Abstract
Rationale: Eosinophil β1-integrin activation correlates inversely with FEV1 and directly with eosinophil-bound P-selectin in subjects with nonsevere allergic asthma.
Objectives: Determine the relationships between β1-integrin activation and pulmonary function or eosinophil-bound P-selectin in subjects with asthma of varying severity and discern the source of eosinophil-bound P-selectin.
Methods: Blood was assayed by flow cytometry for P-selectin and activated β1-integrin on eosinophils and platelets. Plasma was analyzed with ELISA for soluble P-selectin, platelet factor 4, and thrombospondin-1.
Measurements and Main Results: Activated β1-integrin correlated with eosinophil-bound P-selectin among all subjects with asthma even though activated β1-integrin was higher in subjects with nonsevere asthma than severe asthma. Activated β1-integrin correlated inversely with FEV1 corrected for FVC only in younger subjects with nonsevere asthma. Paradoxically, platelet surface P-selectin, a platelet activation marker, was low in subjects with severe asthma, whereas plasma platelet factor 4, a second platelet activation marker, was high. Correlations indicated that P-selectin–positive platelets complexed to eosinophils are the major source of the eosinophil-bound P-selectin associated with β1-integrin activation. After whole-lung antigen challenge of subjects with nonsevere asthma, a model of asthma exacerbation known to cause platelet activation, circulating eosinophils bearing P-selectin and activated β1-integrin disappeared.
Conclusions: The relationship between eosinophil β1-integrin activation and pulmonary function was replicated only for younger subjects with nonsevere asthma. However, we infer that platelet activation and binding of activated platelets to eosinophils followed by P-selectin–mediated eosinophil β1-integrin activation occur in both nonsevere and severe asthma with rapid movement of platelet–eosinophil complexes into the lung in more severe disease.
Keywords: asthma, blood platelets, eosinophils, P-selectin, integrins
At a Glance Commentary
Scientific Knowledge on the Subject
Activated β1-integrins on blood eosinophils of young patients with nonsevere allergic asthma correlate with decreased pulmonary function in two study paradigms, inhaled corticosteroid withdrawal and lung antigen challenge. The relationship of β1-integrin activation to lung function and eosinophil P-selectin in the full range of patients with asthma is unknown.
What This Study Adds to the Field
A correlation between β1-integrin activation and decreased pulmonary function was shown to be restricted to the subpopulation of young subjects with nonsevere disease, whereas a correlation between eosinophil β1-integrin activation and surface-associated P-selectin was shown for the asthmatic population as a whole. Activated P-selectin–bearing platelets associating with eosinophils were the likely source of eosinophil surface-associated P-selectin. Eosinophils with activated α4β1-integrin appear to enter the lungs quickly in severe asthma.
Airway inflammation with eosinophilic infiltration of the bronchial mucosa is a characteristic feature of atopic asthma (1–4). Preferential movement of circulating eosinophils to airway is facilitated by induction of vascular cell adhesion molecule-1 (VCAM-1) in bronchial endothelium in response to Th2 immunity mediators (1, 5); VCAM-1 is a ligand for eosinophil α4β1 integrin (6). Integrins exist in different conformations (7), which can be monitored by certain monoclonal antibodies (mAbs). For example, the epitope for activation-sensitive anti-β1 N29 (8) is exposed in the extended active conformations (9).
High N29 epitope expression on circulating eosinophils is associated with a decrease from baseline of FEV1 in young adult subjects with nonsevere asthma undergoing inhaled corticosteroid (ICS) withdrawal or lung antigen challenge (10, 11). This finding is potentially important inasmuch as eosinophil α4β1 activation is expected to synergize with lung endothelial VCAM-1 expression to direct eosinophils to airway. Recently, it was demonstrated that the amount of P-selectin bound to the surface of circulating eosinophils correlates with β1-integrin activation in subjects with nonsevere asthma and that addition of soluble P-selectin to blood in vitro enhances eosinophil β1-integrin activation and adhesion to VCAM-1 (12).
P-selectin is sequestered in Weibel-Palade granules of endothelial cells and α-granules of platelets and translocated to the surface in response to various inflammatory and thrombogenic mediators (13, 14). It is found soluble in plasma at 20 to 40 ng/ml in normal subjects (14–17) with increases described in various disease states (15), including in asthma after whole-lung antigen challenge or exercise (18, 19). Thus, eosinophil-bound P-selectin in asthma may be derived directly from interactions with activated platelets or endothelial cells or indirectly from platelets or endothelial cells via plasma.
The goals of the present study were to determine whether the correlations between eosinophil β1-integrin activation and eosinophil-bound P-selectin (12) or pulmonary function (10, 11) are true for subjects with severe and nonsevere asthma and to learn the source of eosinophil-bound P-selectin, the likely cause of β1-integrin activation (12). Blood was assayed by flow cytometry for P-selectin, activated β1-integrin, the abundant platelet-surface protein αIIb integrin subunit (CD41) as platelet marker (12, 20), and the P-selectin counter-receptor P-selectin glycoprotein ligand-1 (PSGL-1, CD162) (21) on eosinophils and for P-selectin on platelets, and by ELISA for soluble P-selectin and platelet α-granule proteins, platelet factor 4 (PF4) (18, 22, 23), and thrombospondin-1 (TSP-1) (24–26). Subjects were those studied locally as part of the Severe Asthma Research Program (SARP) (27–29) or undergoing whole-lung antigen challenge. The results indicate that platelet activation and binding of activated platelets bearing surface P-selectin to eosinophils followed by P-selectin–mediated eosinophil β1-integrin activation occur in both nonsevere and severe asthma and are in accord with previous studies linking platelets to airway inflammation and hyperreactivity in humans and animal models (18, 30–41). Some of the results have been reported previously in the form of an abstract (42).
Methods
Subjects and Assessments
Subjects with severe or nonsevere asthma judged by American Thoracic Society criteria (43) were screened and enrolled in SARP (27, 29) at the University of Wisconsin (28) and were analyzed by flow cytometry and ELISA; subjects with no asthma enrolled in SARP were also studied. Data on SARP subjects, grouped by severity, are shown in Table 1 and based on methods described in the online supplement. Non-SARP subjects with allergic nonsevere asthma were studied in the setting of whole-lung antigen challenge as described in the online supplement. These subjects had the following values before antigen challenge: mean FEV1 of 96 % predicted (± SD of 18% predicted), median provocative concentration of methacholine producing a 20% fall in FEV1 of 0.9 mg/ml (with 25th and 75th percentiles of 0.4 and 4.4 mg/ml, respectively), mean of 180 (± 100) blood eosinophils/μl, mean of 220 (± 60) × 103 platelets/μl, median sputum eosinophils of 0.3 % (0.0, 2.2%), and median fraction of exhaled nitric oxide of 43 ppb (26, 60 ppb). None of the SARP or antigen challenge subjects participated in our earlier studies (10–12). Both studies were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Written consent was obtained from each subject.
TABLE 1.
