Abstract
Rationale: Surfactant protein (SP)-D and SP-A have been implicated in immunomodulation in the lung. It has been reported that patients with idiopathic pulmonary fibrosis (IPF) often have elevated serum levels of SP-A and SP-D, although their role in the disease is not known.
Objectives: The goal of this study was to test the hypothesis that SP-D plays an important role in lung fibrosis using a mouse model of fibrosis induced by bleomycin (BLM).
Methods: Triple transgenic inducible SP-D mice (iSP-D mice), in which rat SP-D is expressed in response to doxycycline (Dox) treatment, were administered BLM (100 U/kg) or saline subcutaneously using miniosmotic pumps.
Measurements and Main Results: BLM-treated iSP-D mice off Dox (SP-D off) had increased lung fibrosis compared with mice on Dox (SP-D on). SP-D deficiency also increased macrophage-dominant cell infiltration and the expression of profibrotic cytokines (transforming growth factor [TGF]-β1, platelet-derived growth factor-AA). Alveolar macrophages isolated from BLM-treated iSP-D mice off Dox (SP-D off) secreted more TGF-β1. Fibrocytes, which are bone marrow–derived mesenchymal progenitor cells, were increased to a greater extent in the lungs of the BLM-treated iSP-D mice off Dox (SP-D off). Fibrocytes isolated from BLM-treated iSP-D mice off Dox (SP-D off) expressed more of the profibrotic cytokine TGF-β1 and more CXCR4, a chemokine receptor that is important in fibrocyte migration into the lungs. Exogenous SP-D administered intratracheally attenuated BLM-induced lung fibrosis in SP-D−/− mice.
Conclusions: These data suggest that alveolar SP-D regulates numbers of macrophages and fibrocytes in the lungs, profibrotic cytokine expression, and fibrotic lung remodeling in response to BLM injury.
Keywords: surfactant, lung fibrosis, macrophage, fibrocyte, growth factor
At a Glance Commentary
Scientific Knowledge on the Subject
Surfactant protein (SP)-D has important innate immune functions in infectious and noninfectious models of lung injury. SP-D is also an important biomarker of idiopathic pulmonary fibrosis (IPF), but the role in fibrotic lung remodeling in the disease is not known.
What This Study Adds to the Field
SP-D deficiency increased numbers of macrophages and fibrocytes in the lungs, profibrotic cytokine expression, and fibrotic lung remodeling in response to bleomycin (BLM) injury. In addition, exogenous SP-D administered intratracheally attenuated BLM-induced lung fibrosis in SP-D−/− mice, suggesting that administration of exogenous SP-D may be a useful antifibrotic therapy for patients with IPF.
Idiopathic pulmonary fibrosis (IPF) is a progressive and lethal disease of the lungs characterized by the proliferation of fibroblasts and deposition of extracellular matrix (1). IPF is a consequence of loss of alveolar–capillary barrier function, disruption of basement membrane integrity by epithelial injury/inflammation, subsequent failure of reepithelialization, and dysregulation of tissue repair driven by profibrotic mediators, including growth factors (1, 2). Moreover, epithelial to mesenchymal transition (EMT) and a bone marrow–derived progenitor cell (the fibrocyte) are critical cellular modulators of fibrosis, as is the resident tissue fibroblast (3–6). Several studies have demonstrated the potential for bone marrow–derived circulating fibrocytes to enter tissues after injury and contribute to wound healing and fibrosis (7–10).
Surfactant protein (SP)-A and SP-D have important innate immune functions as a result of their abilities to evoke both proinflammatory and antiinflammatory responses (11, 12). In addition, SP-A and SP-D are also important biomarkers of IPF (13). Recent studies showed that increased serum SP-A or SP-D levels are independent predictors of mortality among patients with IPF (14, 15). Furthermore, both bleomycin (BLM)-treated SP-A– and SP-D–deficient mice each have enhanced lung injury compared with wild-type (WT) mice. We have shown SP-A plays important roles in modulating inflammation, apoptosis, and epithelial integrity in the BLM-challenged lung (16). Casey and colleagues also reported that mortality, bronchoalveolar lavage cellularity, pulmonary parenchymal inflammation, and tissue levels of 3-nitrotyrosine (NO2 Y) were increased to a greater extent in BLM-treated SP-D knockout (SP-D−/−) mice than in WT mice, suggesting an antiinflammatory role for SP-D in response to noninfectious, subacute lung injury via modulation of oxidative–nitrative stress (17).
Although studies with SP-D−/− mice have provided important information about the role of SP-D in infectious and noninfectious models of lung injury (17, 18), interpretation of the results obtained with these mice is complicated by the fact that they have an altered lung phenotype at baseline marked by hyperplastic type II cells; progressive alveolar lipoproteinosis; numerous enlarged alveolar macrophages; increased matrix metalloproteinases (MMPs), such as MMP-2, MMP-9, and MMP-12; and abnormal morphology that mimics emphysematous changes (19, 20). Zhang and colleagues showed that some abnormalities of surfactant and macrophages are reversible using triple transgenic mice, termed inducible (i) SP-D mice, in which doxycycline (Dox) administration induces expression of the rat SP-D transgene within 3 d of initiation of treatment (21). Therefore, to minimize confounding variables present in the lung phenotype of constitutively deficient SP-D null mouse, we used iSP-D mice subjected to manipulation of exogenous Dox treatment (21, 22) to test the role of SP-D in the pathogenesis of lung fibrosis by examining its effects on regulation of macrophages, fibrocytes, and profibrotic mediators in the pathogenesis of BLM-induced fibrosis.
Some of the results of these studies have been previously reported in the form of an abstract (23).
Methods
Detailed methods are described in the online supplement.
Animals
Conditional (inducible) SP-D mice designated iSP-D mice, a triple transgenic mouse (FVB/N background) in which a rat SP-D transgene is expressed in the distal airway epithelia in response to doxycycline (Dox), and SP-D knockout (SP-D−/−) mice (C57BL/6 background) were generated as previously described (20, 22). WT FVB/N, WT C57BL/6 mice, green fluorescent protein (GFP)-transgenic mice (C57BL/6 Background) were purchased from The Jackson Laboratory and bred in-house to account for environmental conditions. Bone marrow–transplanted mice were prepared as previously described with minor modifications (3). Bone marrow cells were collected from donor GFP-transgenic C57BL/6 mice. After irradiation, bone marrow cells were injected retroorbitally into recipient C57BL/6 WT and SP-D−/− mice.
Bleomycin Treatment
Mini-osmotic pumps (ALZET 2001; DURECT Corporation, Cupertino, CA) containing 200 μl saline with or without BLM (Bedford Laboratories, Bedford, OH) were implanted subcutaneously.
Exogenous SP-D Administration In Vivo
Recombinant rat SP-D (2 μg in 50 μl saline) or 50 μl saline as control was administered intratracheally into BLM-treated SP-D−/− mouse by oropharyngeal aspiration as previously described (24) twice weekly from Days 0 to 28 of exogenous subcutaneous BLM administration.
