Abstract
Zebrafish wnt2bb mutants initially fail to form a liver, but surprisingly the liver eventually forms in a majority of these embryos which then develop into fertile adults. This unexpected result raised the possibility that identifying the mechanisms of liver formation in wnt2bb mutants could provide insights into the poorly understood yet general principle of regulative development, a process by which some cells can change fate in order to compensate for a deficiency. Here, we identify two factors that underlie the regulative capacity of endodermal tissues: an intrinsic factor, Sox32, a transcription factor of the SoxF subfamily, and an extrinsic factor, Fgf10a. sox32 is expressed in the extrahepatic duct primordium which is not affected in wnt2bb mutants. Blocking Sox32 function prevented liver formation in most wnt2bb mutants. fgf10a, which is expressed in the mesenchyme surrounding non-hepatic endodermal cells, negatively impacts the regulative capacity of endodermal tissues. In Wnt/β-catenin signaling deficient embryos, in which the liver completely fails to form, the repression of Fgf10a function allowed liver formation. Altogether, these studies reveal that there is more than one way to form a liver, and provide molecular insights into the phenomenon of tissue plasticity.
Keywords: sox17, sox32, fgf10a, developmental plasticity, endoderm, zebrafish
Introduction
Regulative development refers to the process by which cells can change fate in order to compensate for a developmental defect (Gilbert, 2006; Lawrence and Levine, 2006). For example, in Hans Driesch’s classic experiments with sea urchin embryos in 1892, destruction of one cell at the two-cell stage did not interfere with normal development (Sander, 1992). Regulation can also be observed at later developmental stages: for example, removal of the node and anterior primitive streak in early chick embryos did not interfere with normal development either (Psychoyos and Stern, 1996). Importantly, not all embryos display regulative development (Gilbert, 2006), highlighting that it is a specific evolutionary adaptation. Gaining insight into the cellular and molecular mechanisms underlying regulative development is likely to inform us about tissue homeostasis and regeneration.
Zebrafish wnt2bb mutants, as well as embryos with defective Bmp signaling, initially fail to form a liver (Ober et al., 2006; Shin et al., 2007). However, in both cases a liver eventually forms, and wnt2bb mutant embryos in fact develop into healthy and fertile adults, illustrating the remarkable regulative capacity of the foregut endoderm and its derivatives to form a liver. It has recently been reported that wnt2, another Wnt ligand gene which, like wnt2bb, is expressed in the lateral plate mesoderm compensates for the loss of Wnt2bb in liver formation (Poulain and Ober, 2011). wnt2 knockdown abolishes liver formation in most wnt2bb mutants, but not in wild-type (Poulain and Ober, 2011). In addition, we have recently reported that the additional blocking of the Wnt/β-catenin signaling pathway completely abolishes liver formation in wnt2bb mutants (Shin et al., 2011). However, it has not been addressed whether liver formation in wnt2bb mutants is different than in wild-type, or whether it is the same but with a delayed onset.
In this study, we have investigated liver formation in wnt2bb mutants and discovered that it is significantly different from the wild-type process. Sox32 appears to be required for liver formation in wnt2bb mutants, but not in wild-type. In addition, repression of Fgf10a signaling allowed hepatic sox17 expression in wnt2bb mutants, but not in wild-type, and could enhance liver formation in wnt2bb mutants but not in wild-type. These findings illustrate the regulative capacity of endodermal tissues to form a liver, and more importantly shed light into the poorly understood regulative capacity of the vertebrate embryo.
Results
Liver and extrahepatic duct formation in wnt2bb mutants
Liver specification is initially impaired in wnt2bb mutants, but surprisingly the liver eventually forms (Ober et al., 2006). We sought to understand the mechanisms that regulate liver formation in these animals. First, we systematically examined liver formation in wnt2bb mutants. Using the Tg(fabp10:dsRed,ela3l:EGFP)gz12 line that expresses dsRed in hepatocytes and GFP in the acinar cells of the pancreas (Korzh et al., 2008), we observed that the liver eventually formed in most wnt2bbs403 mutants (80-100%; see Table S1). At 4 days post-fertilization (dpf), the liver size in the mutant larvae was variable and much smaller than wild-type, whereas the pancreas was of wild-type size (Fig. 1A-C). We next examined in detail the expression of two hepatoblast markers, hhex and prox1, in wnt2bb mutants during liver development. hhex expression in the liver-forming region was weakly reduced in wnt2bb mutants compared to wild-type at 30 and 36 hours post-fertilization (hpf) (Fig. 1E-H, arrows), whereas prox1 expression was more severely affected (Fig. 1M-P, arrows). Interestingly, at later stages in wild-type embryos, hhex, but not prox1, is expressed in the extrahepatic duct (Fig. 1I and K, arrows), and this expression appeared unaffected in wnt2bb mutants (Fig. 1J and L, arrows).
Fig. 1. Liver formation and the expression patterns of two hepatoblast markers, hhex and prox1, in wnt2bb mutants.
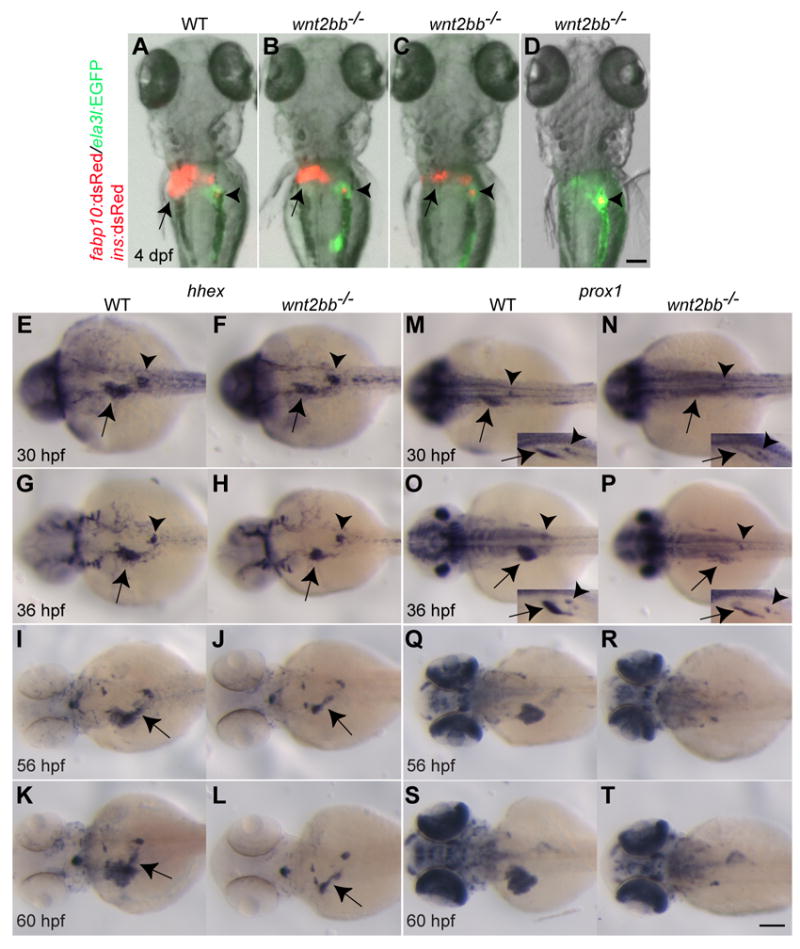
(A-D) The Tg(fabp10:dsRed,ela3l:EGFP);Tg(ins:dsRed) line was used to reveal the liver (red), acinar pancreas (green), and pancreatic beta cells (red). Arrows point to the liver, arrowheads to the endocrine pancreas. The liver formed in most wnt2bb mutants (B and C), but its size was variable and smaller than wild-type. Dorsal views, anterior up. (E-T) hhex expression in the liver-forming region was weakly reduced in wnt2bb mutants compared to wild-type at 30 and 36 hpf (F and H, arrows), whereas prox1 expression was greatly reduced (N and P, arrows). hhex expression in the dorsal pancreas (E-H, arrowheads) and prox1 expression in the interrenal primordium (M-P, arrowheads) appeared unaffected in wnt2bb mutants compared to wild-type. To better visualize hepatic prox1 expression, side-view images are shown in an inset (M-P). Both hhex and prox1 are expressed in the developing liver, but only hhex is expressed in the extrahepatic duct (I-L, arrows). This extrahepatic duct expression appears unaffected in wnt2bb mutants (J and L, arrows). Dorsal views, anterior to the left. Scale bars, 100 μm.