SEVERE ASTHMA RESEARCH PROGRAM SUBJECT CHARACTERISTICS
| Group |
|||
| Variable | Severe Asthma | Nonsevere Asthma | No Asthma |
| N | 10 | 21 | 6 |
| Sex, female, male | 6, 4 | 12, 9 | 3, 3 |
| Age, yr | 34 ± 13 | 29 ± 10 | 25 ± 8 |
| FEV1, % predicted | 74 ± 25 | 92 ± 13 | 94 ± 6 |
| FEV1/FVC, % predicted | 88 ± 20 | 91 ± 8 | 94 ± 5 |
| FVC, % predicted | 82 ± 16*† | 100 ± 12 | 102 ± 6 |
| PC20, mg/ml | 1.2 (0.7, 4.9)‡ | 1.5 (1.0, 6.8)‡ | 50 (50, 50) |
| IgE, IU/ml | 160 (74, 270)‡ | 100 (36, 300)‡ | 9 (2, 56) |
| Positive skin tests, No. | 6 ± 3† | 6 ± 3‡ | 0 ± 0 |
| Blood eosinophils, per μl | 210 ± 70† | 240 ± 170† | 110 ± 60 |
| Blood neutrophils, per μl | 3,500 ± 1,100 | 3,500 ± 980 | 3,500 ± 1,360 |
| Platelets, thousands per μl | 250 ± 30 | 240 ± 40† | 290 ± 40 |
| Sputum eosinophils, % of WBC | 0.7 (0.0, 7.2) | 0.4 (0.1, 1.2) | 0.1 (0.0, 0.2) |
| Sputum neutrophils, % of WBC | 43 ± 21 | 35 ± 17 | 53 ± 23 |
| FeNO, ppb | 30 (22, 84)† | 24 (17, 47) | 13 (11, 21) |
| No. (%) on ICS | 10 (100)§|| | 14 (67)‡ | 0 (0) |
| No. (%) on oral CS | 4 (40)* | 0 (0) | 0 (0) |
| ICS dose, μg/d | 1,000 (940, 1,000)‡¶ | 250 (0, 500) | 0 (0, 0) |
Definition of abbreviations: CS = corticosteroid; FeNO = fraction of exhaled nitric oxide; ICS = inhaled CS; PC20 = provocative concentration of methacholine producing a 20% fall in FEV1; SARP = Severe Asthma Research Program; WBC = white blood cells.
Data are presented as mean ± SD or median (25th, 75th percentiles) unless otherwise noted. The allergen extracts used for skin testing were Eastern seven-tree mix, grass mix, short ragweed, common weed mix, dog, cat, Alternaria, Cladosporium, Aspergillus mix, Dermatophagoides farinae, Dermatophagoides pteronyssinus, and American cockroach, with histamine as positive control and diluting fluid as negative control, and were from Greer Laboratories (Lenoir, NC). ICS dose is expressed as fluticasone equivalent, based on the equivalence of 1,200 μg budesonide or 1,260 μg beclomethasone with 880 μg fluticasone (43). Twenty-one subjects were on fluticasone, two on budesonide, and one on beclomethasone. Of the 28 subjects with asthma with reliable sputum data (see the online supplement for exclusion criterion), 20 subjects (71%) had a paucigranulocytic asthma inflammatory phenotype, 6 (21%) were eosinophilic, 2 (7%) neutrophilic, and 0 (0%) mixed granulocytic, using the cutoffs 2% eosinophils and 61% neutrophils as described by Gibson and colleagues (60). Alternatively, using the cutoffs 2% eosinophils and 40% neutrophils as done in a larger SARP cohort (59), 13 subjects (46%) were paucigranulocytic, 2 (7%) eosinophilic, 9 (32%) neutrophilic, and 4 (14%) mixed.
P ≤ 0.01 versus nonsevere asthma.
P ≤ 0.05 versus no asthma.
P ≤ 0.01 versus no asthma.
P ≤ 0.05 versus nonsevere asthma.
P ≤ 0.001 versus no asthma.
P ≤ 0.001 versus nonsevere asthma.
Antibodies, ELISA, and Flow Cytometry
Antibodies, ELISA, and flow cytometry are described in the online supplement.
Statistics and Time-to-Time and Subject-to-Subject Variability of Flow Cytometry and ELISA Measurements
The Mann-Whitney or χ2 tests were used to compare ordinal or nominal data, respectively, between two groups; the Wilcoxon test or the Friedman two-way analysis of variance to compare data from different visits of single subjects or single visits of different subjects. The Spearman rank test was used to analyze correlations. A P value ≤ 0.05 was considered significant. Analyses were performed using Prism (GraphPad; La Jolla, CA) or as described (44). Group data are reported as mean ± SD if the variable was normally distributed (passed Prism's normality test) and as median with 25th and 75th percentiles if the variable was nonnormally distributed.
Individual-to-individual versus visit-to-visit variability was analyzed in three SARP subjects who had blood analyzed at three different visits. Coefficients of variation (SD/mean) for N29 reactivity and plasma P-selectin measured at different visits in the same subject were smaller than the coefficients of variation of the means of the subjects such that there were significant differences among subjects but not among visits.
Results
Description of SARP Subjects
To learn the relationships among β1-integrin activation, eosinophil-bound P-selectin, and pulmonary function in subjects with asthma of variable severity, we studied 37 SARP subjects, 10 with severe asthma, 21 with nonsevere asthma, and 6 normal control subjects (Table 1). Criteria for severe and nonsevere asthma were as described (27, 28, 43). Power analyses of sample sizes required to detect possible correlations were performed based on previously observed correlations in nonsevere asthma between N29 reactivity and FEV1 (Spearman rank correlation coefficients [rs] = −0.50 to −0.56) (10) or eosinophil-associated P-selectin (rs < 0.8) (12). The analyses estimated that a sample size of 8 or 22 subjects was needed to detect with 80% power a true correlation with r = ±0.8 or 0.5, respectively, with the alternative hypothesis r = 0. Thus, the target was to study at least eight subjects in each asthma status group. Subject status was blinded until after flow cytometry was performed. Accrual was discontinued when 10 subjects with severe asthma and 22 with nonsevere asthma had been enrolled. One of these subjects with nonsevere asthma was later removed from analysis due to the interim diagnosis of possible sarcoidosis.
The mean age was higher in subjects with severe asthma than nonsevere asthma and lowest in the normal subjects. Subjects with severe asthma had lower FVC than subjects with nonsevere asthma, as described in larger studies (27, 28). The concentrations of serum IgE were higher in both asthma groups than in normal subjects. Mean platelet count was lower in nonsevere asthma than normal with a trend to a lower count also in severe asthma. Only one subject had a count below the lower end of the normal range, at 1.1 × 106/ml. All the subjects with severe and a majority of those with nonsevere asthma were receiving inhaled corticosteroids (ICS). Some subjects with severe asthma also received oral corticosteroids. The daily ICS dose in the severe asthma population was higher than that of the nonsevere asthma group. All the subjects with asthma except two (one with severe asthma and one with nonsevere asthma) had at least one positive skin test. The two skin test–negative subjects had experienced allergic symptoms. Thus, no subjects were nonallergic. Plasma from additional 54 SARP subjects (25 subjects with nonsevere asthma, 18 with severe asthma, and 11 normal subjects) was studied by ELISA for P-selectin and PF4. The demographics and screening results were similar to the 37 SARP subjects described in Table 1.