Bronchoalveolar Lavage
The trachea was cannulated and bronchoalveolar lavage (BAL) was performed six times with 1 ml of 0.1 mM ethylenediaminetetraacetic acid/phosphate-buffered saline. After counting cell number of the BAL fluid, cells were cytospun onto glass slides and stained with Hemacolor (EMD Chemicals, Gibbstown, NJ) for differential cell counting.
Collagen Assay
The left lungs harvested on Day 35 were used for collagen assay. Total lung collagen was determined using the Sircol Collagen Assay kit (Biocolor Ltd., Belfast, Northern Ireland) according to the manufacturer's instructions.
Immunohistochemistry
The sections of snap-frozen lung samples were stained with the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method by using TACS 2 TdT-Fluor In Situ Apoptosis Detection Kit following manufacturer's instruction (Trevigen, Gaithersburg, MD). TUNEL-positive index was calculated by dividing the TUNEL-positive by 4′,6-diamidino-2-phenylindole (DAPI)-positive cell numbers (magnification ×20). Fluorescence images were analyzed using a confocal laser scanning microscope (Zeiss LSM 710; Zeiss, Germany; magnification ×20).
Fluorescence-activated Cell Sorter Analysis
The minced lungs were digested, and the harvested cells were stained with fluorescein isothiocyanate–labeled anti-mouse CD45 Ab, phycoerythrin-labeled anti-mouse CXCR4 Ab, and biotin-conjugated anti-mouse collagen I (Col-I) Ab (Rockland, Gilbertsville, PA), followed by Streptavidin-Texas Red. The stained cells were analyzed by fluorescence-activated cell sorter (FACS) using a BD LSRII (San Diego, CA) for acquisition.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was extracted from murine fibrocytes isolated from the lungs using TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse transcribed into cDNA according to the manufacturer's instructions. Quantitative real-time polymerase chain reaction (PCR) amplification was performed using i-Cycler iQ Detection System (Bio-Rad, Hercules, CA).
Statistical Analysis
Comparisons among multiple groups were analyzed using the one-way analysis of variance with Newman-Keuls post hoc correction (GraphPad Prism, version 5.0; GraphPad Software, Inc., San Diego, CA). Differences were considered statistically significant if P values were less than 0.05.
Results
BLM Injury Increased SP-D and SP-A in BAL Fluid
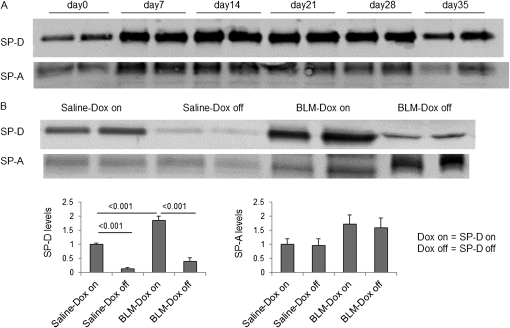
We examined SP-D and SP-A levels in BAL fluid of BLM-treated iSP-D mice on or off Dox (SP-D on or off) by Western blot. Dox treatment resulted in expression of SP-D as expected. After 7 days of BLM treatment, SP-D and SP-A were elevated in BAL fluid of iSP-D mice on Dox (SP-D on) compared with untreated mice (Figure 1A). SP-D levels were greatly reduced by discontinuance of Dox treatment in saline-treated mice compared with mice on Dox on Day 21. In some mice in which Dox treatment was discontinued, SP-D was not detectable by Western blot of BAL fluid. However, some SP-D levels in BALF of BLM-treated iSP-D mice off Dox (SP-D off) were detectable on Day 21 (Figure 1B). On the other hand, SP-A levels were similar between BLM-treated iSP-D mice on and off Dox (SP-D on and off) on Day 21 (see Figure E1B in the online supplement), suggesting that the absence of SP-D does not change the amount of SP-A in the lungs.
Figure 1.
Increased surfactant protein (SP)-D in bronchoalveolar lavage (BAL) fluid of inducible (i) SP-D mice on doxycycline (Dox) (SP-D on) after bleomycin (BLM) treatment. BAL fluid was collected from iSP-D mice on or off Dox (SP-D on or off) on Days 0, 7, 14, 21, or 35 after saline or BLM treatment. Equal amounts of BAL fluid from each mouse were resolved by sodium dodecyl sulfate– polyacrylamide gel electrophoresis, and SP-D or SP-A expression was detected by Western blot analysis. (A) SP-D and SP-A levels in BAL fluid of iSP-D mice on Dox (SP-D on) at the indicated time point after BLM treatment are shown. (B) SP-D and SP-A levels in BAL fluid of iSP-D mice off Dox (SP-D off) on Day 21 after saline or BLM treatment are shown. Data in the lower panel show the average intensity of bands relative to that of saline-Dox on ± SEM using an ImageJ program. n = 6 in each group.
SP-D Deficiency Enhances Fibrotic Response in the Lungs of BLM-treated iSP-D Mice
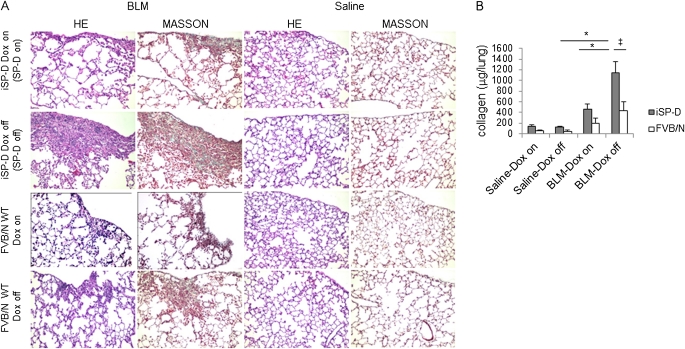
As an initial assessment, histological and biochemical analysis of fibrotic response in mice receiving BLM was performed in the presence/absence of SP-D. Lung histology obtained on Day 35 showed increased fibrosis and collagen deposition in the subpleural areas of the lungs of BLM-treated iSP-D mice off Dox (SP-D off) compared with BLM-treated iSP-D mice on Dox (SP-D on) and saline-treated control mice (Figure 2A). The collagen content of the lung was also significantly higher in BLM-treated iSP-D mice off Dox (SP-D off) compared with the mice on Dox (SP-D on) (Figure 2B). In addition, a histological study for BLM-treated FVB/N WT mice with or without Dox administration was also performed to test whether Dox treatment itself reduces the lung fibrotic response. Dox treatment tended to reduce lung fibrosis in FVB/N WT mice, but lung histology of BLM-treated iSP-D mice off Dox (SP-D off) showed significantly increased fibrosis and collagen depositions, compared with that of BLM-treated FVB/N WT mice without Dox (Figures 2A and 2B). Thus, the increased lung fibrosis in iSP-D mice off Dox predominantly depends on the absence of SP-D rather than the absence of Dox.
Figure 2.
Surfactant protein D (SP-D)–deficient mice develop exaggerated bleomycin (BLM)-induced lung fibrosis. Mice were implanted with miniosmotic pumps containing saline or BLM. (A) Typical photomicrographs of hematoxylin and eosin (HE) and Masson trichrome staining of the lungs from inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) or FVB/N wild-type (WT) mice with or without Dox on Day 35 after saline or BLM treatment. Magnification ×10. (B) Collagen content of the lungs from iSP-D mice on or off Dox (SP-D on or off) or FVB/N WT mice with or without Dox on Day 35 after saline or BLM treatment by Sircol collagen assay. Data are presented as mean ± SEM. n = 4–6 for saline and 6–12 for BLM treatment groups. *P < 0.001; ‡P < 0.01.