We have recently reported that the additional blocking of the Wnt/β-catenin signaling pathway completely blocks liver formation in wnt2bb mutants (Shin et al., 2011) using the Tg(hsp70l:dkk1-GFP)w32 line that expresses Dkk1, an inhibitor of the Wnt/β-catenin signaling pathway, upon heat-shock (Stoick-Cooper et al., 2007). In Wnt/β-catenin signaling deficient embryos (Tg(hsp70l:dkk1-GFP);wnt2bb-/- embryos heat-shocked at 18 hpf) compared with wnt2bb mutants, both hhex and prox1 expression were profoundly downregulated at 42 hpf (Fig. 2C and F, arrows). Furthermore, when assessed with the pan-endodermal marker foxa3 at 56 hpf, a liver bud was absent in Wnt/β-catenin signaling deficient embryos (Fig. 2I, arrow), but present in wnt2bb mutants (Fig. 2H, arrow). Whole-mount immunostaining with anti-Prox1 and 2F11 antibody, which labels the extrahepatic and extrapancreatic ducts, additionally revealed that the extrahepatic duct as well as the liver bud was absent in Wnt/β-catenin signaling deficient embryos (Fig. 2L). Altogether, these data show that in the absence of Wnt2bb function, the extrahepatic duct forms normally and the liver eventually recovers, whereas a more severe block in Wnt/β-catenin signaling blocks the formation of the extrahepatic duct as well as the liver.
Fig. 2. Loss of Wnt/β-catenin signaling does not affect the formation of the extrapancreatic duct.
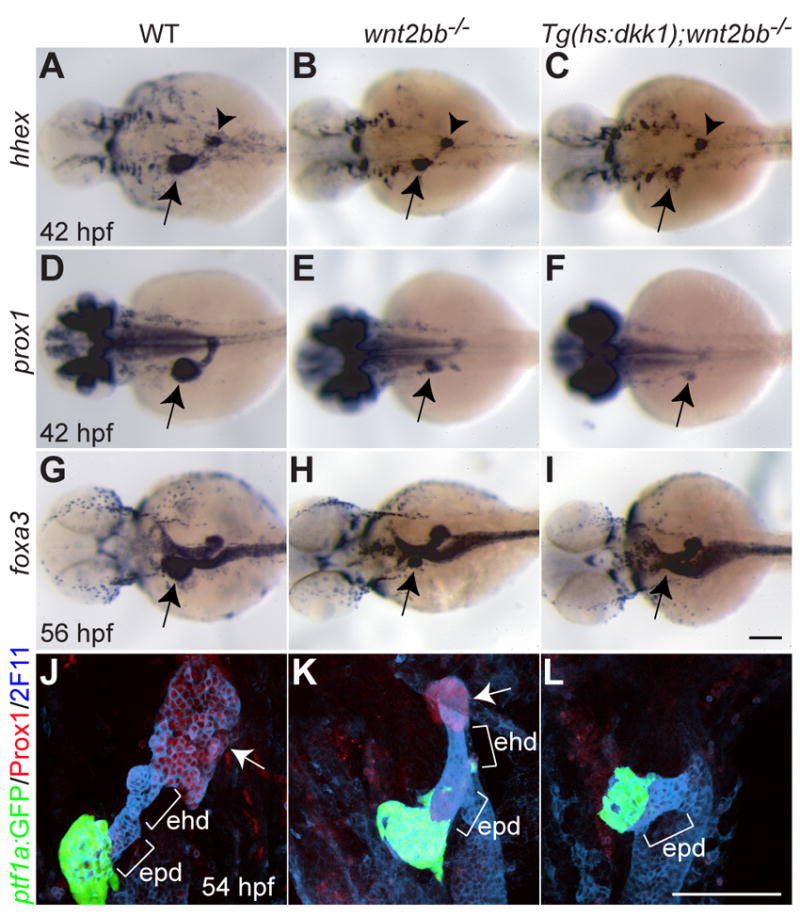
(A-F) hhex (A-C) and prox1 (D-F) expression in the liver-forming region (arrows) at 42 hpf was further reduced in Wnt/β-catenin signaling deficient embryos compared to wnt2bb mutants. However, hhex expression in the dorsal pancreas appeared unaffected (A-C, arrowheads). (G-I) foxa3 expression was examined to reveal the overall gut morphology. A small liver bud formed in wnt2bb mutants at 56 hpf (H), whereas it did not form in Wnt/β-catenin signaling deficient embryos (I). Arrows point to the liver-forming region. Dorsal views, anterior to the left. (J-L) The extrahepatic and extrapancreatic ducts (ehd and epd) were revealed by mAb 2F11 staining, the exocrine pancreas by Tg(ptf1a:GFP) expression, and the liver by Prox1 expression (arrows). Both the liver and the extrahepatic duct failed to form in Wnt/β-catenin signaling deficient embryos (L). Ventral views, anterior up. Scale bars, 100 μm.
sox17 and sox32 are expressed in the forming liver
In order to investigate molecular mechanisms of liver formation in wnt2bb mutants, we sought to identify transcription factor genes expressed in their late forming liver bud. We previously reported that sox17, a transcription factor gene of the SoxF subfamily expressed in all endodermal cells during gastrulation (Alexander and Stainier, 1999), was re-expressed in a ventrally located group of cells on the left side of 48 hpf embryos (Alexander and Stainier, 1999), but we did not investigate the precise identity of these cells. In resolving this issue, we observed that sox17 expression is off by the 6-somite stage through at least 25 hpf (Fig. 3A). By 36 hpf, it is re-expressed in a small ventral region of the liver close to the extrahepatic duct (Fig. 3G), and by 60 hpf, it is expressed in the gallbladder (Fig. 3I, arrowhead). This expression is transient and is gone by 5 dpf (data not shown). Since sox32 is also expressed in all endodermal cells during gastrulation and regulates sox17 expression in these cells (Kikuchi et al., 2001; Sakaguchi et al., 2001), we also examined sox32 expression. sox17 and sox32 (sox17/32) expression patterns in the liver appeared very similar to each other (Fig. 3A-F).
