Abstract
Eosinophils function in murine allergic airways inflammation as professional antigen-presenting cells (APCs). In murine professional APC cell types, optimal functioning of MHC Class II depends on its lateral association in plasma membranes and colocalization with the tetraspanin CD9 into detergent-resistant membrane microdomains (DRMs). With human eosinophils, we evaluated the localization of MHC Class II (HLA-DR) to DRMs and the functional significance of such localization. In granulocyte-macrophage colony-stimulating factor–stimulated human eosinophils, antibody cross-linked HLA-DR colocalized by immunofluorescence microscopy focally on plasma membranes with CD9 and the DRM marker ganglioside GM1. In addition, HLA-DR coimmunoprecipitates with CD9 after chemical cross-linking of CD9. HLA-DR and CD9 were localized by Western blotting in eosinophil DRM subcellular fractions. DRM disruption with the cholesterol-depleting agent methyl-β-cyclodextrin decreased eosinophil surface expression of HLA-DR and CD9. We show that CD9 is abundant on the surface of eosinophils, presenting the first electron microscopy data of the ultrastructural immunolocalization of CD9 in human eosinophils. Disruption of HLA-DR–containing DRMs decreased the ability of superantigen-loaded human eosinophils to stimulate CD4+ T-cell activation (CD69 expression), proliferation, and cytokine production. Our results, which demonstrate that eosinophil MHC Class II localizes to DRMs in association with CD9 in a functionally significant manner, represent a novel insight into the organization of the antigen presentation complex of human eosinophils.
Keywords: eosinophils, antigen presentation, HLA-DR, CD9, immunoelectron microscopy
Eosinophils have been increasingly recognized to have important immunoregulatory functions that extend beyond their traditional characterization as end-stage mediators of antiparasitic activity and of allergic inflammation (1). One such function is the ability of eosinophils to act as antigen-presenting cells (APCs). When exposed to the proper stimuli, human and murine eosinophils have been demonstrated to express MHC Class II and the requisite costimulatory molecules necessary to act as professional APCs (2–4). Moreover, murine eosinophils have been shown to act as professional APCs, as defined by their ability to stimulate naive antigen-specific T cells in in vitro experiments and in murine models (5–7).
Detergent-resistant membrane microdomains (DRMs), often referred to as lipid rafts, have important roles in the organization and activity of MHC Class II molecules in a variety of APCs (8). Cross-linking HLA-DR results in colocalization with markers of DRMs microscopically in a myelomonocytic cell line (9). MHC Class II in murine B cells has been observed in DRMs, with DRM disruption inhibiting their ability to present antigen (10). In human monocyte–derived dendritic cells (DCs), HLA-DR similarly coaggregates with DRM markers when cross-linked, and biochemical disruption of DRMs inhibits T-cell activation by DCs (11). The role of DRMs in antigen presentation by eosinophils is unknown. DRMs have in general been poorly studied in eosinophils. A study by Yoon and colleagues describing the presence of the granulocyte activation marker CD66b in DRMs is, to our knowledge, the only prior study that evaluated DRMs in eosinophils (12).
The tetraspanin CD9 is abundantly expressed on the surface of eosinophils and intracellularly, yet the function of CD9 in eosinophils remains unclear (13). Matsumoto and colleagues demonstrated that cross-linking CD9 in isolated human eosinophils with a monoclonal antibody followed by a secondary antibody caused eosinophil “activation,” as measured by homotypic aggregation by flow cytometry (14). In another study, cross-linking CD9 with anti-CD9 antibody immobilized on tissue culture plates caused degranulation of human eosinophils as well as increased survival (15). CD9 has been found to associate with HLA-DR in DRMs of monocytes and with CD1a in DRMs of DCs (16, 17). CD9 localization on murine DCs, in contrast to other murine APCs, was shown to have a central role in mediating the lateral membrane association of MHC Class II molecules (18).
We sought to characterize the importance of DRMs and CD9 in MHC Class II–restricted antigen presentation by human eosinophils. The present study investigates the hypotheses that MHC Class II and CD9 associate in DRMs of human eosinophils and that the presence of MHC Class II in DRMs is functionally meaningful. We used microscopic colocalization studies with and without DRM disruption, as well as subcellular isolation of DRMs from whole cell lysates, to test for the presence of MHC Class II and CD9 in DRMs of human eosinophils. We then tested if the aggregation of MHC Class II to DRMs is functionally meaningful through coculture experiments of CD4+ T cells with superantigen-loaded eosinophils, assessing whether DRM disruption of eosinophils decreased their ability to stimulate CD4+ T cells. We also present the first ultrastructural immunolocalization by electron microscopy (EM) of CD9 in human eosinophils. Parts of these data were previously presented in abstract form (19, 20).