SP-D Deficiency Alters the Profile of Profibrotic Cytokines and Cells in BAL Fluid
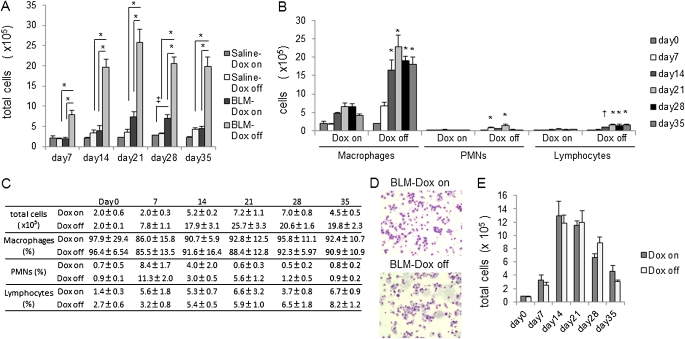
We examined the BAL fluid to test whether the increase in lung fibrosis in the absence of SP-D correlated with increased inflammation. As early as 7 days after BLM treatment, total cell numbers in BAL fluid from iSP-D mice off Dox (SP-D off) were significantly increased compared with that in mice on Dox (SP-D on) (Figures 3A and 3C). In control groups (saline treatment), the total cell number from iSP-D mice off Dox (SP-D off) also trended to be higher than that of the mice on Dox (SP-D on) after Day 21 but failed to achieve statistical significance. Differential cell counts revealed that in iSP-D mice off Dox (SP-D off) after BLM, an increase in macrophages was the primarily contributor to the increase in total cell counts; however, increases in polymorphonuclear neutrophils and lymphocytes were also evident (Figures 3B and 3C). In addition, more foamy macrophages were observed in BAL cells of iSP-D mice off Dox (SP-D off) after BLM treatment (Figure 3D).
Figure 3.
Increased inflammatory cell infiltrates in surfactant protein (SP)-D–deficient mice after bleomycin (BLM) treatment. Bronchoalveolar lavage (BAL) fluid was collected from inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) on Day 0, 7, 14, 21, 28, or 35 after saline or BLM treatment. (A, C) Total cell and (B, C) differential cell counts were performed. BAL fluid was collected from FVB/N wild-type (WT) mice on or off Dox on Day 0, 7, 14, 28, or 35 after BLM treatment. (D) Representative pictures of BAL cells from BLM-treated iSP-D mice on or off Dox (SP-D on or off) on Day 21 after BLM treatment are shown. Magnification ×10. iSP-D mice off Dox (SP-D off) had a higher degree of macrophage-dominant inflammatory cells including foamy macrophages. (E) Total cell counts were performed. Data are presented as mean ± SEM. n = 4–5 in each group. *P < 0.001; ‡P < 0.01; †P < 0.05 versus the Dox on (SP-D on) group. PMNs = polymorphonuclear neutrophils.
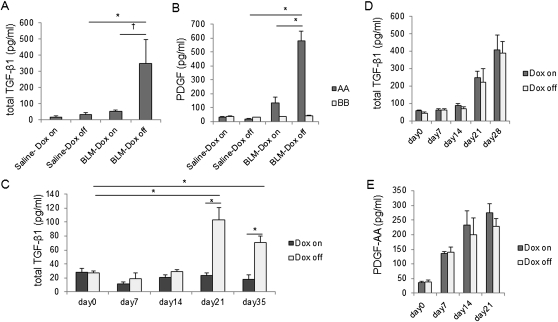
To evaluate which cytokines were primarily associated with the increased lung fibrosis in iSP-D mice off Dox (SP-D off) after BLM treatment, we measured proinflammatory cytokines and profibrotic cytokines in BAL fluid by ELISA. SP-D deficiency resulted in enhanced levels of total transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF)-AA in BAL fluid, whereas the level of PDGF-BB did not change (Figures 4A–4C). On the other hand, tumor necrosis factor-α (TNF-α) and IL-1β levels were not elevated after BLM treatment in either iSP-D mice on and off Dox (SP-D on and off) (data not shown). Our data show that SP-D deficiency contributes to an increase in profibrotic cytokine expression rather than proinflammatory cytokine expression in this model and suggest that the elevated profibrotic cytokine expression may predominantly contribute to the increased lung fibrosis in iSP-D mice off Dox (SP-D off) after BLM treatment. On the other hand, active TGF-β1 levels were not increased in BAL fluid of BLM-treated iSP-D mice off Dox compared with the mice on Dox (data not shown), which suggests that SP-D may not be involved in activation of latent TGF-β. We also performed total cell counts and measured cytokine levels in BAL fluid of BLM-treated FVB/N WT mice with or without Dox administration to test whether Dox treatment changes inflammatory cell infiltration and cytokine expression. BLM treatment resulted in increases in total cell counts and levels of total TGF-β1 and PDGF-AA in BAL fluid of FVB/N WT mice, whereas Dox treatment did not change the total cell number, profibrotic cytokine levels (TGF-β1, PDGF-AA) (Figures 3E, 4D, and 4E) and proinflammatory cytokine levels (TNF-α, IL-1β) (data not shown).
Figure 4.
Increased fibrotic cytokine expression in surfactant protein (SP)-D–deficient mice after bleomycin (BLM) treatment. The levels of (A) total transforming growth factor (TGF)-β1 and (B) platelet-derived growth factor (PDGF)-AA and -BB protein in bronchoalveolar lavage (BAL) fluid from inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) were measured by ELISA on Day 28 (TGF-β1) or Day 21 (PDGF) after saline or BLM treatment. (C) The levels of total TGF-β1 protein in BAL fluid from iSP-D mice on or off Dox (SP-D on or off) were measured by ELISA on days 0, 7, 14, 21, and 35 after BLM treatment. The levels of (D) total TGF-β1 and (E) PDGF-AA protein in the BAL fluid from FVB/N wild-type (WT) mice on or off Dox were measured by ELISA at the indicated time points after BLM treatment. *P < 0.001; †P < 0.05. Data are presented as mean ± SEM. n = 4–5 in each group.