Fig. 3. sox17 and sox32 are expressed in the forming liver of wild-type and wnt2bb mutant embryos.
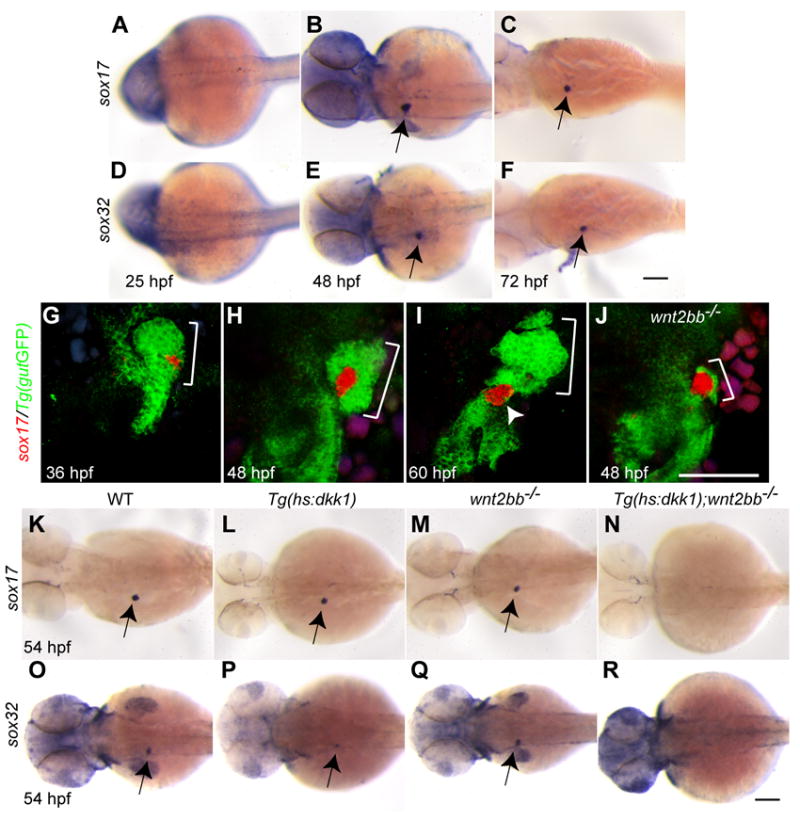
(A-F) sox17 (A-C, arrows) and sox32 (D-F, arrows) are re-expressed in the endoderm at 48 and 72 but not 25 hpf. Dorsal views, anterior to the left. (G-J) sox17 in situ hybridization combined with anti-GFP immunostaining in Tg(gutGFP)s854 embryos reveals sox17 expression (red) in the liver (brackets) and later in the gallbladder (I, arrowhead). At 48 hpf, most of the hepatic bud tissue (brackets) in wnt2bb mutants expressed sox17 (J). Ventral views, anterior up. (K-R) Hepatic sox17 and sox32 expression was absent in Wnt/β-catenin signaling deficient embryos (N and R), whereas it was present in wnt2bb mutants (M and Q) and Tg(hs:dkk1-GFP) embryos heat-shocked at 18 hpf (L and P). Dorsal views, anterior to the left. Scale bars, 100 μm.
We next investigated hepatic sox17/32 expression in wnt2bb mutants and Wnt/β-catenin signaling deficient embryos. sox17/32 expression was present in wnt2bb mutants, but not in Wnt/β-catenin signaling deficient embryos (Fig. 3N and R). Interestingly, most of the late forming liver tissue in wnt2bb mutants expressed sox17 (Fig. 3J) and sox32 (data not shown). This correlation between hepatic sox17/32 expression and liver formation prompted us to investigate the role of these genes in this process.
Sox32 positively regulates liver formation in wnt2bb mutants
Since sox32 is necessary and sufficient for endoderm formation during gastrulation (Dickmeis et al., 2001; Kikuchi et al., 2001; Sakaguchi et al., 2001), it was critical to be able to manipulate Sox32 levels, or activity, after gastrulation in order to investigate its function in later events including liver formation. To block sox17/32 function after gastrulation, we generated a line that expresses a dominant-negative Sox32 upon heat-shock, Tg(hs:dnsox32)s921 (Fig. 4A). The injection of dnsox32 mRNA into Tg(sox17:GFP) s870 embryos, which express GFP under the control of the sox17 promoter (Chung and Stainier, 2008), resulted in a profound reduction in sox17:GFP expression (Fig. 4D), indicating that dnSox32 blocks Sox32 function. Since sox17 expression during gastrulation is regulated by Sox32 (Kikuchi et al., 2001), we asked whether hepatic sox17 expression was also dependent on Sox32. In Tg(hs:dnsox32) embryos heat-shocked at 26 hpf, sox17 expression was absent or greatly reduced (Fig. 4G and H), indicating that hepatic sox17 expression is regulated by Sox32. Importantly, a heat-shock at 26 hpf blocked liver formation in most Tg(hs:dnsox32);wnt2bb-/- embryos (88%, n = 25; Fig. 4L), but not in Tg(hs:dnsox32) embryos (Fig. 4J). Thus, Sox32 function appears to be required for liver formation in wnt2bb mutants.
Fig. 4. Sox32 positively regulates liver formation in wnt2bb mutants.