Materials and Methods
Materials
Anti–HLA-DR, anti-CD9, and anti-CD69 mAbs (BD Biosciences, San Jose, CA); polyclonal anti–flotillin-1 antibody; and antitransferrin receptor mAb (Abcam, Cambridge, MA) were used. Anti–HLA-DR and anti-CD9 mAbs for immunoblotting were from Santa Cruz Biotechnologies (Santa Cruz, CA). Alexa 488–labeled cholera toxin B (CTB) and Alexa-labeled goat antimouse antibodies were from Invitrogen (Carlsbad, CA). Anti-CD9 (clone 209306; R&D Systems, Minneapolis, MN) and irrelevant mouse IgG isotype control purified mAbs were used for EM immunodetection studies. The secondary Ab for immunoEM was an affinity-purified goat antimouse Fab fragment conjugated to 1.4 nm gold (1:100) (Nanogold; Nanoprobes, Stony Brook, NY).
Eosinophil Purification
Venous blood was collected from normal donors under protocols approved by our institution's Committee on Clinical Investigations. Eosinophils were isolated by negative selection (StemCell, Vancouver, BC, Canada) as previously described (21) and incubated in 100 pM granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems) for 24 to 72 hours unless otherwise specified.
Microscopy and Flow Cytometry
Immunofluorescence microscopy and immunoEM were performed as described in the online supplement. Flow cytometry data were obtained with a BD Biosciences FACScan and analyzed with FlowJo 8.5.2 (Tree Star, Ashland, OR).
DRM Isolation
GM-CSF–stimulated eosinophils (20 × 106) were lysed in 1 ml of 1% Triton X-100 (Sigma, St. Louis, MO) in TNE buffer (10 mM Tris, 150 mM NaCl, 5 mM EDTA) with protease inhibitor cocktail (Roche, Basel, Switzerland). Lysate was mixed with 1 ml of 85% sucrose. Six milliliters of 35% sucrose was overlaid, followed by 4 ml of 3.5% sucrose. Samples were ultracentrifuged at 211,400 × g for 16 hours. Fractions (1 ml), including buoyant DRM fractions, were collected from the top.
Protein was precipitated with 20% TCA followed by two acetone washes. Pellets were resuspended in 1% SDS/0.1 M NaOH solution. For CD9 solubilization, 1% Triton X-100 was added, and samples were incubated for 20 minutes on ice. Western blots were performed under reducing conditions.
DRM Disruption
Eosinophils were incubated with 10 mM methyl-β-cyclodextrin (MβCD) (Sigma) or with 50 μg/ml nystatin (Sigma) in RPMI-1640 (Mediatech, Manassas, VA) with 2% FBS for 30 minutes at 37°C.
Eosinophil and T-Cell Coculture
CD4+ T cells were purified by negative selection (StemCell) from peripheral blood. CD4+ T cells were incubated overnight concurrent with GM-CSF stimulation of eosinophils from the same donor. Eosinophils were incubated with 100 ng/ml Staphylococcal Enterotoxin A (SEA) (Sigma) for 30 minutes at 37°C and washed, followed by DRM disruption. Eosinophils were washed before inclusion in coculture.
CD69 expression.
A total of 2 × 105 eosinophils and 5 × 104 CD4+ T cells per well were incubated overnight in round-bottom, 96-well tissue culture plates.
Proliferation.
T cells were labeled with 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen). Eosinophils (1 × 105) and 1 × 105 CD4+ T cells were cocultured for 4 days.
Cytokine assays.
A total of 1 × 105 fixed (2% paraformaldehyde) and washed eosinophils and 1 × 105 CD4+ T cells were cocultured for 48 hours. Cytokine concentrations of culture supernatants were measured by multiplex assays (BioRad, Hercules, CA).
Statistical Analysis
Paired t tests were performed. Significance was accepted at P < 0.05.
Results
MHC Class II and CD9 Associate with DRMs in Human Eosinophils
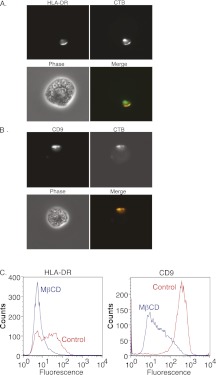
Resting eosinophils stimulated with GM-CSF exhibit enhanced plasma membrane expression of MHC Class II (e.g., HLA-DR) (2). To evaluate whether eosinophil MHC Class II might undergo membrane localization within DRMs, we used anti-MHC Class II antibody cross-linking to induce MHC Class II protein “capping.” We first studied by immunofluorescence microscopy the colocalization of cross-linked MHC Class II with fluorescent-labeled CTB. CTB binds to ganglioside GM1 and serves as a marker for DRMs (22). Human eosinophils were stimulated with 100 pM GM-CSF for 48 hours. MHC Class II cross-linked by primary antibody followed by fluorescent-labeled secondary antibody extensively colocalized with CTB on the surface of eosinophils (Figure 1A). The tetraspanin CD9 also colocalized with CTB (Figure 1B) when cross-linked by primary and secondary antibodies. We further investigated the association of CD9 with DRMs due to a previous description of its association with MHC Class II in DRMs of antigen-presenting cells (17). The distribution of GM1 signal in human eosinophils with GM-CSF did not change at 1, 2, or 3 days compared with unstimulated cells (data not shown).