SP-D Regulates Alveolar Macrophage Profibrotic Cytokine Expression
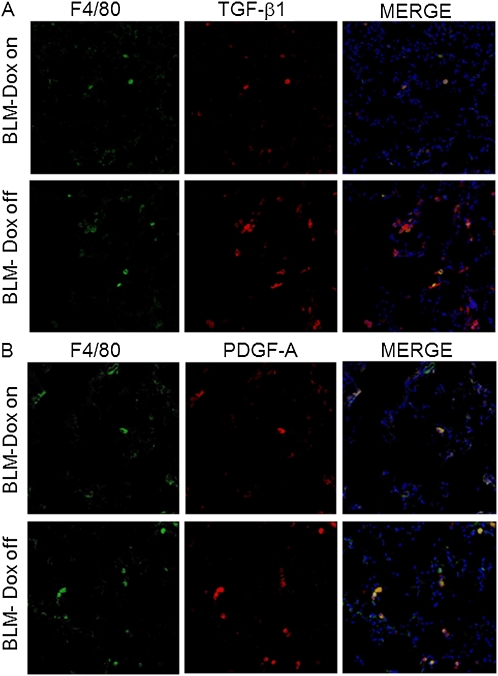
To determine the cellular source of TGF-β1 and PDGF-AA, we performed immunohistochemical staining for TGF-β1 and PDGF-A on lung sections of BLM-treated iSP-D mice on or off Dox (SP-D on or off) on Day 21. TGF-β and PDGF-A were expressed predominantly in alveolar macrophages, defined here as F4/80-positive cells. In addition, an increased number of macrophages expressing TGF-β1 and PDGF-A were observed in iSP-D mice off Dox compared with the mice on Dox (SP-D on) (Figures 5A and 5B). These findings suggest that alveolar macrophages are an important source of TGF-β1 and PDGF-AA and that SP-D is involved in regulating the expression from macrophages in BLM-induced lung fibrosis.
Figure 5.
Surfactant protein (SP)-D deficiency enhances the expression of fibrotic cytokines from alveolar macrophages after bleomycin (BLM) treatment. F4/80 (green) and transforming growth factor (TGF)-β1 or platelet-derived growth factor (PDGF)-A (red) staining was performed with lung sections from inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) on Day 21 after BLM treatment. Representative pictures are shown. Merge (yellow) is costaining for (A) F4/80 and TGF-β1, or (B) F4/80 and PDGF-A indicating TGF-β1– or PDGF-A–expressing macrophages. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Magnification ×20.
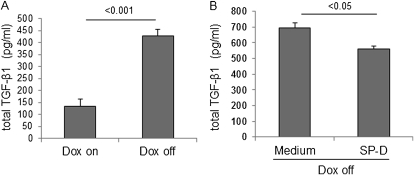
TGF-β is one of the most important profibrotic cytokines in the pathogenesis of pulmonary fibrosis (25, 26), and production of TGF-β by alveolar macrophages has been shown to be involved in fibroblast migration and collagen production (27–29). To test whether SP-D regulates TGF-β1 secretion from alveolar macrophages directly, alveolar macrophages were isolated from untreated iSP-D mice on or off Dox (SP-D on or off) by collecting adherent cells from BAL fluid. Total TGF-β1 levels were measured in culture medium of the macrophages incubated with or without exogenously added SP-D for 3 days. Macrophages from iSP-D mice off Dox (SP-D off) secreted more TGF-β1 compared with macrophages from the mice on Dox (SP-D on) (Figure 6A). The addition of exogenous SP-D significantly reduced TGF-β1 secretion from macrophages isolated from iSP-D mice off Dox (SP-D off) (Figure 6B). These data suggest that SP-D regulates TGF-β1 secretion from macrophages directly.
Figure 6.
Exogenous surfactant protein (SP)-D reduces the secretion of transforming growth factor (TGF)-β1 from alveolar macrophages ex vivo. Macrophages were isolated from bronchoalveolar lavage (BAL) fluid of untreated inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off). TGF-β1 was measured by ELISA in culture medium of the macrophages incubated with or without exogenous SP-D for 3 days. (A) Macrophages from iSP-D mice off Dox (SP-D off) secreted more TGF-β compared with the mice on Dox (SP-D on). (B) Exogenous SP-D reduced TGF-β1 secretion from macrophages from iSP-D mice off Dox (SP-D off). Data are presented as mean ± SEM. n = 4 in each group.
SP-D Regulates Bone Marrow–derived Fibrocyte Recruitment into the Lungs after BLM Treatment
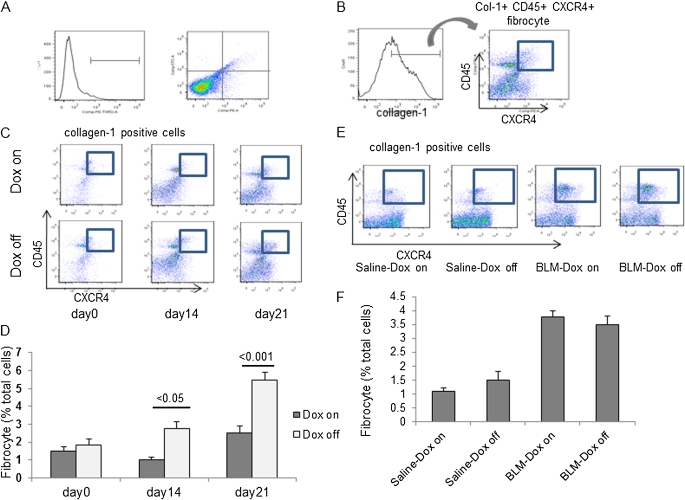
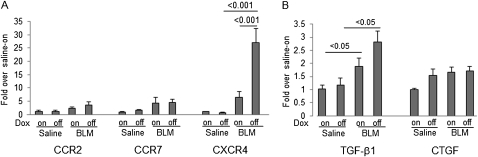
A number of studies reported that bone marrow–derived circulating fibrocytes migrate into the lungs after injury and contribute to fibrosis in the BLM model (3, 5, 8). To test whether SP-D regulates the fibrocyte pool size in the lungs after BLM treatment, we examined the number of fibrocytes in lung digests of BLM-treated iSP-D mice on or off Dox on days 0, 14, and 21 by cell staining and FACS analysis for expression of collagen I (Col- I), CD45, and CXCR4. Fibrocytes were increased to a greater extent in the lungs of iSP-D mice off Dox (SP-D off) compared with the mice on Dox (SP-D on) on Days 14 and 21 (Figures 7C and 7D). Next, we examined the expression of representative chemokine receptors and profibrotic cytokines in fibrocytes isolated from BLM-treated iSP-D mice on and off Dox (SP-D on and off) on Day 21 by real-time PCR. Levels of CCR2 and CCR7 mRNA in fibrocytes were not significantly increased by BLM treatment and were similar in iSP-D mice on and off Dox (SP-D on and off). On the other hand, expression of CXCR4 mRNA, which plays an important role in fibrocyte recruitment into the lungs, was significantly increased in fibrocytes isolated from BLM-treated iSP-D mice off Dox (SP-D off) compared with the mice on Dox (SP-D on) and saline-treated control mice (Figure 8A). In addition, BLM treatment significantly increased TGF-β1 mRNA expression from fibrocytes. SP-D deficiency also tended to increase the expression of TGF-β1 from fibrocytes after BLM treatment, although there was not a significant difference in TGF-β1 levels between iSP-D mice on and off Dox (SP-D on and off) (Figure 8B). Expression of connective tissue growth factor mRNA, which is recognized as a downstream mediator of TGF-β, was similar between each group. These data suggest that SP-D regulates the fibrocyte pool size in the lungs in BLM-induced lung fibrosis via regulating CXCR4 expression on fibrocytes and that fibrocytes secret profibrotic cytokines, thereby promoting fibroblast activation.
Figure 7.