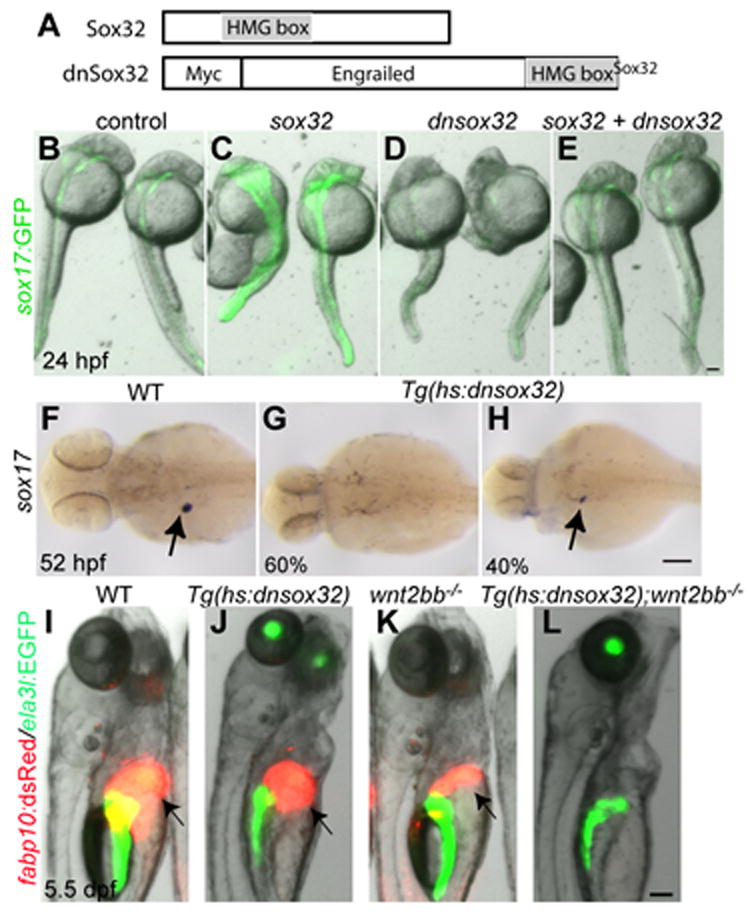
(A) Schematic of the dominant-negative Sox32 construct. The transcriptional repressor domain of Drosophila Engrailed was fused to the HMG box (gray box) of Sox32. (B-E) mRNA injections into Tg(sox17:GFP) embryos reveal that dnSox32 blocks Sox32 function. Injections of sox32 mRNA enhanced GFP expression driven by the sox17 promoter; injections of dnsox32 mRNA reduced it. Co-injections of sox32 and dnsox32 mRNA blocked GFP induction by Sox32. Dorsal views, anterior up. (F-H) Hepatic sox17 expression was almost absent (G, 60%) or greatly reduced (H, 40%) in Tg(hs:dnsox32) embryos heat-shocked at 26 hpf (n = 10). Dorsal views, anterior to the left. (I-L) Tg(hs:dnsox32);wnt2bb-/- embryos were heat-shocked at 26 hpf, and examined at 5.5 dpf. The liver (arrows) formed in all Tg(hs:dnsox32), but failed to form in most Tg(hs:dnsox32);wnt2bb-/- embryos (L, 22 out of 25). Side views, anterior up. Scale bars, 100 μm.
We next investigated whether sox32 could induce liver formation in Wnt/β-catenin signaling deficient embryos. To overexpress sox32 after gastrulation, we generated a line that expresses Sox32 upon heat-shock, Tg(hs:sox32)s926 (Fig. 4A). In Tg(hs:sox32) embryos heat-shocked at 5 hpf, sox17 expression was greatly induced (data not shown), validating this line. The overexpression of Sox32 with heat-shocks at 18 and 26 hpf did not induce liver formation in Wnt/β-catenin signaling deficient embryos (data not shown), suggesting that sox32 is not sufficient for liver formation in this context.
Fgf signaling negatively regulates liver formation in wnt2bb mutants
Since Fgf signaling positively regulates liver specification (Jung et al., 1999; Zhang et al., 2004; Calmont et al., 2006; Shin et al., 2007), we next investigated its role during liver formation in wnt2bb mutants. To block Fgf signaling, we used the Fgfr inhibitor SU5402 at a concentration sufficiently low to allow the animals to survive up to 5 dpf when liver formation was assessed. Surprisingly, we found that reducing Fgf activity appeared to enhance liver formation in wnt2bb mutants (data not shown). Since liver formation occurs in most wnt2bb mutants, we analyzed the effect of Fgf signaling in Wnt/β-catenin signaling deficient embryos in which liver formation is completely blocked. Strikingly, reducing Fgf activity from 26 hpf onward allowed liver formation in 100% of the Wnt/β-catenin signaling deficient embryos (Fig. 5A; Table S2). These data show that Fgf signaling negatively regulates liver formation in wnt2bb mutants as well as in Wnt/β- catenin signaling deficient embryos.
Fig. 5. fgf10a signaling negatively regulates liver formation in wnt2bb mutants.
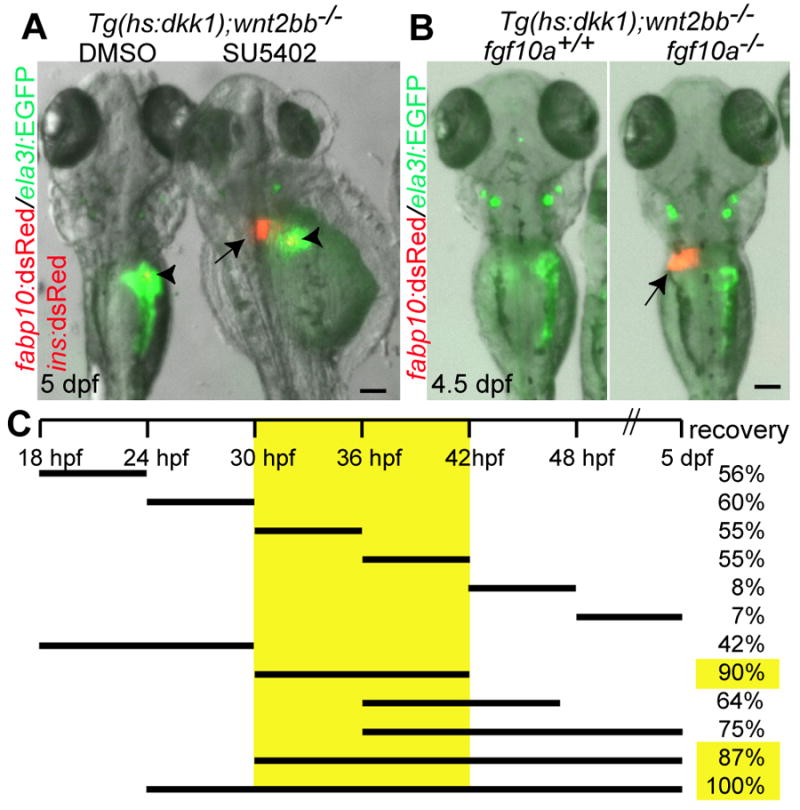
(A) Wnt/β-catenin signaling deficient embryos were treated with SU5402 (1μM) from 26 hpf onward, and examined at 5 dpf. The liver (arrow) formed in SU5402-treated embryos despite severe morphological defects in the head and trunk. Arrowheads point to the endocrine pancreas. (B) The liver (arrow) also formed in fgf10a-/-;Wnt/β-catenin signaling deficient embryos. Dorsal views, anterior up. Scale bars, 100 μm. (C) The time-window critical for Fgf function in liver formation was delineated by treating Wnt/β-catenin signaling deficient embryos with SU5402 at multiple time points and for different duration (black bars); recovery indicates the percentage of embryos showing a liver at 5 dpf (n = 9-23 for each treatment).
We next investigated when Fgf signaling acts to repress liver formation in embryos with compromised Wnt/β-catenin signaling. Specifically, we treated Wnt/β-catenin signaling deficient embryos with SU5402 at multiple time points and for different duration. These experiments revealed that the critical window for the effect of Fgf signaling on liver formation in Wnt/β-catenin signaling deficient embryos is between 30 and 42 hpf, and that inhibiting Fgf signaling after 42 hpf has no effect on this process (Fig. 5C).
