Abstract
The production of tumor necrosis factor alpha (TNF alpha) and IL-1 beta by the monocytic cell line THP-1, productively infected with HIV-1, was investigated using specific RIA and Northern blot analysis. HIV-infected cells, like uninfected cells, did not constitutively produce any detectable amounts of protein or mRNA for TNF alpha or IL-1 beta. After stimulation with LPS or a combination of LPS plus IFN-gamma, TNF alpha and IL-1 beta were detected in tissue culture supernatants and cell lysates and transcripts for both cytokines were seen on Northern blots. No significant difference in production of these two cytokines was observed between uninfected and chronically infected cells. Acutely HIV-infected cells, however, showed phenotypic changes compatible with maturation and an increase in TNF alpha and IL-1 beta mRNA production, and released significantly higher levels of TNF alpha and IL-1 beta compared with chronically infected or uninfected cells. Furthermore, LPS stimulation of HIV-infected cells increased virus production. These results suggest that HIV-infected monocytic cells may produce increased amounts of TNF alpha and IL-1 beta in response to stimuli that could be present in vivo.
Full text
PDF
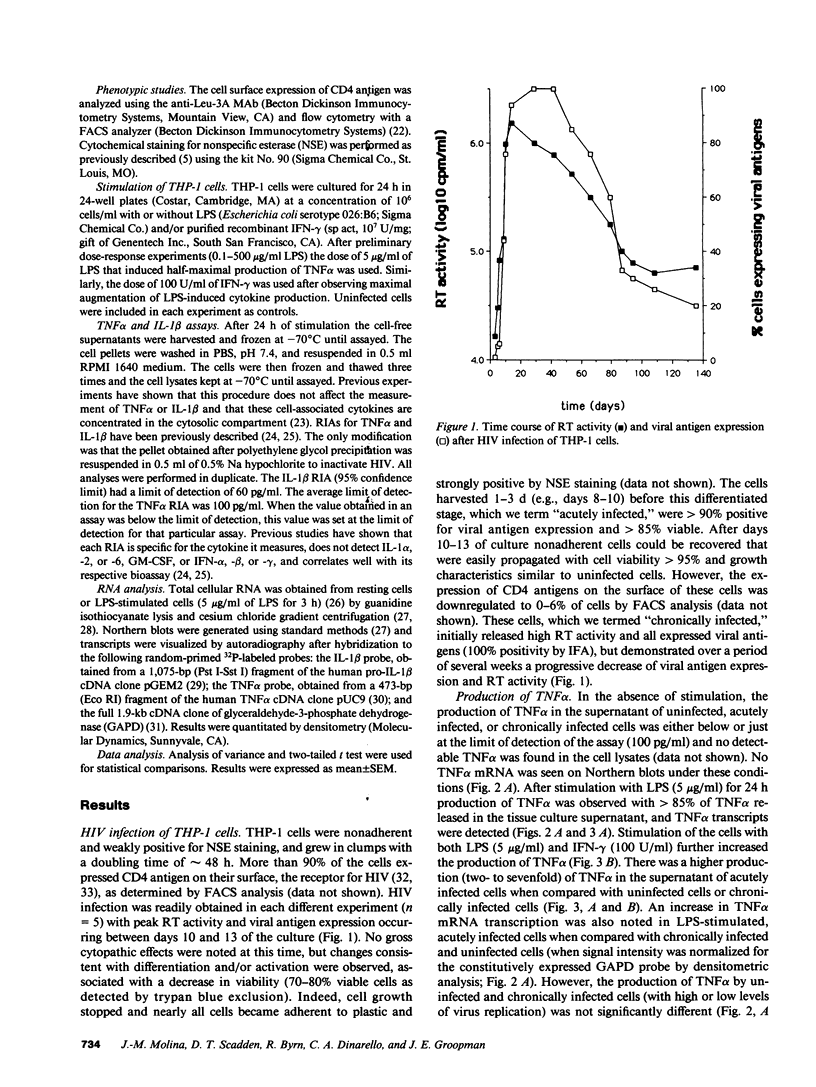
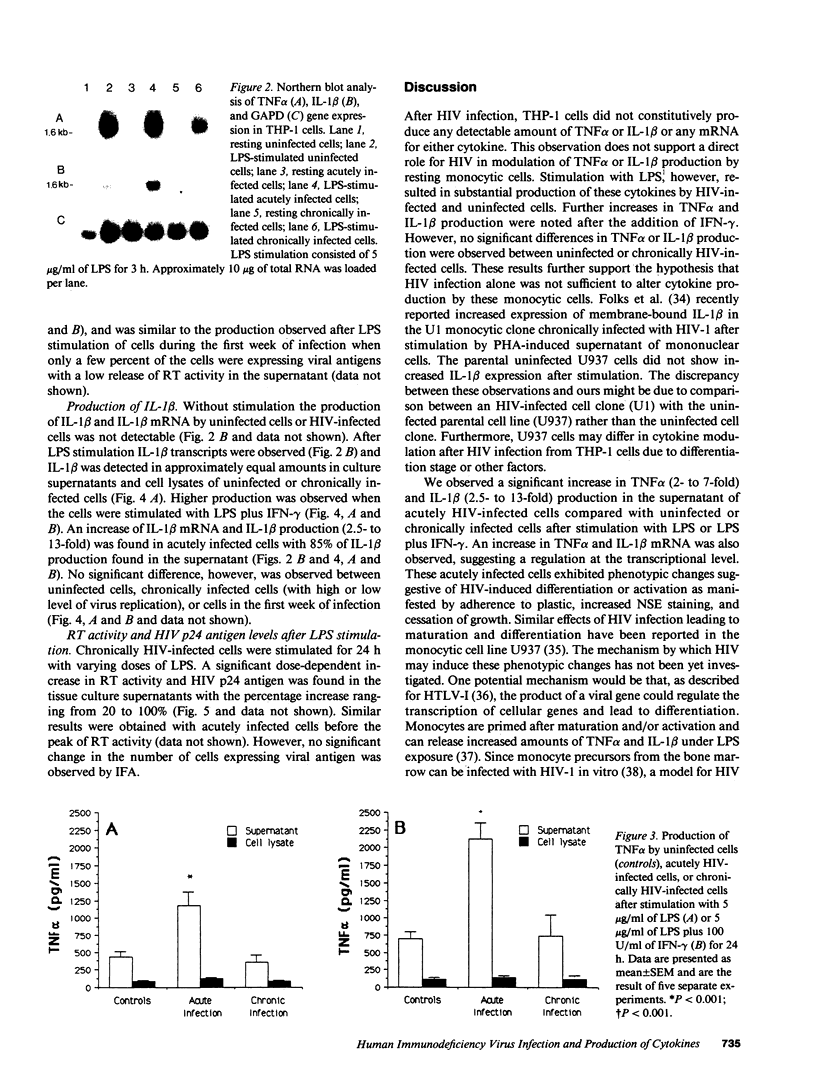
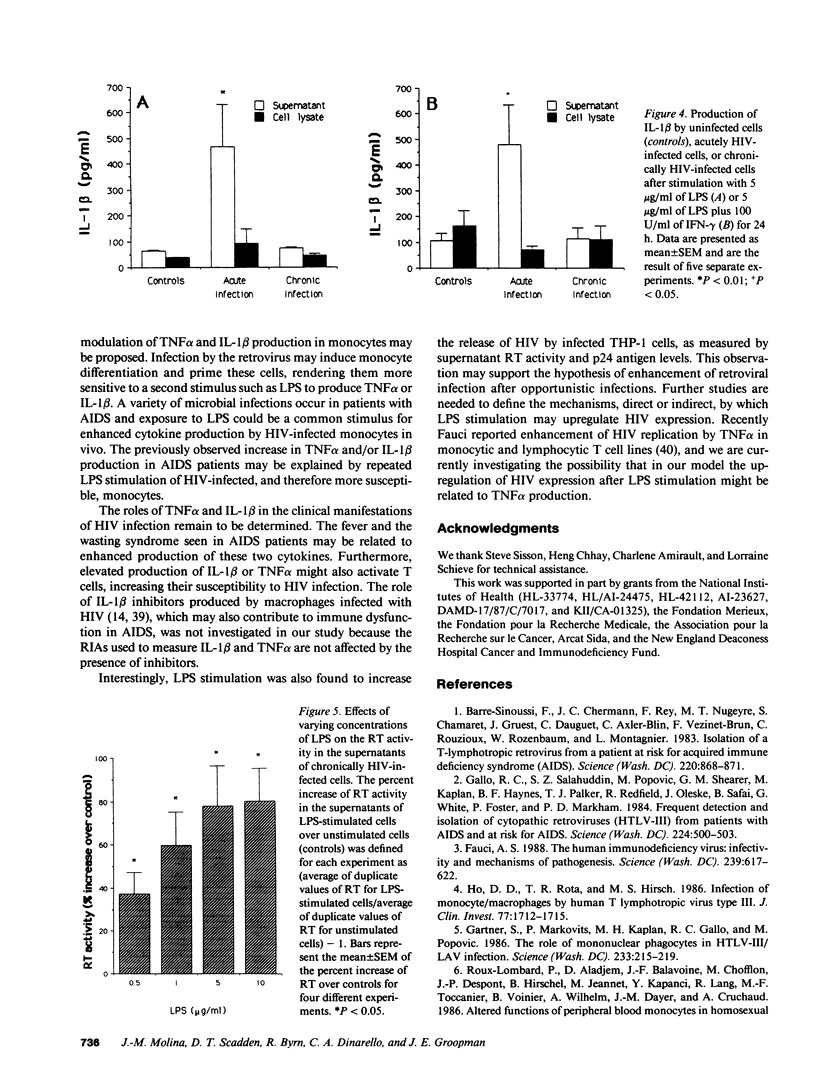

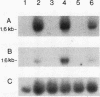
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bender B. S., Davidson B. L., Kline R., Brown C., Quinn T. C. Role of the mononuclear phagocyte system in the immunopathogenesis of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Rev Infect Dis. 1988 Nov-Dec;10(6):1142–1154. doi: 10.1093/clinids/10.6.1142. [DOI] [PubMed] [Google Scholar]