Eosinophil β1-Integrin Activation Correlates with Eosinophil-Bound P-Selectin in Subjects with Asthma as a Whole but with FEV1/FVC Only in Young Subjects with Nonsevere Asthma
For the 31 subjects with asthma grouped together or the 21 subjects with nonsevere asthma, mean eosinophil β1-integrin activation on circulating eosinophils was significantly increased by 1.9-fold or 2.2-fold, respectively, when compared with normal subjects (Table 2). Eosinophil surface P-selectin was not significantly different in subjects with asthma as compared with normal subjects, but there was a trend to a lower level in subjects with severe asthma (Table 2). Results were similar for eosinophil surface-associated αIIb integrin, which is an abundant platelet surface protein (20) and which we used as a platelet marker as before (12), with a trend to lower signal in severe asthma (Table 2). We also measured eosinophil expression of PSGL-1, the eosinophil surface counter-receptor for P-selectin (21). PSGL-1 expression was high in all groups and not significantly different among them (Table 2).
TABLE 2.
EOSINOPHIL β1-INTEGRIN ACTIVATION, P-SELECTIN, PLATELET FACTOR 4, AND THROMBOSPONDIN-1 IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH ASTHMA, NONSEVERE ASTHMA, SEVERE ASTHMA, OR NO ASTHMA
| Measurement | All Asthma | Nonsevere Asthma | Severe Asthma | No Asthma |
| Eosinophil N29, specific gMCF | 210 ± 110* (31) | 240 ± 100* (21) | 150 ± 110 (10) | 110 ± 100 (6) |
| Eosinophil P-selectin, specific gMCF | 250 ± 180 (31) | 260 ± 200 (21) | 210 ± 120 (10) | 250 ± 150 (6) |
| Eosinophil αIIb integrin, specific gMCF | 640 ± 350 (31) | 670 ± 390 (21) | 560 ± 260 (10) | 640 ± 420 (6) |
| Eosinophil PSGL-1, specific gMCF | 1,250 ± 230 (31) | 1,230 ± 260 (21) | 1,280 ± 150 (10) | 1,180 ± 250 (6) |
| Platelet P-selectin, specific gMCF | 110 (60, 590)* (31) | 150 (60, 780) (21) | 90 (0, 460)* (10) | 500 (240, 1,220) (6) |
| Plasma P-selectin, ng/ml | 35 ± 14 (31) | 36 ± 11 (21) | 33 ± 19 (10) | 40 ± 8 (6) |
| 25 ± 13 (74) | 25 ± 12 (46) | 26 ± 14 (28) | 23 ± 12 (17) | |
| Platelet factor 4 (PF4), ng/ml | 9.7 (5.5, 36) (31) | 7.9 (5.2, 16) (21) | 31 (12, 62)† (10) | 16 (9.4, 130) (6) |
| 7.2 (4.0, 17) (74) | 6.3 (3.7, 13)* (46) | 13 (5.2, 22)‡ (28) | 12 (5.8, 21) (17) | |
| Thrombospondin-1 (TSP-1), ng/ml | 250 ± 200 (30) | 230 ± 160 (20) | 290 ± 270 (10) | 300 ± 200 (5) |
Definition of abbreviations: gMCF = geometric mean channel fluorescence; mAb = monoclonal antibody; PF4 = platelet factor 4; PSGL-1, P-selectin glycoprotein ligand-1; SARP = Severe Asthma Research Program; TSP-1 = thrombospondin-1.
Data are presented as mean ± SD (n) or median (25th, 75th percentiles) (n). The reactivity of blood eosinophils with activation-sensitive anti-β1 integrin mAb N29, the level of blood eosinophil surface-associated P-selectin and αIIb integrin subunit, blood eosinophil expression of PSGL-1, and platelet surface expression of P-selectin were measured by flow cytometry; and the concentrations of soluble plasma P-selectin, PF4, and TSP-1 were measured by ELISAs as described in the online supplement. Expression level values for platelet P-selectin are not comparable to those for eosinophil N29, P-selectin, αIIb, and PSGL-1 due to different settings of detector sensitivity for platelets and leukocytes, as described in the online supplement. Data are from the SARP subjects described in Table 1, except that the data in the bottom entry for plasma P-selectin and PF4 also include the additional 54 SARP subjects mentioned in the text under Subject Accrual and Characteristics.
P ≤ 0.05 versus no asthma.
P ≤ 0.01, versus nonsevere asthma.
P ≤ 0.05 versus nonsevere asthma.
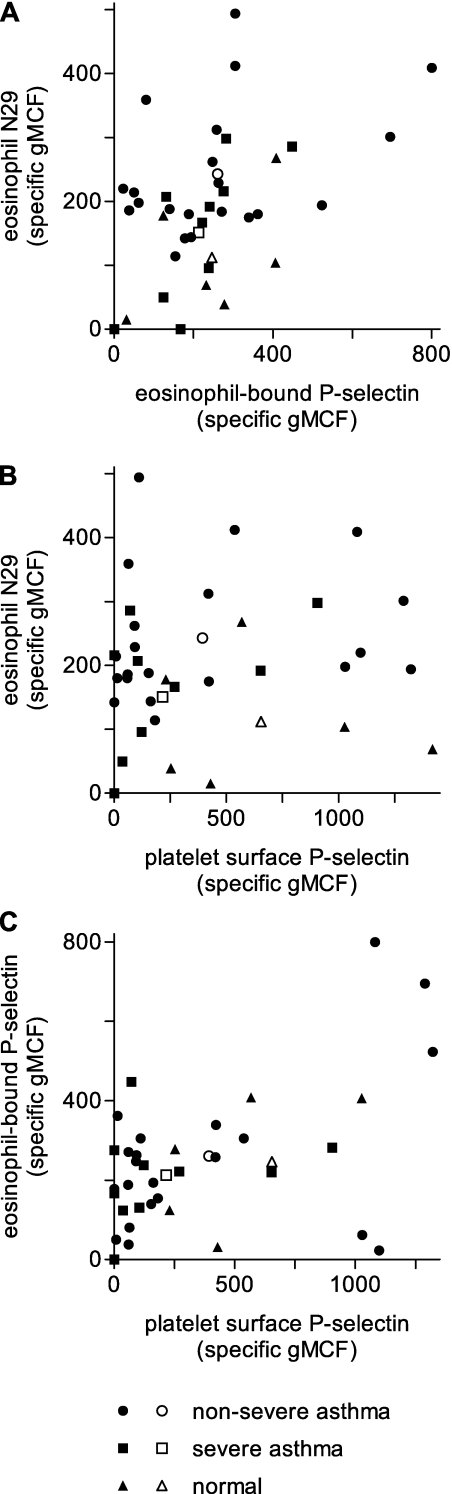
A plot of β1-integrin activation versus eosinophil-surface P-selectin demonstrated considerable scatter, with more variability for subjects with nonsevere asthma (Figure 1A). Nevertheless, β1-integrin activation correlated directly with eosinophil surface P-selectin in subjects with asthma (rs = 0.42, P = 0.02) (Table 3). The correlation was less in subjects with nonsevere asthma (rs = 0.23, P = 0.32) than in severe asthma (rs = 0.81, P = 0.006).
Figure 1.
Scatter plots of relations among eosinophil β1-integrin activation, assessed by reactivity with activation-sensitive anti-β1 integrin monoclonal antibody (mAb) N29, level of eosinophil-bound P-selectin, and platelet surface P-selectin expression in Severe Asthma Research Program subjects. (A) Eosinophil N29 reactivity versus eosinophil-bound P-selectin. (B) Eosinophil N29 reactivity versus platelet surface P-selectin. (C) Eosinophil-bound P-selectin versus platelet surface P-selectin. Solid symbols, individual subjects; open symbols, means (see Table 3). Correlation coefficients and P values are described in Table 3 and the text. gMCF = geometric mean channel fluorescence.