Figure 1.
MHC Class II undergoes lateral membrane movement to associate with detergent-resistant membrane microdomains (DRMs) in human eosinophils. (A) Immunofluorescence colocalization of MHC Class II with fluorescent-labeled cholera toxin B (CTB). The CTB subunit binds to ganglioside GM1 and is a marker for DRMs. Eosinophils were incubated with anti–HLA-DR Ab followed by Alexa 594–conjugated secondary Ab; cells were then incubated with Alexa 488–conjugated CTB. Cells were fixed in 2% paraformaldehyde for 5 minutes before analysis. Cross-linked MHC Class II extensively colocalized with CTB on the surface of eosinophils. (B) The tetraspanin CD9 also colocalized with CTB. CD9 was cross-linked with primary and secondary antibody using the same staining protocol as above. Data for panels A and B were obtained with 40× magnification and are representative of three separate experiments from three different donors. (C) Eosinophils were incubated with control medium or 10 mM MβCD to compare normal cells with DRM-disrupted cells. Flow cytometry analyses showed that HLA-DR and CD9 expression were reduced from the surface of eosinophils after MβCD treatment. Data are representative of four separate experiments from four different blood donors.
To further demonstrate that MHC Class II is associated with DRMs on the surface of human eosinophils, we performed experiments using the DRM-disrupting agent, MβCD. MβCD, commonly used for this purpose, depletes plasma membranes of cholesterol (23). Eosinophils were incubated with control medium or 10 mM MβCD to compare normal cells with DRM-disrupted cells. Flow cytometric analyses showed that HLA-DR and CD9 were reduced from the surface of eosinophils after MβCD treatment (Figure 1C). Flow cytometry for surface expression of a non-DRM protein, complement receptor 1, did not show reduction after MβCD treatment (data not shown). GM1 surface expression by flow cytometry using Alexa 488–labeled CTB also did not demonstrate reduction after MβCD treatment (data not shown).
HLA-DR and CD9 Can Be Identified in DRM Fractions of Human Eosinophils
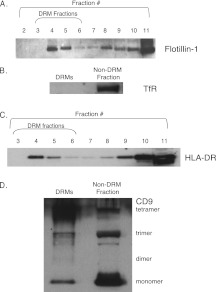
To further demonstrate in a more direct fashion that MHC Class II is present in DRM domains in human eosinophils and not merely in close proximity to them, DRMs from GM-CSF–stimulated eosinophil lysates were isolated by subjecting lysates to a sucrose density gradient fractionation protocol that has successfully isolated DRM fractions from human eosinophils (12). DRM fractions were defined as fractions 3 through 6 as numbered from the top of the sucrose gradient. To confirm the isolation of DRM fractions, Western blot analyses were performed on fractions for the protein flotillin-1, which is enriched in DRMs. Flotillin-1 was enriched as expected in DRM fractions, most abundantly in fractions 4 and 5, and was also detected in non-DRM fractions of human eosinophils (Figure 2A). Western blot analyses of pooled DRM fractions and a non-DRM fraction (fraction 11) were performed for the transferrin receptor, which is fully excluded from DRMs (24); as expected, the transferrin receptor was not detectable in pooled DRM fractions (Figure 2B).
Figure 2.
Western blot of DRM fractions for HLA-DR. (A) To confirm the isolation of DRMs, Western blot analysis was performed of fractions for the DRM-enriched protein flotillin-1 and the DRM-excluded protein transferrin receptor (TfR). Flotillin-1 was readily detectable in DRM fractions, most abundantly in fractions 4 and 5. Molecular weight of flotillin-1, 48 kD. (B) TfR was not detected in pooled DRM fractions (fractions 3–6) but was present in non-DRM fractions (fraction 11). Molecular weight of TfR = 95 kD. (C) HLA-DR was readily detectable by Western blot in DRM fractions (fractions 3–6). Molecular weight of HLA-DR = 34 kD. (D) CD9 was detectable by Western blot in pooled DRM fractions (fractions 3–6). Tetramers (77 kD) and monomers (24 kD) were most prominent in DRM fractions, but trimers (50kD) and dimers (40 kD) were visualized as well. CD9 tetramers, trimers, and monomers were readily detected in non-DRM fraction 11. Total protein loading concentrations were comparable for the pooled DRM and non-DRM fractions as measured by spectrophotometry (data not shown).