Fgf10a signaling negatively regulates liver formation in wnt2bb mutants
To identify the Fgf ligand(s) mediating this effect, we used morpholino antisense oligonucleotides (MO) to reduce the expression of three candidate Fgf ligands: Fgf5, Fgf10a and Fgf24. The genes encoding these ligands are normally expressed around the liver- and/or pancreas-forming region (Fig. S1). fgf10a regulates hepatopancreatic ductal system patterning and differentiation (Dong et al., 2007) and hepatic competence in the endoderm posterior to the liver-forming region (Shin et al., 2011). Both fgf10a and fgf24 have been implicated in exocrine pancreas specification (Manfroid et al., 2007). fgf5 was identified in an analysis of gene expression profiles of 26 hpf trunk endodermal cells (Shin and Stainier, unpublished data). MO knockdown of fgf10a, but not fgf5 or fgf24 (data not shown), allowed liver formation in 100% of Wnt/β-catenin signaling deficient embryos (n = 26; Table S2). The same phenotype was seen in Wnt/β-catenin signaling deficient embryos that were fgf10atbvbo homozygous mutant (n = 9; Fig. 5B and Table S2). Since the extrahepatic duct is also absent when the liver fails to form, we investigated whether the extrahepatic duct did form as well as the liver following Fgf10a repression. Whole-mount immunostaining with 2F11 antibody clearly revealed that the extrahepatic duct also formed in Wnt/β-catenin signaling deficient embryos knocked down for fgf10a (Fig. 6D and E)
Fig. 6. Both the liver and the extrahepatic duct form in Wnt/β-catenin signaling deficient embryos lacking Fgf10a function.
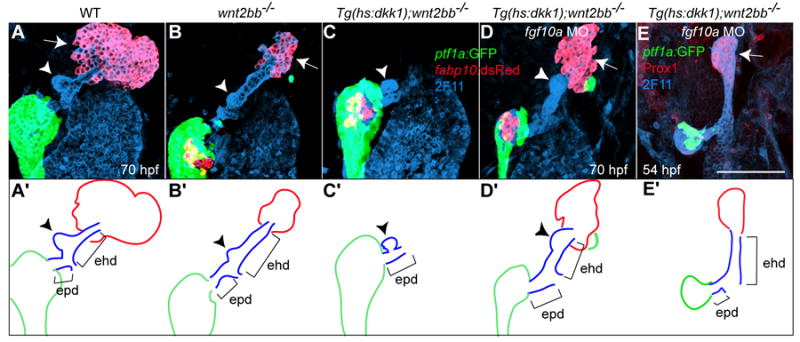
Tg(hs:dkk1-GFP);wnt2bb-/- embryos were injected with fgf10a MO, heat-shocked at 18 hpf, and examined at 54 (E) or 70 (D) hpf. The extrahepatic duct was revealed by mAb 2F11 staining, the exocrine pancreas by Tg(ptf1a:GFP) expression, and the liver by Tg(fabp10:RFP) (A-D) or Prox1 (E) expression. Both the liver (arrows) and the extrahepatic duct (ehd) failed to form in Wnt/β-catenin signaling deficient embryos (C), whereas they did form when these embryos were injected with fgf10a MO (D and E). The gallbladder (arrowheads) formed in the extrahepatic duct when the liver formed, whereas it formed in the extrapancreatic duct (epd) when the liver failed to form (C). (A’-E’) The liver, the extrahepatic and extrapancreatic ducts as well as the pancreas are schematically illustrated. Ventral views, anterior up. Scale bar, 100 μm.
During the fgf10a mutant analysis, we noticed that some of the Wnt/β-catenin signaling deficient embryos forming a liver were heterozygous for the fgf10a mutation, suggesting that a partial loss of Fgf10a was sufficient to rescue liver formation in the absence of Wnt/β-catenin signaling. To further investigate this possibility, we examined 202 Wnt/β-catenin signaling deficient embryos from wnt2bb-/-;fgf10a+/- by Tg(hs:dkk1-GFP);wnt2bb-/- crosses, and genotyped all the GFP+ embryos that formed a liver. Nineteen out of 24 such embryos were fgf10a heterozygous, and the other five were fgf10a wild-type. We also examined whether the reduction of Fgf10a level enhanced liver formation in wnt2bb mutants. We examined 1726 embryos at 5 dpf from wnt2bb-/-;fgf10a+/- by wnt2bb-/- crosses, and genotyped all the embryos that failed to form a liver. Only one out of 25 such embryos was heterozygous for fgf10a and the rest were wild-type. Altogether, these data indicate that a reduction in Fgf10a levels enhances liver formation in wnt2bb mutants as well as in Wnt/β-catenin signaling deficient embryos.
sox32 is induced, but not required for liver formation, in wnt2bb mutants compromised in Fgf signaling
Since hepatic sox17/32 expression correlates with liver formation in wnt2bb mutants and Wnt/β-catenin signaling deficient embryos (Fig. 3K-R), we investigated the expression and role of these genes in wnt2bb mutants and Wnt/β-catenin signaling deficient embryos compromised in Fgf signaling. Hepatic sox17 expression was present in Wnt/β-catenin signaling deficient embryos treated with SU5402 from 30 to 44 hpf (Fig. 7B, arrow), whereas it was absent in controls (Fig. 7A). Hepatic sox17/32 expression was also present in fgf10a-/-;Wnt/β-catenin signaling deficient (Figs. 7D and F, arrows), but not in fgf10a+/+;Wnt/β-catenin signaling deficient embryos (Figs. 7C and E). In addition, the hepatic expression domain of sox17 was often expanded into the extrahepatic duct region in fgf10a-/-;wnt2bb-/- embryos (Fig. 7J, arrow), but not in wild-type, wnt2bb-/- or fgf10a-/- embryos (Fig. 7G-I). These data indicate that loss of Fgf10a function allows hepatic sox17/32 expression in Wnt/β-catenin signaling deficient embryos, and suggest that lack of initial liver formation together with Fgf10a repression leads to the expansion of sox17 expression into the neighboring endoderm.
Fig. 7. Hepatic sox17 and sox32 expression in wnt2bb mutant and Wnt/β-catenin signaling deficient embryos lacking Fgf10a function.