TABLE 3.
CORRELATIONS AMONG P-SELECTIN, PLATELET FACTOR 4, THROMBOSPONDIN-1, EOSINOPHIL β1-INTEGRIN ACTIVATION, AND MEASUREMENTS OF PULMONARY FUNCTION IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH ASTHMA
| PF4 | TSP1 | Eos N29 | Eos PS | Eos αIIb | Platelet PS | FEV1 | FEV1/FVC | |
| Protein/mAb | rs/P Value | rs/P Value | rs/P Value | rs/P Value | rs/P Value | rs/P Value | rs/P Value | rs/P Value |
| Plasma PS | 0.02/0.93 | 0.01/0.97 | 0.23/0.22 | 0.14/0.46 | 0.13/0.48 | 0.09/0.62 | −0.03/0.80 | 0.02/0.88 |
| PF4 | 0.44/0.02 | −0.38/0.04 | −0.32/0.08 | −0.20/0.28 | −0.26/0.16 | 0.03/0.83 | 0.08/0.51 | |
| TSP-1 | −0.47/0.01 | −0.19/0.33 | −0.08/0.68 | −0.20/0.31 | 0.05/0.80 | 0.02/0.91 | ||
| Eos N29 | 0.42/0.02 | 0.17/0.37 | 0.42/0.02 | −0.10/0.60 | −0.26/0.15 | |||
| Eos PS | 0.61/0.0003 | 0.35/0.05 | 0.07/0.72 | −0.01/0.96 | ||||
| Eos αIIb | 0.32/0.08 | 0.36/0.05 | 0.28/0.13 | |||||
| Platelet PS | 0.05/0.78 | −0.01/0.95 | ||||||
| FEV1 | 0.71/<0.0001 |
Definition of abbreviations: Eos = eosinophil; mAb = monoclonal antibody; PF4 = platelet factor 4; PS = P-selectin; rs = Spearman rank correlation coefficient; TSP-1 = thrombospondin-1.
For measurements, see Table 2.
In earlier studies correlating eosinophil β1 activation with pulmonary function (10, 11), subjects had median ages of 21 years, with only one of 27 subjects older than 30 years. The measure of function was change in FEV1 from baseline before steroid withdrawal or lung antigen challenge (10, 11). No intervention was taken in the present study to warrant a baseline, and therefore the measure of pulmonary function here was FEV1 divided by FVC, both expressed as percent of the predicted value. As the subjects in the previous studies all had nonsevere asthma and most were less than 30 years of age, a question was whether the correlation between eosinophil β1 activation and lung function would hold up across all subjects with asthma or would be restricted to such a subgroup. To address this issue, we analyzed both the whole population with asthma and subgroups. For all subjects with asthma, rs for N29 reactivity versus FEV1/FVC was −0.26, which was not significant (Table 3). For subjects with nonsevere asthma, rs was −0.38, still nonsignificant but a greater absolute value than the correlation of rs of −0.18 for subjects with severe asthma. When we analyzed the 14 subjects with nonsevere asthma and aged 30 years or younger, activated β1 correlated inversely with FEV1/FVC with an rs of −0.54 (P = 0.05).
The results were initially puzzling. On the one hand, we found that the previously described relationship between activated eosinophil β1-integrin and airway physiology (10, 11) is specific for younger subjects with nonsevere asthma. On the other hand, we found that the correlation between activated eosinophil β1 integrin and eosinophil-bound P-selectin holds for all subjects with asthma as a group.
The Primary Source of the Eosinophil-Bound P-Selectin Associated with Eosinophil β1-Integrin Activation in Asthma Is Likely the Activated Platelet
Activated platelets bind to leukocytes, including eosinophils, via P-selectin (45) binding its counter-receptor PSGL-1 (21). Studies of platelet-depleted mice reconstituted with activated wild-type or P-selectin–deficient platelets implicate platelet P-selectin as a cause of airway eosinophilic inflammation in the ovalbumin-sensitization model (38, 40). An in vitro study observed enhanced complex formation between activated P-selectin–bearing platelets and eosinophils from subjects with allergic asthma and indicated that platelet association contributes to the enhanced tethering of such eosinophils to activated endothelium in a P-selectin–dependent manner (46). Previous studies have demonstrated variable increases in P-selectin in plasma in subjects with asthma after antigen challenge or exercise (18, 19) and on the platelet surface in aspirin-sensitive subjects or associated with acute disease (33, 34). In immunofluorescence microscopic studies of P-selectin associated with circulating eosinophils from subjects with nonsevere asthma, P-selectin was found in a patchy distribution, with some but not all of the patches colocalizing with platelet markers (12). A possible explanation for the platelet-free P-selectin–positive sites is the transient nature of the selectin-dependent association of platelets to eosinophils; activated platelets may “deliver” P-selectin to the eosinophil surface and then dissociate. To learn the possible source(s) of the eosinophil-bound P-selectin, we measured the concentration of soluble P-selectin in plasma by ELISA and P-selectin on the surface of activated platelets by flow cytometry. Samples were processed to minimize artifactual platelet activation due to blood collection and handling, and results were correlated with activated β1-integrin and P-selectin on the surface of eosinophils.
ELISA estimates of plasma P-selectin concentration were no different from normal subjects for the subjects with asthma as a whole or for the groups with nonsevere or severe asthma (Table 2). The failure to demonstrate differences in plasma P-selectin persisted when additional individuals were studied (Table 2). The variability in measurements of platelet-surface P-selectin by flow cytometry was considerable (Table 2, Figures 1B and 1C), as has been found before (33). Nevertheless, flow cytometry revealed a significant decrease in platelet surface P-selectin in subjects with severe asthma (Table 2). Platelet surface P-selectin, but not plasma P-selectin concentration, correlated directly with β1-integrin activation (rs = 0.42, P = 0.02) and eosinophil-bound P-selectin (rs = 0.35, P = 0.05) in subjects with asthma as a whole (Table 3, Figures 1B and 1C). The correlations had similar rs values for both nonsevere and severe subjects (activated β1-integrin versus platelet surface P-selectin: rs = 0.34, P = 0.13 for nonsevere and rs = 0.50, P = 0.14 for severe; eosinophil P-selectin versus platelet surface P-selectin: rs = 0.33, P = 0.15 for nonsevere and rs = 0.42, P = 0.22 for severe). The results suggest that the major source of eosinophil-bound P-selectin associated with eosinophil β1-integrin activation in asthma is activated platelets that associate with eosinophils. In addition, platelet association with eosinophils assessed by eosinophil-bound αIIb integrin correlated with rs = 0.61, P = 0.0003 with eosinophil P-selectin in subjects with asthma (Table 3), further strengthening the conclusion that platelets associating with eosinophils are the source of eosinophil-bound P-selectin.
