Abstract
1,25-Dihydroxyvitamin D (1,25-(OH)2D3) directly suppresses the secretion and synthesis of PTH in vivo and in cell culture. This compound has been used to treat secondary hyperparathyroidism associated with renal failure, but in some patients prolonged treatment with 1,25-(OH)2D3 results in hypercalcemia. An analogue of 1,25-(OH)2D3 with little or no calcemic activity, 22-oxacalcitriol (OCT), was recently developed. We confirmed this lack of calcemic activity by acute and chronic administration to normal rats. A single intraperitoneal injection of vehicle (propylene glycol), OCT, or 1,25-(OH)2D3 (1.0 micrograms/rat) increased calcium by 0.32, 0.30, and 1.40 mg/dl, respectively. When rats were given daily injections of vehicle or 0.5 micrograms of either 1,25-(OH)2D3 or OCT for 4 d, calcium did not change in the rats receiving vehicle or OCT, but increased from 8.4 to 11.4 mg/dl in the rats treated with 1,25-(OH)2D3. In primary cultures of bovine parathyroid cells, 10 nM OCT was as active as 10 nM 1,25-(OH)2D3, suppressing PTH release by 33%. This suppression is due, at least in part, to blocking of transcription of the PTH gene. Using a probe prepared by random prime labeling of an Msp I fragment of plasmid PTHm122, we found that a single 40-ng dose of OCT or 1,25-(OH)2D3 depressed PTH mRNA levels by 70-80% by 48 h when compared with vehicle. Thus, OCT is a very effective suppressor of PTH secretion with virtually no calcemic activity. This analogue may be a valuable tool for the treatment of secondary hyperparathyroidism.
Full text
PDF

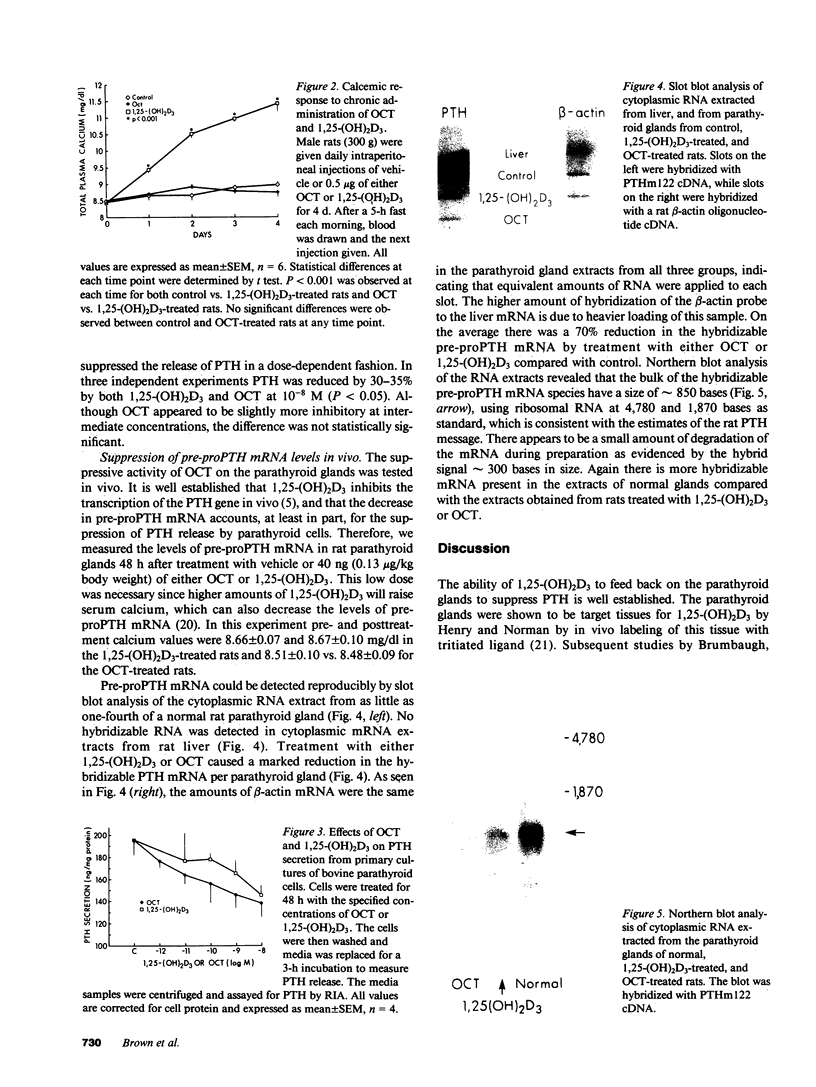


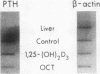
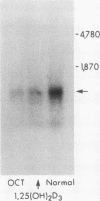
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe J., Morikawa M., Miyamoto K., Kaiho S., Fukushima M., Miyaura C., Abe E., Suda T., Nishii Y. Synthetic analogues of vitamin D3 with an oxygen atom in the side chain skeleton. A trial of the development of vitamin D compounds which exhibit potent differentiation-inducing activity without inducing hypercalcemia. FEBS Lett. 1987 Dec 21;226(1):58–62. doi: 10.1016/0014-5793(87)80550-1. [DOI] [PubMed] [Google Scholar]
- Berl T., Berns A. S., Hufer W. E., Hammill K., Alfrey A. C., Arnaud C. D., Schrier R. W. 1,25 dihydroxycholecalciferol effects in chronic dialysis. A double-blind controlled study. Ann Intern Med. 1978 Jun;88(6):774–780. doi: 10.7326/0003-4819-88-6-774. [DOI] [PubMed] [Google Scholar]
- Binderup L., Bramm E. Effects of a novel vitamin D analogue MC903 on cell proliferation and differentiation in vitro and on calcium metabolism in vivo. Biochem Pharmacol. 1988 Mar 1;37(5):889–895. doi: 10.1016/0006-2952(88)90177-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Aurbach G. D. Preparation of viable isolated bovine parathyroid cells. Endocrinology. 1976 Dec;99(6):1582–1588. doi: 10.1210/endo-99-6-1582. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Wilson R. E., Eastman R. C., Pallotta J., Marynick S. P. Abnormal regulation of parathyroid hormone release by calcium in secondary hyperparathyroidism due to chronic renal failure. J Clin Endocrinol Metab. 1982 Jan;54(1):172–179. doi: 10.1210/jcem-54-1-172. [DOI] [PubMed] [Google Scholar]