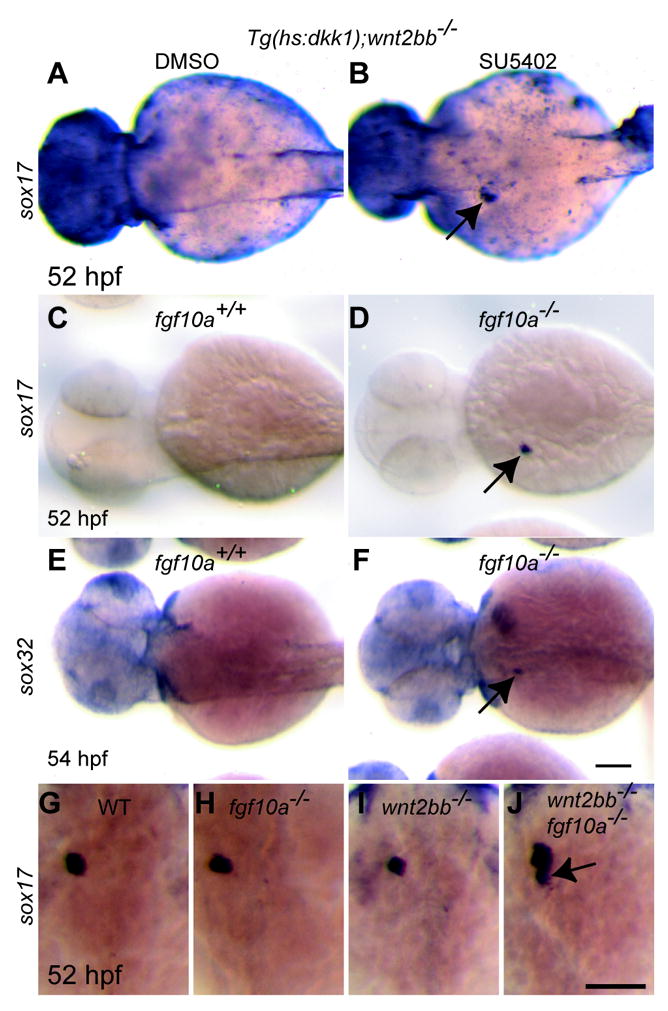
(A-F) Hepatic sox17 expression was present in Wnt/β-catenin signaling deficient embryos treated with SU5402 (1μM) from 30 to 44 hpf (B) as well as in Wnt/β-catenin signaling deficient embryos lacking Fgf10a function (D). Hepatic sox32 expression was also present in these embryos (F). Dorsal views, anterior to the left. (G-J) There was no clear difference in the hepatic sox17 expression domain among wild-type, fgf10a-/- and wnt2bb-/- embryos (G-I). However, it expanded into the extrahepatic duct region in most fgf10a-/-;wnt2bb-/- (J, 5 out of 7 embryos). Dorsal views, anterior up. Scale bars, 100 μm.
We also examined whether liver formation upon loss of Fgf10a function required Sox32 function. We blocked Sox32 function, using the previously described Tg(hs:dnsox32) line, in wnt2bb mutants treated with SU5402 or injected with fgf10a MO, but the liver formed in these embryos (data not shown). These data suggest that the downregulation of Fgf10a signaling can regulate liver formation independently of Sox32.
Discussion
In this study, we identified two novel ways to form a liver during development by investigating liver formation in zebrafish wnt2bb mutants. The first is a Sox32-dependent way that occurs in wnt2bb mutants, in which initial liver specification is defective. The second is an Fgf10a-regulated way that can be observed in Wnt/β-catenin signaling deficient embryos, in which liver formation is completely blocked. These two ways illustrate the regulative capacity of the vertebrate embryos, and shed light into the mechanisms of this poorly understood process.
Liver formation in wnt2bb mutants
Sox17 is the earliest endodermal marker; moreover, Sox17 in mouse (Kanai-Azuma et al., 2002) and Xenopus (Hudson et al., 1997) and sox32 in zebrafish (Dickmeis et al., 2001; Kikuchi et al., 2001; Sakaguchi et al., 2001) are required for endoderm specification. Sox17 expression in part of the liver primordium, and later in the gallbladder, is also observed in other vertebrates including Xenopus (Zorn and Mason, 2001), chick (Chapman et al., 2007), and mouse (Matsui et al., 2006). The role of Sox17 in the formation of the extrahepatic duct and gallbladder has recently been reported (Spence et al., 2009; Uemura et al., 2010). In mouse, SOX17 is initially broadly expressed in the ventral foregut progenitor cells, and is gradually repressed in HHEX+ liver progenitor cells at E8.5 and in PDX1+ pancreatic progenitor cells at E9.5 (Spence et al., 2009; Uemura et al., 2010). Thus, at E9.5, SOX17 expression is present in extrahepatic ductal progenitor cells, but not in the liver or pancreatic progenitor cells. Deletion of Sox17 in the ventral foregut endoderm at E8.5 results in the loss of extrahepatic ductal structures, while Sox17 overexpression induces ectopic extrahepatic ductal tissues (Spence et al., 2009). SOX17 is also expressed in respiratory epithelial cells in mouse (Park et al., 2006). Overexpression of SOX17 in adult alveolar epithelial cells was reported to endow them with characteristics of bronchiolar epithelial cells, suggesting that SOX17 can reprogram adult lung epithelial cells (Park et al., 2006). Intriguingly, Sox17 is also expressed in fetal and neonatal but not adult hematopoietic stem cells in mouse, and is required for their maintenance (Kim et al., 2007).
In wnt2bb mutants, hhex expression appeared unaffected in the extrahepatic duct, and the extrahepatic duct did form. However, in Wnt/β-catenin signaling deficient embryos, hhex expression was further reduced, and both the liver and the extrahepatic duct failed to form. These data raise the possibility that in wnt2bb mutants when liver formation is initially defective, hhex+ extrahepatic ductal cells can later give rise to the liver. Furthermore, sox17/32 expression in the liver bud appeared unaffected in wnt2bb mutants, but was absent in Wnt/β-catenin signaling deficient embryos. The sox17/32+ cells occupied most of the liver bud in wnt2bb mutants, and importantly blocking Sox32 function abolished liver formation. Based on these data and the known functions of Sox17 in mouse, we speculate that the sox17/32+ cells in the liver bud function as progenitor cells and can generate an entire, functional liver, when the wild-type process is defective. However, it is also possible that sox17/32+ cells activate surrounding endodermal cells to give rise to the liver in these embryos. Lineage tracing of sox17/32+ cells should allow one to investigate these possibilities, and will also reveal whether sox17/32+ cells contribute to the liver in wild-type embryos as well as in wnt2bb mutants.
Liver formation in Wnt/β-catenin signaling deficient embryos with Fgf10a repression
Here, we also show that Wnt/β-catenin signaling deficient embryos failed to form a liver, but that repression of Fgf signaling mediated by Fgf10a allowed liver formation in these embryos. We have recently reported that fgf10a is expressed in the mesenchyme surrounding Pdx1+ non-hepatic endodermal cells, and that Fgf10a signaling negatively regulates hepatic competence in these non-hepatic endodermal cells (Shin et al., 2011). In those experiments, the Tg(hsp70l:wnt8a-GFP)w34 line that expresses wnt8a upon heat-shock (Weidinger et al., 2005) was used to provide a hepatic inducing signal. However, in the current study, no ectopic hepatic inducing signal was provided to Wnt/β-catenin signaling deficient embryos. The observation in chick embryos that Hensen’s node regenerates after its removal led to the suggestion that cells adjacent to the node are normally suppressed to become nodal tissue but are released from this suppression when the node is ablated (Psychoyos and Stern, 1996). The factor produced by the node that inhibits neighboring cells to become nodal tissue was subsequently identified as anti-dorsalizing morphogenetic protein (Joubin and Stern, 1999). Similarly, hepatoblasts might produce a factor that inhibits neighboring cells from adopting a liver fate. The absence of hepatoblasts would lead to the activation of the adjacent endodermal cells, which would then contribute to the liver.
