.K.C., and G.M.H.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201108-1574OC on January 12, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997;278:313–321 [PubMed] [Google Scholar]
- 2. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22. [DOI] [PubMed]
- 3. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389. [PubMed]
- 4.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335:1001–1009 [DOI] [PubMed] [Google Scholar]
- 5.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol 2006;176:7657–7665 [DOI] [PubMed] [Google Scholar]
- 6.Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 2004;109(Suppl. 1):II18–II26 [DOI] [PubMed] [Google Scholar]
- 7.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax 2006;61:729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed]
- 9.Tan A, Levrey H, Dahm C, Polunovsky VA, Rubins J, Bitterman PB. Lovastatin induces fibroblast apoptosis in vitro and in vivo. A possible therapy for fibroproliferative disorders. Am J Respir Crit Care Med 1999;159:220–227 [DOI] [PubMed] [Google Scholar]
- 10.Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim KH, et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol 2010;37:1055–1063 [DOI] [PubMed] [Google Scholar]
- 11.Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes. J Immunol 2006;176:5284–5292 [DOI] [PubMed] [Google Scholar]
- 12.Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med 2009;179:903–913 [DOI] [PubMed] [Google Scholar]
- 13.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 2010;207:535–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H. Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am J Respir Cell Mol Biol 2009;41:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez AB, Karas RH, Alsheikh-Ali AA, Thompson PD. Statins and interstitial lung disease: a systematic review of the literature and of food and drug administration adverse event reports. Chest 2008;134:824–830 [DOI] [PubMed] [Google Scholar]
- 16.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011;364:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009;180:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San Jose Estepar R, Silverman EK, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol 2010;17:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Nakahira K, Li H, Ryter S, Choi AM. Statins promote NALP3 inflammasome activation through increased mitochondrial ROS generation in macrophages. Presented at the American Thoracic Society International Conference. May, 2011, Denver, Colorado.
- 20.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011;12:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 2006;203:2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, Morse D. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol 2005;166:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;475:122. [DOI] [PubMed] [Google Scholar]
- 24.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 25.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313 [DOI] [PubMed] [Google Scholar]
- 26.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria; 2009.
- 27.White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol 2002;42:963–970 [PubMed] [Google Scholar]
- 28.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006;112:71–105 [DOI] [PubMed] [Google Scholar]
- 29.Ponnuswamy A, Manikandan R, Sabetpour A, Keeping IM, Finnerty JP. Association between ischaemic heart disease and interstitial lung disease: a case-control study. Respir Med 2009;103:503–507 [DOI] [PubMed] [Google Scholar]
- 30.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med 2008;178:1257–1261 [DOI] [PubMed] [Google Scholar]
- 31.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117:3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA 2008;105:9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol 2006;291:C1208–C1212 [DOI] [PubMed] [Google Scholar]
- 35.Ito M, Adachi T, Pimentel DR, Ido Y, Colucci WS. Statins inhibit beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes via a Rac1-dependent mechanism. Circulation 2004;110:412–418 [DOI] [PubMed] [Google Scholar]
- 36.Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, Piquard F, Geny B, Zoll J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a mitohormesis mechanism involving reactive oxygen species and PGC-1. Eur Heart J (In press) [DOI] [PMC free article] [PubMed]
- 37.Ou XM, Feng YL, Wen FQ, Huang XY, Xiao J, Wang K, Wang T. Simvastatin attenuates bleomycin-induced pulmonary fibrosis in mice. Chin Med J (Engl) 2008;121:1821–1829 [PubMed] [Google Scholar]
- 38.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Berghe TV, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1{alpha} and the Nlrp3/caspase-1/IL-1{beta} axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J 2011;38:1019–1028 [DOI] [PubMed] [Google Scholar]
- 39.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol 2008;180:1169–1178 [DOI] [PubMed] [Google Scholar]
- 40.Montero MT, Matilla J, Gomez-Mampaso E, Lasuncion MA. Geranylgeraniol regulates negatively caspase-1 autoprocessing: implication in the Th1 response against Mycobacterium tuberculosis. J Immunol 2004;173:4936–4944 [DOI] [PubMed] [Google Scholar]
- 41.Montero MT, Hernandez O, Suarez Y, Matilla J, Ferruelo AJ, Martinez-Botas J, Gomez-Coronado D, Lasuncion MA. Hydroxymethylglutaryl-coenzyme A reductase inhibition stimulates caspase-1 activity and Th1-cytokine release in peripheral blood mononuclear cells. Atherosclerosis 2000;153:303–313 [DOI] [PubMed] [Google Scholar]
- 42.Kuijk LM, Mandey SH, Schellens I, Waterham HR, Rijkers GT, Coffer PJ, Frenkel J. Statin synergizes with LPS to induce IL-1beta release by THP-1 cells through activation of caspase-1. Mol Immunol 2008;45:2158–2165 [DOI] [PubMed] [Google Scholar]
- 43.Massonnet B, Normand S, Moschitz R, Delwail A, Favot L, Garcia M, Bourmeyster N, Cuisset L, Grateau G, Morel F, et al. Pharmacological inhibitors of the mevalonate pathway activate pro-IL-1 processing and IL-1 release by human monocytes. Eur Cytokine Netw 2009;20:112–120 [DOI] [PubMed] [Google Scholar]
- 44.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009;113:2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:928–934 [DOI] [PubMed] [Google Scholar]
- 46.Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol 2008;26:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP, Jr, May RM, Wu HP, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005;172:1146–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald SD, Travis WD, Stylianou MP, Rosas IO. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008;168:159–166 [DOI] [PubMed] [Google Scholar]
- 50.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289:1681–1690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.