Surfactant protein (SP)-D regulates fibrocyte pool size in the lungs. Lung digests stained for collagen I (Col-I), CD45, and CXCR4 were examined by fluorescence-activated cell sorter (FACS). (A) Lung digests were stained for isotype control IgG with fluorescein isothiocyanate, phycoerythrin, or phycoerythrin-Texas Red. (B) Col-I + fibrocytes were examined for dual expression of CD45 and CXCR4 using the logical gates depicted. Col-I + CD45 + CXCR4 + fibrocytes in lung digests from (C) inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) on Day 0, 14, and 21 after bleomycin (BLM) treatment, or (E) FVB/N wild-type (WT) mice on or off Dox on Day 21 after saline or BLM treatment were examined by FACS. The percent fibrocytes relative to total cells of lung digests ± SEM n = 4 in each group in (D) iSP-D mice at the indicated time points or (F) FVB/N WT mice on Day 21 after BLM treatment.
Figure 8.
Surfactant protein (SP)-D regulates chemokine receptor and growth factor expression from fibrocytes. Fibrocytes were isolated from the lungs of inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) on Day 21 after saline or bleomycin (BLM) treatment. mRNA levels of (A) representative chemokine receptors (CCR2, CCR7, and CXCR4) and (B) fibrotic cytokines (transforming growth factor [TGF]-β1 and connective tissue growth factor [CTGF]) were measured in the cells by real time polymerase chain reaction. Fibrocytes isolated from BLM-treated iSP-D mice off Dox (SP-D off) expressed more CXCR4 mRNA and TGF-β1 mRNA. Data are shown as target gene expression fold over saline–wild-type (WT) (Dox on) group relative to cyclophilin ± SEM. n = 4 in each group.
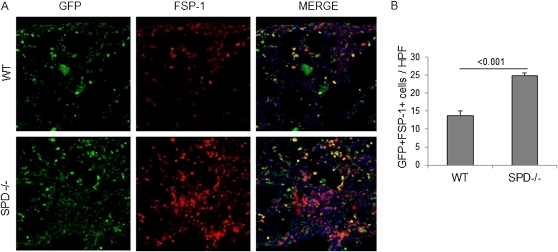
To confirm that the increased fibrocytes in the lungs of BLM-treated iSP-D mice off Dox (SP-D off) are derived from bone marrow, we assessed the number of fibroblast-specific protein-1 (FSP-1)/S100A4–positive bone marrow–derived cells in the lungs of C57BL/6 WT and SP-D−/− mice by using GFP-positive bone marrow cell–transplanted mice (GFP chimeric mice). We performed immunohistochemical staining for GFP and FSP-1 for the lung sections of GFP chimeric WT and SP-D−/− mice on Day 28 after BLM treatment. In quantitative counts, GFP and FSP-1 double-positive cells, which are recognized as bone marrow–derived fibroblasts, were significantly increased to a greater extent in SP-D−/− mice compared with WT mice (Figures 9A and 9B). These data suggest that most of the fibrocytes in SP-D–deficient lungs after BLM treatment are derived from bone marrow.
Figure 9.
Fibroblasts derived from bone marrow can be detected in surfactant protein (SP)-D−/− mice treated with bleomycin (BLM). Analysis of bone marrow–derived mesenchymal cell migration after BLM treatment by immunohistochemistry for green fluorescent protein (GFP) and fibroblast-specific protein (FSP)-1. GFP (green) and FSP-1 (red) staining were performed with lung sections from GFP+ bone marrow cell–transplanted wild-type (WT) or SPD−/− mice on Day 28 after BLM treatment. Merge (yellow) is costaining for GFP and FSP-1 indicating bone marrow–derived cells expressing fibroblast marker, FSP-1. (A) Representative pictures are shown. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Magnification ×20. (B) GFP and FSP-1 double-positive cells were counted from 10 random fields per section at ×20 magnification. GFP and FSP-1 double-positive cells were increased in SP-D−/− mice. Data are presented as mean ± SEM. n = 5 in each group.
Decreased Epithelial Integrity in the Lungs from BLM-treated iSP-D Mice off Dox
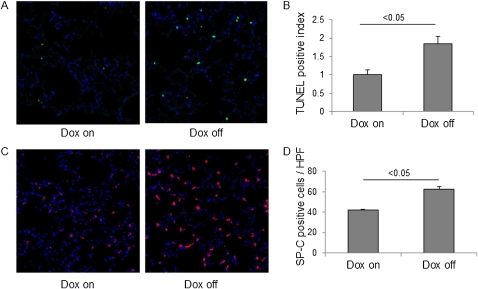
A number of studies now support the hypothesis that epithelial apoptosis contributes to the pathogenesis of lung fibrosis, particularly in the initiation of fibrotic foci (30, 31). Moreover, we recently reported that significantly more apoptotic cells were present in the lungs of intratracheal BLM-challenged SP-A−/− mice compared with WT mice (16). Therefore, to test whether SP-D also inhibits epithelial apoptosis induced by BLM injury, we assessed the level of cell death in the lungs of iSP-D mice on or off Dox (SP-D on or off) by immunohistochemical staining for DNA fragmentation using the TUNEL assay. In quantitative counts, TUNEL-positive cells were significantly increased in the lungs of iSP-D mice off Dox (SP-D off) on Day 7 compared with the mice on Dox (SP-D on) (Figures 10A and 10B). Next, we assessed the number of alveolar type II cells in the lungs by immunohistochemical staining for SP-C. SP-C–positive alveolar type II cells were larger and hyperplastic and had significantly increased numbers in iSP-D mice off Dox (SP-D off) on Day 7 after BLM treatment (Figures 10C and 10D), which indicates that more alveolar epithelial proliferative responses exist in the absence of SP-D after BLM treatment. These results suggest that SP-D potentially protects alveolar epithelium from BLM injury.
Figure 10.
Increased apoptotic cells and hyperplastic type II cells in the lungs in absence of surfactant protein (SP)-D. For analysis of epithelial injury after bleomycin (BLM) treatment, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) and SP-C staining were performed with lung sections from inducible (i) SP-D mice on or off doxycycline (Dox) (SP-D on or off) on Day 7 after BLM treatment. Representative pictures of (A) TUNEL staining and (C) SP-C staining are shown. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Magnification ×20. (B) TUNEL-positive cells and (D) SP-C–positive cells were counted from 10 random fields per section at ×20 magnification, and TUNEL-positive index was calculated as indicated in Methods. Significant increase of TUNEL-positive cells or SP-C–positive hyperplastic type II cells were seen in iSP-D mice off Dox (SP-D off). Data are presented as mean ± SEM. n = 3 in each group.