The observations that subjects with severe asthma have low platelet surface P-selectin and their eosinophils have trends to low eosinophil-bound P-selectin and αIIb and do not have increased β1-integrin activation (Table 2) raise the possibility that platelet and eosinophil β1-integrin activation, and platelet-eosinophil complex formation, are ongoing in severe asthma but not detected by flow cytometry of venous blood because of decreased entry into the circulation or increased clearance. P-selectin is a type I membrane protein that is sequestered in platelet α-granules and mobilized to the platelet surface upon platelet activation (13, 14). We therefore quantified plasma PF4 (18, 22, 23) and TSP-1 (24–26), which also originate in α-granules and are released into the circulation upon platelet activation. Plasma PF4 has previously been demonstrated to be higher in patients with asthma when symptomatic (32, 39), after exercise (30), or after whole-lung antigen challenge (18, 32). Plasma PF4 was significantly higher and TSP-1 trended to a higher level in subjects with severe asthma than with nonsevere asthma; the difference in PF4 persisted when a larger number of subjects was tested (Table 2). Remarkably, there was an inverse correlation of rs of −0.38 or −0.47 between plasma PF4 or TSP-1 and activated eosinophil β1-integrin for the asthmatic group as a whole and trends to inverse correlations between plasma PF4 or TSP-1 and eosinophil or platelet surface P-selectin (Table 3).
Eosinophil β1-Integrin Activation and Eosinophil-Bound P-Selectin after Whole-Lung Antigen Challenge
The results described above on subjects with stable asthma of variable severity suggest that platelet activation, association of activated platelets with eosinophils, and binding of P-selectin to eosinophils lead to activation of eosinophil β1-integrin and are linked to increased vascular clearance of eosinophils with surface P-selectin and activated β1-integrin. To examine trafficking of such eosinophils in a second experimental paradigm, we studied 16 subjects with mild allergic asthma undergoing whole-lung inhaled antigen challenge. Such challenge has been shown to lead to platelet activation, a decrease in the number of eosinophils in the circulation at 0.5 to 6 hours followed by a recovery at 24 hours, and a decrease in the platelet count at 0.5 hours (and 6 h in subjects with a dual-response phenotype) followed by recovery at 6 hours (at 24 h in dual responders) (18), and an increase in soluble VCAM-1 and eosinophils in the airway at 4 to 48 hours (47–49).
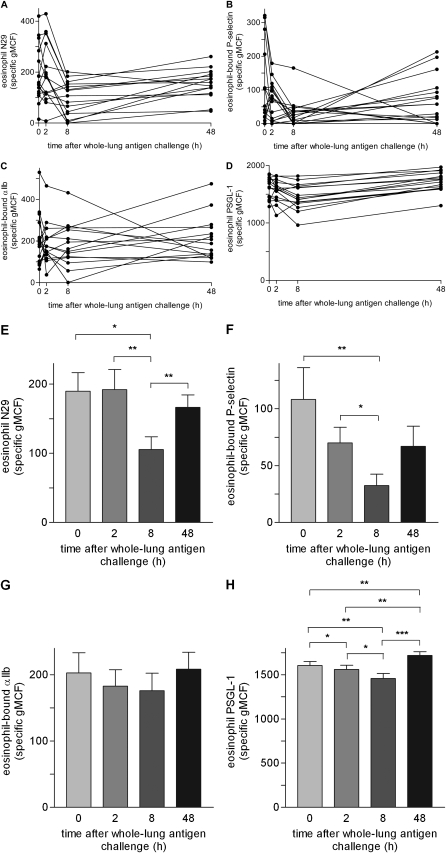
There was a significant decrease in β1-integrin activation at 8 hours after challenge followed by a significant recovery at 48 hours (Figures 2A and 2E). Eosinophil-bound P-selectin was also significantly decreased at 8 hours, followed by a trend to a recovery at 48 hours (Figures 2B and 2F). The two measurements tracked together (rs = 0.42, P = 0.0005 assuming that the 64 samples are independent of one another). Eosinophil-bound αIIb similarly showed trends to a greatest decrease at 8 hours followed by recovery at 48 hours (Figures 2C and 2G). Eosinophil PSGL-1 expression had a minor but significant decrease at 2 hours, a somewhat greater decrease at 8 hours, and a significant recovery at 48 hours (Figures 2D and 2H). Mean blood eosinophil concentration, measured by a Unopette method, was decreased at 2 hours (150 per μl vs. 180 per μl at 0 h, P = 0.31; then recovered to 180 and 220 per μl at 8 and 48 h, respectively; P = 0.03 for 48 h versus 2 h). In contrast, the percentage of eosinophils in sputum increased from a median of 0.3% (25th and 75th percentiles, 0.0 and 2.2%, respectively) at 0 hours to 7.0% (4.0, 8.8%) at 48 hours (P = 0.002), and the number of eosinophils per weight sputum increased from a median of 1,100 (0, 19,000) per g at 0 hours to 38,000 (14,000, 62,000) per g at 48 hours (P = 0.02). Platelet counts are available from 14 of these 16 subjects; mean platelet concentration was 2.2 × 106/μl at 0 hours and 2.4 × 106/μl at 2, 8, and 48 hours. Thus, whereas Kowal and colleagues recorded a transient decrease in platelet count in all their subjects at 0.5 hours (and in dual responders at 6 h) (18), we did not observe a decrease in platelet numbers. Perhaps a decrease in platelets occurred also in our subjects at 0.5 hours, which we did not record. The disappearance of circulating eosinophils bearing surface P-selectin, high PSGL-1 expression, and activated β1-integrin; the trend for disappearance of eosinophils bearing αIIb; and appearance of more eosinophils in sputum suggest that those eosinophils with the highest PSGL-1 level that have associated with activated P-selectin–bearing platelets triggering β1-integrin activation preferentially traffic to the airway.
Figure 2.
Time courses of eosinophil β1-integrin activation, assessed by reactivity with activation-sensitive anti-β1 integrin monoclonal antibody (mAb) N29, levels of eosinophil-bound P-selectin and the platelet marker αIIb integrin, and P-selectin glycoprotein ligand-1 (PSGL-1) expression after whole-lung inhaled antigen challenge in non–Severe Asthma Research Program subjects with mild allergic asthma. (A) Individual time courses of eosinophil N29 reactivity. (B) Individual time courses of eosinophil-bound P-selectin. (C) Individual time courses of eosinophil-bound αIIb; (D) Individual time courses of eosinophil PSGL-1. (E–H) Means of values (± SEM) of (E) eosinophil N29 reactivity, (F) eosinophil-bound P-selectin, (G) eosinophil-bound αIIb, and (H) eosinophil PSGL-1 at indicated times. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. gMCF = geometric mean channel fluorescence.
Discussion
Asthma is a heterogeneous disease based on symptoms, allergen sensitization, airflow limitation, response to medication, airway inflammation, and outcomes; and establishing subgroups and relating subgroups to pathophysiology are considered important steps in determining optimal treatments (2, 3, 29, 50–52). Working from studies demonstrating associations between blood eosinophil β1-integrin activation and lung function (10, 11) and effects of P-selectin binding to eosinophils on β1-integrin activation (12) in young subjects with nonsevere asthma, we undertook the present study of subjects with allergic asthma of variable severity. We reproduced the association between eosinophil β1-integrin activation and pulmonary function only in subjects with nonsevere asthma similar to those studied previously. Nevertheless, the association between β1-integrin activation and eosinophil-bound P-selectin was true for the asthmatic population as a whole, suggesting that similar pathophysiology underlies eosinophil β1-integrin activation in both nonsevere and severe allergic asthma. Platelet surface P-selectin correlated with eosinophil β1-integrin activation and eosinophil-bound P-selectin, whereas soluble P-selectin did not. Furthermore, degree of platelet association with eosinophils, assessed by αIIb integrin subunit, correlated strongly with eosinophil-bound P-selectin. Thus, the major source of eosinophil-bound P-selectin that likely triggers β1-integrin activation in allergic asthma appears to be activated platelets that associate with eosinophils.