Since Fgf10a repression allowed liver formation in Wnt/β-catenin signaling deficient embryos, it could be hypothesized that liver formation in wnt2bb mutants is facilitated by the downregulation of Fgf10a signaling. However, the expression of fgf10a and five general Fgf target genes, dusp6, sef, pea3, erm and etv5, appeared unaffected in wnt2bb mutants (Fig. S2, and data not shown). Nevertheless, at a purely genetic level, wnt2bb mutants were more likely to form a liver if they had one, or two, fgf10a mutant alleles.
Materials and methods
Zebrafish strains
Embryos and adult fish were raised and maintained under standard laboratory conditions (Westerfield, 2000). We used the following mutant and transgenic lines: wnt2bbs403 (Ober et al., 2006), fgf10atbvbo (Norton et al., 2005), Tg(hsp70l:dkk1-GFP)w32 (Stoick-Cooper et al., 2007), Tg(fabp10:dsRed,ela3l:EGFP)gz12 (Korzh et al., 2008), Tg(ptf1a:eGFP)jh1 (Godinho et al., 2005), Tg(gutGFP)s854 (Field et al., 2003), Tg(sox17:GFP)s870 (Chung and Stainier, 2008), Tg(ins:dsRed)m1081 (gift of W. Driever), Tg(hs:dnsox32)s921, and Tg(hs:sox32)s926.
Generation of the Tg(hs:dnsox32) and Tg(hs:sox32) line
The dnsox32 construct was made by fusing the myc-tagged transcriptional repressor domain of Drosophila Engrailed to the HMG box of zebrafish Sox32 (KAPV…MPSS, 94 amino acids). This construct was inserted into an I-SceI meganuclease vector containing the zebrafish heat shock cognate 70-kd protein (hsp70) promoter. To facilitate the identification of germline transmission, the crystallin:venus construct, which drives strong Venus expression in the eye (Kurita et al., 2003), was also inserted into the vector. 1-2 nl of the solution containing the final construct and I-SceI meganuclease was microinjected into one-cell stage embryos to generate a transgenic line as previously described (Grabher et al., 2004). Embryos expressing Venus in the eye were raised to adulthood, and crossed to identify founder fish with germline integration based on Venus expression in the eye. Embryos from four founder fish were heat-shocked and processed for sox32 in situ hybridization. One founder, which expressed the highest level of dnsox32 RNA following heat-shock induction, was selected and used to establish the line. In case of the Tg(hs:sox32) line, a sox32 cDNA covering the entire coding region was inserted into the I-SceI meganuclease vector. The best founder was selected out of two founder fish based on the level of sox32 RNA following heat-shock induction.
Heat-shock conditions
Embryos were heat-shocked at various stages by transferring them into egg water pre-warmed at 37°C (Tg(hsp70l:dkk1-GFP)), 38°C [Tg(hs:dnsox32)) or 39°C (Tg(hs:sox32)], and kept at this temperature for 25 minutes. After heat-shock, the plate containing the embryos was transferred into a 28°C incubator.
In situ hybridization and immunohistochemistry
Whole-mount in situ hybridization was performed as previously described (Alexander et al., 1998), using the following probes: hhex (Ho et al., 1999), prox1 (Glasgow and Tomarev, 1998), foxa3 (Odenthal and Nusslein-Volhard, 1998), fgf10a (Norton et al., 2005), fgf24 (Fischer et al., 2003), fgf5, dusp6 (Tsang et al., 2004), sef (Tsang et al., 2002), pea3 (Munchberg et al., 1999), erm (Munchberg et al., 1999), etv5 (Kudoh et al., 2001), sox17 (Alexander et al., 1999), and sox32 (Kikuchi et al., 2001). Whole-mount immunohistochemistry was performed as previously described (Dong et al., 2007), using the following antibodies: chicken polyclonal anti-GFP (1:1000; Aves Labs, Inc.), rabbit polyclonal anti-Prox1 (1:1000; Chemicon), mouse monoclonal 2F11 (1:100; Abcam), rabbit polyclonal anti-dsRed (1:200; Clontech), and fluorescently conjugated Alexa antibodies (1:250; Molecular Probes).
Morpholino and mRNA injections
Embryos were injected at the one- or two-cell stage with 4 ng of fgf10a splicing MO (Shin et al., 2011), fgf24 splicing MO (Draper et al., 2003), or fgf5 splicing MO (5’-GATCAGACTTACAGTGGCATGAAGC-3’). Tg(sox17:GFP) embryos were injected with 50 pg of sox32 and/or 50-200 pg of dnsox32 mRNA, then GFP expression was analyzed at 24 hpf.
Genotyping of wnt2bb and fgf10a mutants
wnt2bb (Shin et al., 2011) and fgf10a (Norton et al., 2005) genotyping were performed as previously described.
Chemical treatment
A 1 mM stock of the Fgf inhibitor SU5402 (EMD Chemicals) was prepared in 100% DMSO and diluted to 1 μM with egg water. As a control, 0.1% DMSO solution in egg water was used.
Supplementary Material
fgf5 (A-C), fgf10a (D-F) and fgf24 (G-I) are expressed in or around the endoderm during liver and pancreas formation. Arrows point to this expression. Dorsal views, anterior to the left. Scale bar, 100 μm.
fgf10a expression around the endoderm (arrows) appears unaffected in wnt2bb mutants and Wnt/β-catenin signaling deficient embryos, whereas its expression in the pectoral fin (arrowheads) was greatly reduced in Wnt/β-catenin signaling deficient embryos, consistent with previous data (Mercader et al., 2006). Dorsal views, anterior to the left. Scale bar, 100 μm.
Five different wnt2bb-/- pairs were crossed until five clutches were analyzed for each pair. Liver formation was assessed at 6 dpf based on Tg(fabp10:dsRed) expression. The number of embryos that formed a liver (w/ liver column) and those that failed to form a liver (w/o liver column) are presented. The percentage of embryos that formed a liver is also presented (% column). Overall the liver formed in 80-100% of wnt2bb-/- embryos. Generally, clutches from the same parents showed a similar percentage of liver formation, suggesting a genetic influence. The 3rd, 4th and 5th pairs represent sibling fish.
All embryos were heat-shocked at 18 hpf. SU5402 was added from 26 hpf + refers to the number of embryos that formed a liver based on Tg(fabp10:dsRed) expression or that expressed sox17 sox32 in the liver region. nd: not determined.
Highlights.
> hhex expression in the extrahepatic duct is not affected in wnt2bb mutants. > sox17 and sox32 are expressed in the extrahepatic duct primordium. > Blocking Sox32 function abolishes liver formation in wnt2bb mutants. > Fgf10a negatively impacts the regulative capacity of endodermal tissues.