Exogenous SP-D Administration Attenuated BLM-induced Lung Fibrosis in SP-D−/− mouse
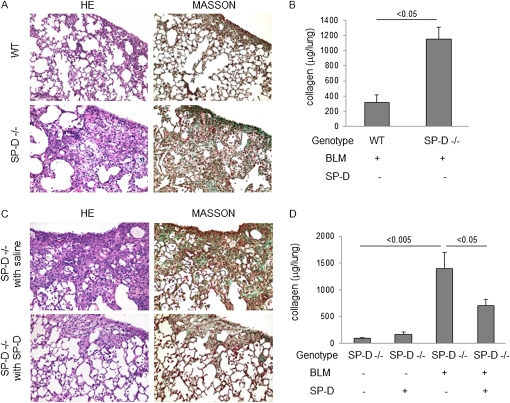
As proof of concept, we next asked the question of whether intratracheally administered exogenous SP-D could reduce BLM-induced lung fibrosis in SP-D−/− mouse. Lung histology obtained on Day 28 showed increased fibrosis and collagen deposition in the lungs of BLM-treated SP-D−/− mice compared with BLM-treated WT mice and decreased fibrosis and collagen deposition in the lungs of BLM-treated SP-D−/− mice treated with exogenous SP-D compared with BLM-treated SP-D−/− mice with control saline administration (Figures 11A and 11C). The collagen content of the lung was also significantly higher in BLM-treated SP-D−/− mice compared with BLM-treated WT mice and significantly lower in BLM-treated SP-D−/− mice given exogenous SP-D compared with BLM-treated SP-D−/− mice with control saline administration (Figures 11B and 11D).
Figure 11.
Exogenous surfactant protein (SP)-D administration attenuates bleomycin-induced lung fibrosis in SP-D−/− mice. SP-D−/− mice were administered exogenous SP-D (2 μg in 50 μl saline) intratracheally twice a week from Days 0 to 28 after bleomycin (BLM) treatment. (A) Typical photomicrographs of hematoxylin and eosin (HE) and Masson trichrome staining of the lungs from BLM-treated wild-type (WT) or SP-D−/− mice on Day 28. (B) Collagen content of the lungs from BLM-treated WT or SP-D−/− mice on Day 28 by Sircol collagen assay. (C) Typical photomicrographs of HE and Masson trichrome staining of the lungs from BLM-treated SP-D−/− mice with control saline or exogenous SP-D administration on Day 28. (D) Collagen content of the lungs in saline or BLM-treated SP-D−/− mice with control saline or exogenous SP-D administration on Day 28 by Sircol collagen assay. Magnification ×10. Data are presented as mean ± SEM. n = 4–6.
Discussion
The purpose of this study was to determine the role of SP-D in the development of lung fibrosis in response to systemic administration of BLM using Alzet miniosmotic pumps. Compared with the intratracheal BLM (ITB) model, the minipump method produces a greater increase in lung hydroxyproline after 6 weeks, produces confluent subpleural fibrosis involving almost 50% of the pleural space (compared with 10–15% in the ITB model), and is generally believed to be more similar to human IPF pathology than the ITB model, although neither fully recapitulates the human disease (32–36). We found that BLM treatment of iSP-D mice off dox (SP-D off) resulted in macrophage-dominant inflammatory cell recruitment and increased levels of profibrotic cytokines (TGF-β1, PDGF-AA), which modulate fibroblast migration, proliferation, and differentiation into myofibroblasts, which in turn produce more extracellular matrix and result in progression of lung fibrosis (32, 37, 38).
Our results with the minipump delivery of BLM extend previous studies reported by Casey and colleagues (17), in that SP-D–deficient mice had enhanced susceptibility to lung fibrosis when challenged with ITB, although the endpoints analyzed are different and there are important differences in the experimental systems, including the use of the ITB model in the Casey and colleagues study (17) and the pump model in our study. In addition, the SP-D−/− mice have an altered lung phenotype at baseline, which we have strived to reduce by Dox treatment before BLM treatment in the current study. Thus, the report of Casey and colleagues (17) highlights the important antiinflammatory role for SP-D in response to noninfectious lung injury via modulation of oxidative–nitrative stress, and our current study highlights the importance of fibrotic mediators in the fibrotic phase using a more chronic mouse model of BLM-induced lung fibrosis.
In this study we have shown that SP-D deficiency increases expression of both TGF-β1 and PDGF-A in vivo from alveolar macrophages, suggesting that SP-D regulates the secretion of these cytokines from alveolar macrophages after BLM treatment. This was confirmed in vitro, where SP-D expression suppressed the elaboration of TGF-β1 from macrophages in primary culture. These results are consistent with those of Khalil and colleagues, who showed that the main source of TGF-β in BLM-treated lungs is alveolar macrophages (27). In another study, both PDGF-A and -B mRNA levels were shown to be markedly increased in alveolar macrophages of patients with IPF at a rate similar to that observed for normal macrophages after in vitro LPS stimulation (39). Considered together, these studies suggest that macrophages are the important source of TGF-β1 and PDGF in lung fibrosis. On the other hand, PDGF-BB levels were not increased in BAL fluid of BLM-treated iSP-D mice off Dox (SP-D off) compared with those of the mice on Dox in our study, although it has been reported that PDGF-BB levels are increased in the lungs of BLM-treated C57BL/6 WT mice and that PDGF-BB is important in lung fibrosis progression (32, 37). It may depend on the different genetic background of mice used in both studies, and SP-D may regulate the expression of PDGF-AA to a greater extent compared with PDGF-BB in lung fibrosis.
Fibrocytes appear to be derived from the differentiation of CD14+ peripheral blood mononuclear cells. They express markers of hematopoietic cells (CD34) and leukocytes (CD11b, CD13, and CD45) as well as fibroblast products (collagens I, III, and fibronectin) (40). Moeller and colleagues found that the percentage of CD45/collagen I (Col-I)–positive fibrocytes is increased in blood of patients with IPF compared with healthy volunteers, and very high circulating fibrocyte percentages are predictive of poor clinical outcome of IPF (41). We have demonstrated increased fibrocytes in the lungs of iSP-D mice off Dox (SP-D off), which contribute to the development of lung fibrosis. Some chemokines (CXCL12, CCL3, CCL12, CCL21) and chemokine receptors (CXCR4, CCR2, CCR5, CCR7) have been shown to correlate with the recruitment of tissue fibrocytes (5, 8, 42), and the CXCL12-CXCR4 biological axis plays an important role in the migration of fibrocytes in BLM-induced lung fibrosis (5). In our study, CXCR4 expression was increased in fibrocytes isolated from the lungs of BLM-treated iSP-D mice off Dox (SP-D off), suggesting that SP-D deficiency increases the number of fibrocytes in the lungs via up-regulating the expression of CXCR4 on fibrocytes. Moreover, other studies have shown that PDGF up-regulates CXCR4 expression on fibrocytes and functions as a strong chemoattractant for fibrocytes (7, 43), which is consistent with our finding of increased fibrocytes in the lungs in the absence of SP-D. In our study, SP-D deficiency resulted in increased levels of PDGF-AA in BALF and PDGF-A expression from alveolar macrophages after BLM treatment. The elevated production of PDGF-AA may contribute to the increased number of fibrocytes in the lungs as well. In addition, a recent study showed that fibrocytes isolated from burned skin produce more TGF-β and connective tissue growth factor, and the culture medium from these fibrocytes promotes the differentiation of fibroblasts into myofibroblasts (44). Another investigation also showed that fibrocytes produce some growth factors, including PDGF, insulin-like growth factor-I, vascular endothelial growth factor, and fibroblast growth factor-2, and the culture medium promotes the proliferation of endothelial cells (45). We also demonstrated that fibrocytes isolated from the lungs express more TGF-β1 in the absence of SP-D after BLM treatment. These data suggest that fibrocytes may regulate fibroblast activation via producing profibrotic cytokines in fibrotic areas and that SP-D may regulate activation of fibrocytes either directly or indirectly.