- Berman M. A., Sandborg C. I., Calabia B. S., Andrews B. S., Friou G. J. Interleukin 1 inhibitor masks high interleukin 1 production in acquired immunodeficiency syndrome (AIDS). Clin Immunol Immunopathol. 1987 Jan;42(1):133–140. doi: 10.1016/0090-1229(87)90180-2. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crowe S., Mills J., McGrath M. S. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Enk C., Gerstoft J., Møller S., Remvig L. Interleukin 1 activity in the acquired immunodeficiency syndrome. Scand J Immunol. 1986 Apr;23(4):491–497. doi: 10.1111/j.1365-3083.1986.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fenton M. J., Clark B. D., Collins K. L., Webb A. C., Rich A., Auron P. E. Transcriptional regulation of the human prointerleukin 1 beta gene. J Immunol. 1987 Jun 1;138(11):3972–3979. [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Kessler S. W., Orenstein J. M., Justement J. S., Jaffe E. S., Fauci A. S. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988 Nov 11;242(4880):919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., de Wolf F., Paul D. A., Epstein L. G., Lange J. M., Krone W. J., Speelman H., Wolters E. C., Van der Noordaa J., Oleske J. M. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986 Jul 26;2(8500):177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Benz P. M., Ferriani R., Mayer K., Allan J. D., Weymouth L. A. Characterization of serum neutralization response to the human immunodeficiency virus (HIV). AIDS Res Hum Retroviruses. 1987 Spring;3(1):71–85. doi: 10.1089/aid.1987.3.71. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K. An alternate B cell activation mechanism mediated by macrophages through the release of cyclo-oxygenase pathway products and interleukin 1. Lymphokine Res. 1987 Fall;6(4):299–308. [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Krakauer T., Oppenheim J. J. Interleukin 1 production by a human acute monocytic leukemia cell line. Cell Immunol. 1983 Sep;80(2):223–229. doi: 10.1016/0008-8749(83)90111-9. [DOI] [PubMed] [Google Scholar]