Expression of P-selectin on the surface of free platelets was significantly lower than normal in subjects with severe asthma but not in subjects with nonsevere asthma. One possible explanation for this phenomenon is that in severe asthma there is rapid shedding of P-selectin from the surface of free circulating activated platelets that have not associated with circulating leukocytes or endothelium. Rapid P-selectin shedding from circulating activated platelets have been described in animal models (53–55). Plasma PF4 was higher in severe than in nonsevere asthma, indicating that, despite low P-selectin on surfaces of platelets and eosinophils, there is more platelet activation in severe asthma.
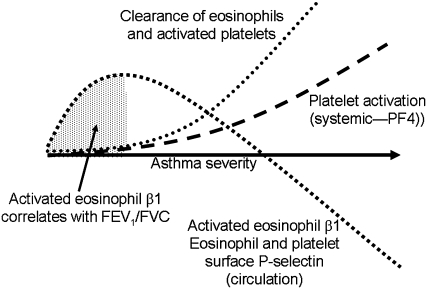
Figure 3 depicts a model that addresses this paradox and is buttressed by the findings that transfusion of platelet-depleted mice with activated wild-type but not P-selectin–deficient platelets enhances eosinophil recruitment to the airway after lung antigen challenge (38, 40) and that in vitro there is enhanced complex formation between activated P-selectin–bearing platelets and eosinophils from subjects with allergic asthma contributing to enhanced P-selectin–dependent tethering of such eosinophils to activated endothelium (46). Circulating eosinophils bearing activated β1-integrins and P-selectin are shown as becoming increasingly less common in more severe asthma even as platelet activation, as assessed by systemic PF4 concentration, increases. This discrepancy occurs because complexes of activated platelets and eosinophils are removed efficiently from the circulation. Efficient removal may be due to greater lung endothelial VCAM-1 expression, as has been observed in bronchial biopsies of subjects with severe compared with nonsevere asthma (56). Increased density of VCAM-1 would create a “sink” for arrest and transmigration of eosinophils with activated α4β1. The model is consistent with the trends toward lower numbers of blood eosinophils in SARP subjects with severe compared with nonsevere asthma (27) and higher sputum eosinophil numbers in severe asthma (Table 1). The model is also consistent with the decreased eosinophil β1-integrin activation, surface P-selectin, and expression of the P-selectin counter-receptor PSGL-1 found after whole-lung inhaled antigen challenge, accompanied by decreased circulating eosinophils and increased sputum eosinophils. The model predicts that increased eosinophil β1-integrin activation correlates with obstructive airway physiology only in patients with the mildest form of asthma (Figure 3). Presumably, β1 activation on circulating eosinophils does not correlate with physiology in more severe asthma due to enhanced extravasation of activated eosinophils. Furthermore, the correlation did not hold up in older subjects, including older subjects with nonsevere asthma. Perhaps pulmonary function becomes less associated with eosinophil activation and recruitment as the disease evolves with time and increased age. Finally, the model is consistent with the lower or trends to lower platelet counts in nonsevere and severe asthma (Table 1). The lower numbers of free platelets may be due to platelets associating with eosinophils and other leukocytes or extravasating along with the complexes or possibly also due to migration of isolated platelets, as has been described in mice (57). In this context, it can be noted that atopic asthma has been reported to be accompanied by shorter platelet survival time (41, 58).
Figure 3.
Model of platelet activation, eosinophil β1-integrin activation, and airway physiology in asthma of increasing severity. Platelet activation and appearance of platelet factor 4 (PF4) in circulation increase with increasing disease severity. P-selectin appears on the surface of platelets upon activation, activated platelets bind to eosinophils via P-selectin, and P-selectin triggers activation of eosinophil β1-integrins. Eosinophils with activated β1 integrins persist in the circulation in stable nonsevere asthma but are lost in more severe asthma or during asthma exacerbation. Loss is likely due to interaction of activated α4β1 integrin with endothelial cell vascular cell adhesion molecule-1 followed by extravasation in the asthmatic lung. Increased eosinophil β1-integrin activation is therefore inversely associated with decreased pulmonary function only in the subpopulation with stable nonsevere asthma and minimal eosinophil clearance.
As mentioned, there was a trend to higher sputum eosinophil numbers in severe asthma. Still, these sputum eosinophil levels were, although variable among subjects, relatively low. Using criteria for the definition of inflammatory phenotypes in asthma (59, 60), a plurality of our subjects can be considered to have paucigranulocytic asthma with smaller groups having eosinophilic or neutrophilic asthma dependent on cutoffs used (see legend of Table 1). Perhaps the relatively low level of sputum eosinophils in both severe and nonsevere asthma is an effect of steroid treatment and maybe more subjects would be considered “eosinophilic” if their steroid dose were reduced; sputum eosinophil percentage tends to increase if ICS are withdrawn in nonsevere asthma (10). An additional possibility may be that these subjects may have significant numbers of lung eosinophils that are retained in the airway wall or tissue and not recovered from induced sputum.
The time courses of eosinophil β1-integrin activation and P-selectin after whole-lung antigen challenge can be compared with that of eosinophil reactivity with mAb A17 after antigen challenge. A17 recognizes an activated form of Fcγ receptor II (CD32) (61). A17 reactivity increased 6 hours after whole-lung antigen challenge and then decreased at 24 hours (62), indicating that this receptor was first activated followed by extravasation of eosinophils with activated CD32. In addition, circulating eosinophils from subjects with allergic asthma have greater responsiveness to chemoattractants, which is more pronounced after allergen challenge (63). Such priming of eosinophils to greater responsiveness is achieved by IL-5 and related cytokines (64). However, we do not believe that IL-5 family cytokines are involved in the platelet P-selectin–β1-integrin activation axis described here. Although P-selectin activates eosinophil β1-integrins in vitro, IL-5 does not (12). On the other hand, IL-5 activates eosinophil β2-integrins, but P-selectin does not (12). Thus, the present in vivo and the previous in vitro data indicate that eosinophil β1 activation reports and is a result of preactivation by P-selectin and not priming by IL-5. Still, it remains possible that activity of eosinophil β2-integrins are also involved in eosinophil arrest and extravasation in vivo to some degree, since αMβ2 together with α4β1 contributed to arrest of purified eosinophils to VCAM-1 under physiological flow conditions in vitro (65), and both α4 (presumably α4β1) and β2 (presumably αMβ2) are required for eosinophil trafficking to the lung and airway lumen in acute and chronic mouse antigen challenge models (66, 67).
The present study has several limitations. First is the trend to an age discrepancy among the severe asthma, nonsevere asthma, and normal groups. Thus, some of the group differences in Table 1 may be due to age or duration of asthma. Future studies are needed to address this issue. Second, the SARP study was observational and a snapshot of disease in only a short time window. Visit-to-visit variability was low and there was concordance of values over the three blood draw visits. However, it would be useful to perform the same analyses over a longer time span (e.g., one to several years) and relate measurements to the evolution of the disease over time as well as comparing periods of stability to asthma exacerbations.
Third, differences in measures of platelet activation have the potential to be misleading due to activation resulting from uneven quality of blood collection and sample preparation. We used a protocol designed to minimize platelet activation and measured three markers of activation. The findings that platelet surface P-selectin and plasma PF4 or TSP-1 trended toward inverse correlations, whereas PF4 and TSP-1 correlated tightly and directly with one another indicate that, despite considerable variability in values, the flow cytometry and ELISA data can be interpreted without worry about systematic changes in post-phlebotomy platelet activation. Thus, we believe that none of the limitations invalidate a model in which platelet activation is an important determinant of the severity of allergic asthma.