Recent studies have demonstrated FSP-1 is useful marker of fibroblasts (46). Tanjore and colleagues showed that one-fifth of FSP-1–positive fibroblasts in the lungs are derived from bone marrow in BLM-induced lung fibrosis (6). We show here that GFP, FSP-1 double-positive (bone marrow–derived) fibroblasts are increased in the lungs of BLM-treated GFP chimeric SP-D−/− mice compared with WT mice, suggesting that SP-D is involved in regulating the migration of bone marrow–derived fibroblasts to the lungs. In addition, many bone marrow–derived fibroblasts were observed around the dense fibrotic areas containing a lot of FSP-1 single-positive (e.g., resident or EMT) fibroblasts. It is reasonable to think that the distribution of bone marrow–derived fibroblasts indicates the profibrotic function of the cells for resident or EMT fibroblasts in fibrotic areas.
Dox is known to have antiinflammatory functions and to inhibit MMPs (47, 48). To test whether Dox treatment itself affected our data, we analyzed BLM-treated FVB/N WT mice with and without Dox treatment. Dox treatment attenuated lung fibrosis, but BLM-treated iSP-D mice off Dox (SP-D off) had a significantly greater increase of lung fibrosis compared with BLM-treated FVB/N WT mice without Dox, suggesting that SP-D is a more significant factor for preventing lung fibrosis compared with Dox. Although MMPs seem to play an important role in fibrocyte migration (43), Dox treatment did not change the number of fibrocytes in the lungs in our study (Figures 7E and 7F). Moreover, Dox treatment did not change inflammatory cell infiltrations and the production of proinflammatory and profibrotic cytokines in the BLM-treated iSP-D mouse lungs. Therefore, the antifibrotic function of Dox seems to be limited in this model.
FVB/N mice, in which an increase in Th2 cytokines has been shown to be dominant in inflammatory models, is not commonly used for lung fibrosis studies. Therefore, we examined BAL fluid in BLM-treated FVB/N WT mice or C57BL/6 WT mice that are most commonly used for lung fibrosis studies to test the difference of macrophage biology between the two strains. An increase in macrophages is dominant in BAL fluid of BLM-treated FVB/N WT mice compared with C57BL/6 mice (Figures E1A–E1D). Thus, in this study, macrophage function in the absence of SP-D may be emphasized partly by the dominant recruitment of macrophage after BLM treatment in FVB/N mouse. We also examined the number of fibrocytes in lung digests of C57BL/6 WT or SP-D−/− mice on Day 14 after saline or BLM treatment by FACS. Fibrocytes were increased in the lungs of BLM-treated SP-D−/− mice compared with C57BL/6 WT mice, which is consistent with the data of FVB/N mice. The percentage of fibrocytes was increased up to 11.08%, which is higher than that in FVB/N mice in absence of SP-D (Figures E2A and E2B).
Multiple studies have demonstrated that SP-D in the lung is increased in response to BLM. Work by Savani and colleagues reported an acute rise in BAL SP-D in rat given intratracheal BLM (49). Pan and colleagues demonstrated that BLM treatment increases SP-D levels in BAL fluid, lung homogenate, and serum in rats. Moreover, they showed that by in situ hybridization, there were focal areas of intense SP-D mRNA expression after BLM treatment (50). We also have found that BLM treatment increases SP-D in BAL fluid of iSP-D mice on Dox (SP-D on). To test whether BAL SP-D levels are different in the rescue studies in BLM versus saline treatment, we examined SP-D levels in BAL fluid of saline or BLM-treated SP-D−/− mice after SP-D administration (Figures E3A and E3B). Decline in BAL SP-D levels from 5 minutes to 1 hour after SP-D administration were about twofold greater in BLM-treated mice than saline-treated mice. On the other hand, decline in BAL SP-D levels from 1 hour to 24 hours after SP-D administration were about twofold less in BLM-treated mice than saline-treated mice. Finally, SP-D levels in BAL fluid of the BLM-treated mice were similar to those in saline-treated mice 24 hours after the administration. We speculate that SP-D clearance may be similar between BLM-treated mice and control mice. Following BLM stimulation, increased SP-D potentially could then protect alveolar epithelium from BLM injury and promote repair. In addition, we found that intratracheal exogenous SP-D administration attenuates BLM-induced lung fibrosis in SP-D−/− mice. Although the dose (2 μg) of SP-D administration resulted in SP-D levels in BAL fluid of untreated SP-D−/− mice that were approximately 1.7-fold greater than those in WT mice, SP-D levels in BAL fluid of BLM-treated SP-D−/− mice were decreased to approximately 50% of those in WT mice 24 hours after SP-D administration and by approximately 13% at 48 hours (Figures E3A and E3B). Herbein and colleagues have reported that SP-D clearance from lavage into lung tissue from 5 minutes to 1 hour was 1.7-fold greater in LPS-treated lungs than in control lungs due to the increased SP-D uptake in tissue neutrophils in LPS-treated mice (51), which is consistent with our data. Because the greater decline in BAL SP-D levels from 5 minutes to 1 hour on Day 7 after BLM treatment may be emphasized by the peak of neutrophil infiltration, the decline may be less after Day 14 than on Day 7. Moreover, Takeda and colleagues showed that allergen-induced airway hyper responsiveness and eosinophilia in BAL fluid were reduced by more than 0.2 μg of rat SP-D administration, which is 15 to 20% of that in WT mice, in SP-D−/− mice (52). Although 2 μg of SP-D administration at these intervals into SP-D−/− mice could not ameliorate BLM-induced lung fibrosis to the level of BLM-treated WT mice due to the decreased SP-D levels in the lungs, it is possible that the doses at these intervals are enough to contribute to partial resolution of BLM-induced lung fibrosis. Although increased levels of serum SP-D have been identified as important biomarkers in patients with IPF, it has been reported that SP-D levels in BAL fluid are decreased in patients with IPF (14, 53). Thus, adequate levels of functional SP-D in the lungs may be important in preventing IPF and administration of exogenous SP-D may be a potential antifibrotic therapy for patients with IPF. Although we did not perform SP-D administration during the fibrotic phase in BLM-treated SPD−/− mice, SP-D expression during fibrotic phase (late-phase Dox on) attenuated BLM-induced lung fibrosis in iSP-D mice comparable to SP-D expression through the experiment (Dox on). Moreover, amelioration of lung fibrosis by late-phase Dox on is greater than that by early-phase Dox on (Figures E4A and E4B), suggesting that administration of exogenous SP-D has not only an antiinflammatory effect but also an antifibrotic effect.
In conclusion, our study has shown that, in the absence of SP-D, BLM-treated mice exhibit increased lung fibrosis and infiltration of macrophage-dominant inflammatory cells and fibrocytes into the lungs accompanied by elevations in expression of TGF-β1 and PDGF-AA from these cells and decreased epithelial integrity in the lungs. Furthermore, reconstitution of SP-D—null mice by intratracheal administration of exogenous SP-D attenuates BLM-induced lung fibrosis in SP-D−/− mice. These results indicate that SP-D regulates the recruitment of macrophages and fibrocytes and the expression of profibrotic cytokines from the cells and that SP-D plays an important role in preventing lung fibrosis. We speculate that administration of exogenous SP-D may be a useful antifibrotic therapy for patients with IPF.