Acknowledgments
We thank Elke Ober for her initial examination of liver formation in wnt2bb mutants, Dan Hesselson and Ryan Anderson for the crystallin:venus and hsp70 promoter constructs, Francesca Mariani for the myc-tagged Engrailed repressor construct, Michael Tsang for in situ probes, Ana Ayala and Milagritos Alva for fish care, Christopher Cain, Francoise Chanut, Amnon Schlegel, Chunyue Yin, Won-Suk Chung and Chong Hyun Shin for discussions and critical reading of the manuscript, and other Stainier lab members for technical help and discussion. DS was supported by NIH institutional NRSA training grants (T32HL007544 and T32DK60414) and the JDRF (3-2008-538). RTM is an Investigator of the HHMI. This work was supported in part by grants from the NIH (DK60322 to DYRS, GM073887 to RTM) and the Packard Foundation (DYRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, Zaret KS. An FGF Response Pathway that Mediates Hepatic Gene Induction in Embryonic Endoderm Cells. Dev Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Matsumoto K, Cai Q, Schoenwolf GC. Specification of germ layer identity in the chick gastrula. Bmc Developmental Biology. 2007;7 doi: 10.1186/1471-213X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Stainier DY. Intra-endodermal interactions are required for pancreatic beta cell induction. Dev Cell. 2008;14:582–593. doi: 10.1016/j.devcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeis T, Mourrain P, Saint-Etienne L, Fischer N, Aanstad P, Clark M, Strahle U, Rosa F. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes & Development. 2001;15:1487–1492. doi: 10.1101/gad.196901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Draper BW, Stock DW, Kimmel CB. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–4654. doi: 10.1242/dev.00671. [DOI] [PubMed] [Google Scholar]
- Field HA, Ober EA, Roeser T, Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fischer S, Draper BW, Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Eighth ed. Sinauer Associates Inc.; Sunderland: 2006. [Google Scholar]
- Glasgow E, Tomarev SI. Restricted expression of the homeobox gene prox 1 in developing zebrafish. Mech Dev. 1998;76:175–178. doi: 10.1016/s0925-4773(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Grabher C, Joly JS, Wittbrodt J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol. 2004;77:381–401. doi: 10.1016/s0091-679x(04)77021-1. [DOI] [PubMed] [Google Scholar]
- Ho CY, Houart C, Wilson SW, Stainier DY. A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Curr Biol. 1999;9:1131–1134. doi: 10.1016/s0960-9822(99)80485-0. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Joubin K, Stern CD. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell. 1999;98:559–571. doi: 10.1016/s0092-8674(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzh S, Pan X, Garcia-Lecea M, Winata CL, Pan X, Wohland T, Korzh V, Gong Z. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. 2008;8:84. doi: 10.1186/1471-213X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, Kiang A, Schultz S, Epstein JA, Toyama R, Dawid IB. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001;11:1979–1987. doi: 10.1101/gr.209601. [DOI] [PubMed] [Google Scholar]
- Kurita R, Sagara H, Aoki Y, Link BA, Arai K, Watanabe S. Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Dev Biol. 2003;255:113–127. doi: 10.1016/s0012-1606(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Levine M. Mosaic and regulative development: two faces of one coin. Curr Biol. 2006;16:R236–239. doi: 10.1016/j.cub.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Manfroid I, Delporte F, Baudhuin A, Motte P, Neumann CJ, Voz ML, Martial JA, Peers B. Reciprocal endoderm-mesoderm interactions mediated by fgf24 and fgf10 govern pancreas development. Development. 2007;134:4011–4021. doi: 10.1242/dev.007823. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. Journal of Cell Science. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Mercader N, Fischer S, Neumann CJ. Prdm1 acts downstream of a sequential RA, Wnt and Fgf signaling cascade during zebrafish forelimb induction. Development. 2006;133:2805–2815. doi: 10.1242/dev.02455. [DOI] [PubMed] [Google Scholar]
- Munchberg SR, Ober EA, Steinbeisser H. Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech Dev. 1999;88:233–236. doi: 10.1016/s0925-4773(99)00179-3. [DOI] [PubMed] [Google Scholar]
- Norton WH, Ledin J, Grandel H, Neumann CJ. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development. 2005;132:4963–4973. doi: 10.1242/dev.02084. [DOI] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Developmental Biology. 2006;294:192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Poulain M, Ober EA. Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development. 2011;138:3557–3568. doi: 10.1242/dev.055921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos D, Stern CD. Restoration of the organizer after radical ablation of Hensen’s node and the anterior primitive streak in the chick embryo. Development. 1996;122:3263–3273. doi: 10.1242/dev.122.10.3263. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Kuroiwa A, Takeda H. A novel sox gene, 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mechanisms of Development. 2001;107:25–38. doi: 10.1016/s0925-4773(01)00453-1. [DOI] [PubMed] [Google Scholar]
- Sander K. Shaking a Concept - Driesch,Hans, and the Varied Fates of Sea-Urchin Blastomeres. Rouxs Archives of Developmental Biology. 1992;201:265–267. doi: 10.1007/BF00592106. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee Y, Poss KD, Stainier DYR. Restriction of hepatic competence by Fgf signaling. Development. 2011;138:1339–1348. doi: 10.1242/dev.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DY. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SCJ, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 Regulates Organ Lineage Segregation of Ventral Foregut Progenitor Cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Tsang M, Friesel R, Kudoh T, Dawid IB. Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol. 2002;4:165–169. doi: 10.1038/ncb749. [DOI] [PubMed] [Google Scholar]
- Tsang M, Maegawa S, Kiang A, Habas R, Weinberg E, Dawid IB. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- Uemura M, Hara K, Shitara H, Ishii R, Tsunekawa N, Miura Y, Kurohmaru M, Taya C, Yonekawa H, Kanai-Azuma M, Kanai Y. Expression and function of mouse Sox17 gene in the specification of gallbladder/bile-duct progenitors during early foregut morphogenesis. Biochem Bioph Res Co. 2010;391:357–363. doi: 10.1016/j.bbrc.2009.11.063. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; 2000. [Google Scholar]
- Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–326. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Mason J. Gene expression in the embryonic Xenopus liver. Mechanisms of Development. 2001;103:153–157. doi: 10.1016/s0925-4773(01)00341-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fgf5 (A-C), fgf10a (D-F) and fgf24 (G-I) are expressed in or around the endoderm during liver and pancreas formation. Arrows point to this expression. Dorsal views, anterior to the left. Scale bar, 100 μm.
fgf10a expression around the endoderm (arrows) appears unaffected in wnt2bb mutants and Wnt/β-catenin signaling deficient embryos, whereas its expression in the pectoral fin (arrowheads) was greatly reduced in Wnt/β-catenin signaling deficient embryos, consistent with previous data (Mercader et al., 2006). Dorsal views, anterior to the left. Scale bar, 100 μm.
Five different wnt2bb-/- pairs were crossed until five clutches were analyzed for each pair. Liver formation was assessed at 6 dpf based on Tg(fabp10:dsRed) expression. The number of embryos that formed a liver (w/ liver column) and those that failed to form a liver (w/o liver column) are presented. The percentage of embryos that formed a liver is also presented (% column). Overall the liver formed in 80-100% of wnt2bb-/- embryos. Generally, clutches from the same parents showed a similar percentage of liver formation, suggesting a genetic influence. The 3rd, 4th and 5th pairs represent sibling fish.
All embryos were heat-shocked at 18 hpf. SU5402 was added from 26 hpf + refers to the number of embryos that formed a liver based on Tg(fabp10:dsRed) expression or that expressed sox17 sox32 in the liver region. nd: not determined.