- Cantley L. K., Russell J., Lettieri D., Sherwood L. M. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology. 1985 Nov;117(5):2114–2119. doi: 10.1210/endo-117-5-2114. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., McKay C., Dye E., Slatopolsky E. The effect of 1,25 dihydroxycholecalciferol on parathyroid hormone secretion by monolayer cultures of bovine parathyroid cells. Calcif Tissue Int. 1986 Jan;38(1):27–32. doi: 10.1007/BF02556591. [DOI] [PubMed] [Google Scholar]
- Chertow B. S., Baylink D. J., Wergedal J. E., Su M. H., Norman A. W. Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretion in vitro by 1,25-dihydroxycholecalciferol. J Clin Invest. 1975 Sep;56(3):668–678. doi: 10.1172/JCI108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmez J. A., Tindira C., Grooms P., Dusso A., Windus D. W., Slatopolsky E. Parathyroid hormone suppression by intravenous 1,25-dihydroxyvitamin D. A role for increased sensitivity to calcium. J Clin Invest. 1989 Apr;83(4):1349–1355. doi: 10.1172/JCI114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Studies on the mechanism of action of calciferol VII. Localization of 1,25-dihydroxy-vitamin D3 in chick parathyroid glands. Biochem Biophys Res Commun. 1975 Feb 17;62(4):781–788. doi: 10.1016/0006-291x(75)90391-5. [DOI] [PubMed] [Google Scholar]
- Hercz G., Kraut J. A., Andress D. A., Howard N., Roberts C., Shinaberger J. H., Sherrard D. J., Coburn J. W. Use of calcium carbonate as a phosphate binder in dialysis patients. Miner Electrolyte Metab. 1986;12(5-6):314–319. [PubMed] [Google Scholar]
- Hruska K. A., Kopelman R., Rutherford W. E., Klahr S., Slatopolsky E., Greenwalt A., Bascom T., Markham J. Metabolism in immunoreactive parathyroid hormone in the dog. The role of the kidney and the effects of chronic renal disease. J Clin Invest. 1975 Jul;56(1):39–48. doi: 10.1172/JCI108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriniere P., Roussel A., Tahiri Y., de Fremont J. F., Maurel G., Jaudon M. C., Gueris J., Fournier A. Substitution of aluminium hydroxide by high doses of calcium carbonate in patients on chronic haemodialysis: disappearance of hyperaluminaemia and equal control of hyperparathyroidism. Proc Eur Dial Transplant Assoc. 1983;19:784–787. [PubMed] [Google Scholar]
- Morrissey J. J., Cohn D. V. The effects of calcium and magnesium on the secretion of parathormone and parathyroid secretory protein by isolated porcine parathyroid cells. Endocrinology. 1978 Dec;103(6):2081–2090. doi: 10.1210/endo-103-6-2081. [DOI] [PubMed] [Google Scholar]