Supplementary Material
Acknowledgments
The authors thank Kathy Evans (Duke University) for the preparation of recombinant rat SP-D, Charles Giamberardino (Duke University) for the technical support in Western blot analysis, and Tim Oliver and Katsuyasu Sakurai (Duke University) for the technical support in immunohistochemistry.
Footnotes
Supported by National Institute of Health grants HL-08491-03 and AI-81672-01 (J.R.W.); KAKENHI (20390231), a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Y.N.); and a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labor, and Welfare, Japan (Y.N.).
Author Contributions: Conception and design: Y.A., P.W.N., J.R.W.; analysis and interpretation: Y.A., M.F.B., J.R.W.; acquisition of data: Y.A., J.G.L., S.M., H.O.; writing and revisions: Y.A., Y.N., S.S., M.F.B., P.W.N., J.R.W.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201103-0561OC on December 23, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151 [DOI] [PubMed] [Google Scholar]
- 2.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest 2009;136:1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009;180:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol 2009;41:1708–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562 [DOI] [PubMed] [Google Scholar]
- 10.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol 2010;38:548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23 [DOI] [PubMed] [Google Scholar]
- 12.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005;5:58–68 [DOI] [PubMed] [Google Scholar]
- 13.Greene KE, King TE, Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, Nagae H, Mason RJ. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J 2002;19:439–446 [DOI] [PubMed] [Google Scholar]
- 14.Barlo NP, van Moorsel CH, Ruven HJ, Zanen P, van den Bosch JM, Grutters JC. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:155–161 [PubMed] [Google Scholar]
- 15.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest 2009;135:1557–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto H, Ledford JG, Mukherjee S, Noble PW, Williams KL, Wright JR. The role of surfactant protein A in bleomycin-induced acute lung injury. Am J Respir Crit Care Med 2010;181:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey J, Kaplan J, Atochina-Vasserman EN, Gow AJ, Kadire H, Tomer Y, Fisher JH, Hawgood S, Savani RC, Beers MF. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am J Respir Crit Care Med 2005;172:869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geunes-Boyer S, Oliver TN, Janbon G, Lodge JK, Heitman J, Perfect JR, Wright JR. Surfactant protein D increases phagocytosis of hypocapsular cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun 2009;77:2783–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA 1998;95:11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 2000;97:5972–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Ikegami M, Dey CR, Korfhagen TR, Whitsett JA. Reversibility of pulmonary abnormalities by conditional replacement of surfactant protein D (SP-D) in vivo. J Biol Chem 2002;277:38709–38713 [DOI] [PubMed] [Google Scholar]
- 22.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L552–L561 [DOI] [PubMed] [Google Scholar]
- 23.Aono Y, Redford JG, Beers MF, Wright JR. The role of surfactant protein D In bleomycin induced pulmonary fibrosis [abstract]. Am J Respir Crit Care Med 2010;181:A2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Card JW, Voltz JW, Carey MA, Bradbury JA, Degraff LM, Lih FB, Bonner JC, Morgan DL, Flake GP, Zeldin DC. Cyclooxygenase-2 deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol 2007;37:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991;88:6642–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med 1989;170:727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 1993;92:1812–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J, Yamamoto K. In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-beta in the lung. Thorax 2006;61:886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997;17:272–278 [DOI] [PubMed] [Google Scholar]
- 31.Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 1997;16:91–101 [DOI] [PubMed] [Google Scholar]
- 32.Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, Izumi K, Sone S. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 2005;171:1279–1285 [DOI] [PubMed] [Google Scholar]
- 33.Azuma M, Nishioka Y, Aono Y, Inayama M, Makino H, Kishi J, Shono M, Kinoshita K, Uehara H, Ogushi F, et al. Role of alpha1-acid glycoprotein in therapeutic antifibrotic effects of imatinib with macrolides in mice. Am J Respir Crit Care Med 2007;176:1243–1250 [DOI] [PubMed] [Google Scholar]
- 34.Harrison JH, Jr, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther 1987;243:1185–1194 [PubMed] [Google Scholar]
- 35.Inayama M, Nishioka Y, Azuma M, Muto S, Aono Y, Makino H, Tani K, Uehara H, Izumi K, Itai A, et al. A novel IkappaB kinase-beta inhibitor ameliorates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 2006;173:1016–1022 [DOI] [PubMed] [Google Scholar]
- 36.Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, Matsumoto K, Nakamura T, Takahashi T, Nukiwa T. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am J Respir Crit Care Med 1997;156:1937–1944 [DOI] [PubMed] [Google Scholar]
- 37.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005;201:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, et al. Il-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest 2002;109:931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest 1990;85:2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest 2002;82:1183–1192 [DOI] [PubMed] [Google Scholar]
- 41.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588–594 [DOI] [PubMed] [Google Scholar]
- 42.Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, Matsushima K, Mukaida N. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol 2007;170:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-de-Alba C, Becerril C, Ruiz V, Gonzalez Y, Reyes S, Garcia-Alvarez J, Selman M, Pardo A. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med 2010;182:1144–1152 [DOI] [PubMed] [Google Scholar]
- 44.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen 2007;15:113–121 [DOI] [PubMed] [Google Scholar]
- 45.Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 2001;15:2215–2224 [DOI] [PubMed] [Google Scholar]
- 46.Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:899–907 [DOI] [PubMed] [Google Scholar]
- 47.Fujita M, Ye Q, Ouchi H, Harada E, Inoshima I, Kuwano K, Nakanishi Y. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob Agents Chemother 2006;50:739–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KS, Jin SM, Kim SS, Lee YC. Doxycycline reduces airway inflammation and hyperresponsiveness in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol 2004;113:902–909 [DOI] [PubMed] [Google Scholar]
- 49.Savani RC, Godinez RI, Godinez MH, Wentz E, Zaman A, Cui Z, Pooler PM, Guttentag SH, Beers MF, Gonzales LW, et al. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins b and c. Am J Physiol Lung Cell Mol Physiol 2001;281:L685–L696 [DOI] [PubMed] [Google Scholar]
- 50.Pan T, Nielsen LD, Allen MJ, Shannon KM, Shannon JM, Selman M, Mason RJ. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2002;282:L824–L832 [DOI] [PubMed] [Google Scholar]
- 51.Herbein JF, Wright JR. Enhanced clearance of surfactant protein d during LPS-induced acute inflammation in rat lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L268–L277 [DOI] [PubMed] [Google Scholar]
- 52.Takeda K, Miyahara N, Rha YH, Taube C, Yang ES, Joetham A, Kodama T, Balhorn AM, Dakhama A, Duez C, et al. Surfactant protein d regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med 2003;168:783–789 [DOI] [PubMed] [Google Scholar]
- 53.Kuroki Y, Takahashi H, Chiba H, Akino T. Surfactant proteins A and D: disease markers. Biochim Biophys Acta 1998;1408:334–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.