Supplementary Material
Acknowledgments
The authors thank Daniel Kolk, Erin Billmeyer, Holly Eversoll, Michele Wolff, Evelyn Failbene, Gina Crisafi, and Loren Denlinger for patient recruitment, screening, and assessments; Gina Crisafi, Katie Gaworski, Helen Holden, Elizabeth Schwantes, Lei Shi, and Arturo Guadarrama for processing blood and sputum samples; Ethan Stortz for help with P-selectin analysis of multiple samples from the same subjects; Douglas Annis for help with the development of the thrombospondin-1 ELISA; Kathleen Schell and Joel Puchalski for advice on flow cytometry; Michael Evans for power analysis and advice on statistics; Martin Humphries for discussion on β1-integrin activation mAbs and integrin conformations; Ronald Sorkness, Gina Crisafi, Helen Holden, Holly Eversoll, Loren Denlinger, Michele Wolff, Michael Evans, Elizabeth Schwantes, and Becky Kelly for providing pulmonary function predicted values, assessment data, data on ICS use, and advice; Maura Robinson and Ruthie Knowles at the SARP Data Coordinating Center for help with the central database; and Gina Crisafi and Cheri Swenson for administrative help.
Footnotes
Supported by Specialized Center of Research grant P50 HL56396 (W.W.B. and D.F.M.), grant R01 HL69116 (W.W.B.), grant 1 U10 HL109168 (N.N.J.), Program Project grant P01 HL88594 (N.N.J. and D.F.M.), ARRA supplement grant P01 HL88594-02S1 (N.N.J. and D.F.M.), General Clinical Research Center grant M01 RR03186 (R.N. Golden), and Clinical and Translational Science Award grant UL1 RR25011 (M.K. Drezner) from the National Institutes of Health; and Robert Draper Technology Innovation Funding (M.W.J.) from the Graduate School, University of Wisconsin-Madison.
Author Contributions: M.W.J. performed flow cytometry data acquisition and analysis, was responsible for statistical analysis, and wrote the manuscript. S.-T.H. developed and performed thrombospondin-1 ELISA and analysis. K.A.G. performed P-selectin and platelet factor 4 ELISAs and analyses and flow cytometry data acquisition. W.W.B. and N.N.J. conceived, designed, and supervised the Severe Asthma Research Program in Wisconsin and helped revise the manuscript before submission. D.F.M. provided ongoing oversight of flow cytometry and ELISA data acquisition and analyses and participated in the preparation of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1712OC on January 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wills-Karp M, Karp CL. Eosinophils in asthma: remodeling a tangled tale. Science 2004;305:1726–1729 [DOI] [PubMed] [Google Scholar]
- 2.Scott KA, Wardlaw AJ. Eosinophilic airway disorders. Semin Respir Crit Care Med 2006;27:128–133 [DOI] [PubMed] [Google Scholar]
- 3.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol 2009;71:489–507 [DOI] [PubMed] [Google Scholar]
- 4.Akuthota P, Xenakis JJ, Weller PF. Eosinophils: offenders or general bystanders in allergic airway disease and pulmonary immunity? J Innate Immun 2011;3:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 2007;119:1303–1310 [DOI] [PubMed] [Google Scholar]
- 6.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol 2008;83:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg N, Patzak I, Willenbrock F. The insider's guide to leukocyte integrin signalling and function. Nat Rev Immunol 2011;11:416–426 [DOI] [PubMed] [Google Scholar]
- 8.Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. J Biol Chem 1996;271:3046–3051 [PubMed] [Google Scholar]
- 9.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem 2005;280:4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta(1) integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol 2006;117:1502–1504 [DOI] [PubMed] [Google Scholar]
- 11.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol 2008;180:7622–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson MW, Mosher DF. Activation of {beta}1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol 2011;45:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre P. P-selectin in haemostasis. Br J Haematol 2004;126:298–306 [DOI] [PubMed] [Google Scholar]
- 14.Kappelmayer J, Nagy B, Jr, Miszti-Blasius K, Hevessy Z, Setiadi H. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med 2004;42:475–486 [DOI] [PubMed] [Google Scholar]
- 15.Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today 1993;14:506–512 [DOI] [PubMed] [Google Scholar]
- 16.Blann AD, Nitu-Whalley IC, Lee CA, Lip GY. Inverse relationship between plasma von Willebrand factor and soluble P selectin in patients with type 1 but not type 2 von Willebrand disease. Am J Hematol 2002;69:135–137 [DOI] [PubMed] [Google Scholar]
- 17.Kamath S, Blann AD, Caine GJ, Gurney D, Chin BS, Lip GY. Platelet P-selectin levels in relation to plasma soluble P-selectin and beta-thromboglobulin levels in atrial fibrillation. Stroke 2002;33:1237–1242 [DOI] [PubMed] [Google Scholar]
- 18.Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Lukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy 2006;36:426–432 [DOI] [PubMed] [Google Scholar]
- 19.Zietkowski Z, Skiepko R, Tomasiak MM, Bodzenta-Lukaszyk A. Soluble CD40 ligand and soluble P-selectin in allergic asthma patients during exercise-induced bronchoconstriction. J Investig Allergol Clin Immunol 2008;18:272–278 [PubMed] [Google Scholar]
- 20.Kishimoto T, Kikutani H, von dem Borne AEGK, Goyert SM, Mason DY, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer TA, et al. Leucocyte typing VI: white cell differentiation antigens. New York: Garland Publishing, Inc.; 1997. [Google Scholar]
- 21.Symon FA, Lawrence MB, Williamson ML, Walsh GM, Watson SR, Wardlaw AJ. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol 1996;157:1711–1719 [PubMed] [Google Scholar]
- 22.Aidoudi S, Bikfalvi A. Interaction of PF4 (CXCL4) with the vasculature: a role in atherosclerosis and angiogenesis. Thromb Haemost 2010;104:941–948 [DOI] [PubMed] [Google Scholar]
- 23.Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010;125:292–296 [DOI] [PubMed] [Google Scholar]
- 24.Mosher DF, Doyle MJ, Jaffe EA. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol 1982;93:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browne PV, Mosher DF, Steinberg MH, Hebbel RP. Disturbance of plasma and platelet thrombospondin levels in sickle cell disease. Am J Hematol 1996;51:296–301 [DOI] [PubMed] [Google Scholar]
- 26.Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci 2008;65:672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007;119:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol 2008;104:394–403 [DOI] [PubMed] [Google Scholar]
- 29.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, et al. Severe asthma: lessons learned from the NHLBI Severe Asthma Research Program. Am J Respir Crit Care Med (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson CE, Belfield PW, Davis S, Cooke NJ, Spencer A, Davies JA. Platelet activation during exercise induced asthma: effect of prophylaxis with cromoglycate and salbutamol. Thorax 1986;41:290–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyle AJ, Page CP, Atkinson L, Flanagan R, Metzger WJ. The requirement for platelets in allergen-induced late asthmatic airway obstruction. Eosinophil infiltration and heightened airway responsiveness in allergic rabbits. Am Rev Respir Dis 1990;142:587–593 [DOI] [PubMed] [Google Scholar]