- Lepe-Zuniga J. L., Mansell P. W., Hersh E. M. Idiopathic production of interleukin-1 in acquired immune deficiency syndrome. J Clin Microbiol. 1987 Sep;25(9):1695–1700. doi: 10.1128/jcm.25.9.1695-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Crowe S., Sadick M. D., Heinzel F. P., Gardner K. D., Jr, McGrath M. S., Mills J. Release of interleukin 1 inhibitory activity (contra-IL-1) by human monocyte-derived macrophages infected with human immunodeficiency virus in vitro and in vivo. J Clin Invest. 1988 Dec;82(6):2097–2105. doi: 10.1172/JCI113831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähdevirta J., Maury C. P., Teppo A. M., Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988 Sep;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Petit A. J., Terpstra F. G., Miedema F. Human immunodeficiency virus infection down-regulates HLA class II expression and induces differentiation in promonocytic U937 cells. J Clin Invest. 1987 Jun;79(6):1883–1889. doi: 10.1172/JCI113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Prill A. H., Mann T. N. Interleukin 1 and interleukin 1 inhibitor production by human macrophages exposed to influenza virus or respiratory syncytial virus. Respiratory syncytial virus is a potent inducer of inhibitor activity. J Exp Med. 1986 Mar 1;163(3):511–519. doi: 10.1084/jem.163.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Jewett A., Mitsuyasu R., Bonavida B. Spontaneous cytotoxicity and tumor necrosis factor production by peripheral blood monocytes from AIDS patients. J Immunol. 1988 Jul 1;141(1):99–104. [PubMed] [Google Scholar]
- van der Meer J. W., Endres S., Lonnemann G., Cannon J. G., Ikejima T., Okusawa S., Gelfand J. A., Dinarello C. A. Concentrations of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukoc Biol. 1988 Mar;43(3):216–223. doi: 10.1002/jlb.43.3.216. [DOI] [PubMed] [Google Scholar]