- Murayama E., Miyamoto K., Kubodera N., Mori T., Matsunaga I. Synthetic studies of vitamin D3 analogues. VIII. Synthesis of 22-oxavitamin D3 analogues. Chem Pharm Bull (Tokyo) 1986 Oct;34(10):4410–4413. doi: 10.1248/cpb.34.4410. [DOI] [PubMed] [Google Scholar]
- Ostrem V. K., Tanaka Y., Prahl J., DeLuca H. F., Ikekawa N. 24- and 26-homo-1,25-dihydroxyvitamin D3: preferential activity in inducing differentiation of human leukemia cells HL-60 in vitro. Proc Natl Acad Sci U S A. 1987 May;84(9):2610–2614. doi: 10.1073/pnas.84.9.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior J. C., Cameron E. C., Ballon H. S., Lirenman D. S., Moriarty M. V., Price J. D. Experience with 1,25-dihydroxycholecalciferol therapy in undergoing hemodialysis patients with progressive vitamin D2-treated osteodystrophy. Am J Med. 1979 Oct;67(4):583–589. doi: 10.1016/0002-9343(79)90238-9. [DOI] [PubMed] [Google Scholar]
- Russell J., Lettieri D., Sherwood L. M. Direct regulation by calcium of cytoplasmic messenger ribonucleic acid coding for pre-proparathyroid hormone in isolated bovine parathyroid cells. J Clin Invest. 1983 Nov;72(5):1851–1855. doi: 10.1172/JCI111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Lettieri D., Sherwood L. M. Suppression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinology. 1986 Dec;119(6):2864–2866. doi: 10.1210/endo-119-6-2864. [DOI] [PubMed] [Google Scholar]
- Salusky I. B., Coburn J. W., Foley J., Nelson P., Fine R. N. Effects of oral calcium carbonate on control of serum phosphorus and changes in plasma aluminum levels after discontinuation of aluminum-containing gels in children receiving dialysis. J Pediatr. 1986 May;108(5 Pt 1):767–770. doi: 10.1016/s0022-3476(86)81064-2. [DOI] [PubMed] [Google Scholar]
- Salusky I. B., Fine R. N., Kangarloo H., Gold R., Paunier L., Goodman W. G., Brill J. E., Gilli G., Slatopolsky E., Coburn J. W. "High-dose" calcitriol for control of renal osteodystrophy in children on CAPD. Kidney Int. 1987 Jul;32(1):89–95. doi: 10.1038/ki.1987.176. [DOI] [PubMed] [Google Scholar]
- Silver J., Naveh-Many T., Mayer H., Schmelzer H. J., Popovtzer M. M. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986 Nov;78(5):1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Russell J., Sherwood L. M. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Weerts C., Lopez-Hilker S., Norwood K., Zink M., Windus D., Delmez J. Calcium carbonate as a phosphate binder in patients with chronic renal failure undergoing dialysis. N Engl J Med. 1986 Jul 17;315(3):157–161. doi: 10.1056/NEJM198607173150304. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Weerts C., Thielan J., Horst R., Harter H., Martin K. J. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984 Dec;74(6):2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]