- 32.Yasuba H, Chihara J, Kino T, Satake N, Oshima S. Increased releasability of platelet products and reduced heparin-induced platelet factor 4 release from endothelial cells in bronchial asthma. J Lipid Mediat 1991;4:5–21 [PubMed] [Google Scholar]
- 33.Wu G, Li F, Li P, Li J, Ruan C. Detection of activated platelets using activation-dependent monoclonal antibody (SZ-51) in clinical disorders. Nouv Rev Fr Hematol 1992;34:31–35 [PubMed] [Google Scholar]
- 34.Taylor ML, Misso NL, Stewart GA, Thompson PJ. Differential expression of platelet activation markers in aspirin-sensitive asthmatics and normal subjects. Clin Exp Allergy 1996;26:202–215 [DOI] [PubMed] [Google Scholar]
- 35.Moritani C, Ishioka S, Haruta Y, Kambe M, Yamakido M. Activation of platelets in bronchial asthma. Chest 1998;113:452–458 [DOI] [PubMed] [Google Scholar]
- 36.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745 [DOI] [PubMed] [Google Scholar]
- 37.Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Rose MJ, Giannini S, Momi S, Spina D, O'Connor B, et al. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol 2003;112:109–118 [DOI] [PubMed] [Google Scholar]
- 38.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood 2005;105:2074–2081 [DOI] [PubMed] [Google Scholar]
- 39.Tutluoglu B, Gurel CB, Ozdas SB, Musellim B, Erturan S, Anakkaya AN, Kilinc G, Ulutin T. Platelet function and fibrinolytic activity in patients with bronchial asthma. Clin Appl Thromb Hemost 2005;11:77–81 [DOI] [PubMed] [Google Scholar]
- 40.Pitchford SC. Defining a role for platelets in allergic inflammation. Biochem Soc Trans 2007;35:1104–1108 [DOI] [PubMed] [Google Scholar]
- 41.Pitchford SC. Novel uses for anti-platelet agents as anti-inflammatory drugs. Br J Pharmacol 2007;152:987–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansson MW, Han S-T, Gunderson KA, Montgomery RR, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin mobilization, and eosinophil beta1 integrin activation occur in asthma and are associated with clinical phenotypes [abstract]. Am J Respir Crit Care Med 2011;183:A4335 [Google Scholar]
- 43.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351 [DOI] [PubMed] [Google Scholar]
- 44.Siegel S. Nonparametric statistics for the behavioral sciences. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- 45.de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood 1992;80:134–142 [PubMed] [Google Scholar]
- 46.Ulfman LH, Joosten DP, van Aalst CW, Lammers JW, van de Graaf EA, Koenderman L, Zwaginga JJ. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol 2003;28:512–519 [DOI] [PubMed] [Google Scholar]
- 47.Calhoun WJ, Jarjour NN, Gleich GJ, Stevens CA, Busse WW. Increased airway inflammation with segmental versus aerosol antigen challenge. Am Rev Respir Dis 1993;147:1465–1471 [DOI] [PubMed] [Google Scholar]
- 48.Zangrilli JG, Shaver JR, Cirelli RA, Cho SK, Garlisi CG, Falcone A, Cuss FM, Fish JE, Peters SP. sVCAM-1 levels after segmental antigen challenge correlate with eosinophil influx, IL-4 and IL-5 production, and the late phase response. Am J Respir Crit Care Med 1995;151:1346–1353 [DOI] [PubMed] [Google Scholar]
- 49.Liu LY, Swensen CA, Kelly EA, Kita H, Busse WW. The relationship of sputum eosinophilia and sputum cell generation of IL-5. J Allergy Clin Immunol 2000;106:1063–1069 [DOI] [PubMed] [Google Scholar]
- 50.Anderson GP. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372:1107–1119 [DOI] [PubMed] [Google Scholar]
- 51.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vijverberg SJ, Koenderman L, Koster ES, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. Biomarkers of therapy responsiveness in asthma: pitfalls and promises. Clin Exp Allergy 2011;41:615–629 [DOI] [PubMed] [Google Scholar]
- 53.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA 1996;93:11877–11882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger G, Hartwell DW, Wagner DD. P-selectin and platelet clearance. Blood 1998;92:4446–4452 [PubMed] [Google Scholar]
- 55.Dole VS, Bergmeier W, Patten IS, Hirahashi J, Mayadas TN, Wagner DD. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thromb Haemost 2007;98:806–812 [PubMed] [Google Scholar]
- 56.Ramos-Barbon D, Fraga-Iriso R, Brienza NS, Montero-Martinez C, Verea-Hernando H, Olivenstein R, Lemiere C, Ernst P, Hamid QA, Martin JG. T cells localize with proliferating smooth muscle alpha-actin+ cell compartments in asthma. Am J Respir Crit Care Med 2010;182:317–324 [DOI] [PubMed] [Google Scholar]
- 57.Pitchford SC, Momi S, Baglioni S, Casali L, Giannini S, Rossi R, Page CP, Gresele P. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am J Respir Crit Care Med 2008;177:604–612 [DOI] [PubMed] [Google Scholar]
- 58.Taytard A, Guenard H, Vuillemin L, Bouvot JL, Vergeret J, Ducassou D, Piquet Y, Freour P. Platelet kinetics in stable atopic asthmatic patients. Am Rev Respir Dis 1986;134:983–985 [DOI] [PubMed] [Google Scholar]
- 59.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010;125:1028–1036e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration 2010;79:147–151 [DOI] [PubMed] [Google Scholar]
- 61.Kanters D, ten Hove W, Luijk B, van Aalst C, Schweizer RC, Lammers JW, Leufkens HG, Raaijmakers JA, Bracke M, Koenderman L. Expression of activated Fc gamma RII discriminates between multiple granulocyte-priming phenotypes in peripheral blood of allergic asthmatic subjects. J Allergy Clin Immunol 2007;120:1073–1081 [DOI] [PubMed] [Google Scholar]
- 62.Luijk B, Lindemans CA, Kanters D, van der Heijde R, Bertics P, Lammers JW, Bates ME, Koenderman L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol 2005;115:997–1003 [DOI] [PubMed] [Google Scholar]
- 63.Warringa RA, Mengelers HJ, Kuijper PH, Raaijmakers JA, Bruijnzeel PL, Koenderman L. In vivo priming of platelet-activating factor-induced eosinophil chemotaxis in allergic asthmatic individuals. Blood 1992;79:1836–1841 [PubMed] [Google Scholar]
- 64.Koenderman L, van der Bruggen T, Schweizer RC, Warringa RA, Coffer P, Caldenhoven E, Lammers JW, Raaijmakers JA. Eosinophil priming by cytokines: from cellular signal to in vivo modulation. Eur Respir J Suppl 1996;22:119s–125s [PubMed] [Google Scholar]
- 65.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem 2006;281:32175–32187 [DOI] [PubMed] [Google Scholar]
- 66.Banerjee ER, Jiang Y, Henderson WR, Jr, Scott LM, Papayannopoulou T. Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol 2007;35:605–617 [DOI] [PubMed] [Google Scholar]
- 67.Banerjee ER, Jiang Y, Henderson WR, Jr, Latchman Y, Papayannopoulou T. Absence of alpha 4 but not beta 2 integrins restrains development of chronic allergic asthma using mouse genetic models. Exp Hematol 2009;37:715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.