HLA-DR was readily detectable by Western blot in DRM fractions (fractions 3–6) (Figure 2C). It was also present in non-DRM fractions. Total protein loading concentrations were comparable for the pooled DRM fractions and non-DRM fraction as measured by spectrophotometry (data not shown).
The presence of CD9 in DRM fractions was also assessed by Western blots (Figure 2D). CD9 is a 24-kD protein in its monomeric form, and the presence of multimeric forms has been previously established (25). A 77-kD band was most prominently detected in DRM fractions, which likely represents CD9 tetramers, based on the previous work of Kovalenko and colleagues (25). Fainter 40- and 50-kD bands, likely representing CD9 dimers and trimers, respectively, were present. Monomeric CD9 at 24 kD was detected in DRM fractions, strongly suggesting the larger molecular weight bands visualized were indeed multimeric forms of CD9.
MHC Class II and CD9 Colocalize in Human Eosinophils
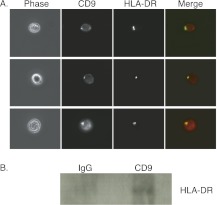
Given that we observed MHC Class II and CD9 associated with the DRM marker ganglioside GM1 in eosinophils, we studied the associations between the two proteins themselves. Immunofluorescence microscopy of eosinophils after HLA-DR cross-linking by primary and secondary antibodies demonstrated colocalization with CD9 on the cell surface (Figure 3A). The pattern of HLA-DR distribution with Ab cross-linking followed by incubation at 37°C was consistent with a “capping” pattern at one pole of the cell.
Figure 3.
Association of CD9 with HLA-DR. (A) Colocalization of HLA-DR and CD9 by immunofluorescence microscopy. HLA-DR was cross-linked with primary antibody and Alexa 488–conjugated secondary antibody. CD9 was identified with Alexa 594–conjugated mAb. Cells were fixed in 2% paraformaldehyde for 5 minutes before analysis. Colocalization of HLA-DR (green) and CD9 (red) shown on images labeled “merge.” Cells displayed are from a single donor but are representative of three separate experiments from three different blood donors. Data were obtained with 40× magnification. (B) Coimmunoprecipitation of HLA-DR with CD9 after chemical cross-linking. Eosinophils were treated with the cross-linking agent DSP followed by cell lysis. HLA-DR was detectable to greater extent after immunoprecipitation with CD9 as opposed to isotype control IgG.
After chemical cross-linking of CD9 in human eosinophils with dithiobis[succinimidylproprionate] (DSP) (details are provided in the online supplement), we observed that HLA-DR coimmunoprecipitated with CD9 (Figure 3B). Lysates of cross-linked eosinophils were serially subjected to immunoprecipitation conditions with IgG isotype control antibody followed by anti-CD9 antibody. Western blot analysis showed detectable HLA-DR in the eluent from CD9 immunoprecipitation, demonstrating the association of CD9 with HLA-DR (Figure 3B).
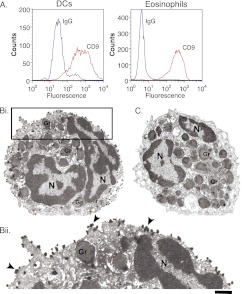
It has been observed in murine models that a high level of CD9 expression may differentiate professional APCs from APCs that do not have the ability to stimulate naive T cells (18). We compared the expression of CD9 on purified eosinophils and monocyte-derived human DCs. Peripheral blood mononuclear cells were enriched for monocyte-derived DCs as described in the online supplement; for flow cytometry analysis, we gated on DCs by their high forward-scatter and side-scatter characteristics. The expression of CD9 was similar on the surface of human eosinophils compared with the surface of monocyte-derived DCs (Figure 4A). The localization of CD9 in human eosinophils was also demonstrated with preembedding immunonanogold EM for precise epitope preservation and secondary antibody Fab fragments specifically conjugated with very small gold particles (1.4 nm) as a probe (Figure 4B). CD9 was detected extensively at the cell surface (Figures 4Bi and 4Bii), with antigen clusters being observed at the plasma membrane (Figure 4Bii, arrowheads). Moreover, a CD9 intracellular pool was detected in association with secretory granules (Figure 4Bi). Control cells in which the primary Ab was replaced by an irrelevant Ab were negative (Figure 4C).
Figure 4.
Abundance of CD9 on the surface of human eosinophils. (A) Flow cytometry analysis of monocyte-derived DCs and eosinophils from the same donor shows that CD9 was highly expressed in both cell types. DCs were identified by their high forward-scatter and side-scatter characteristics. (Bi) Immunonanogold electron microscopy revealed a substantial pool of CD9 at the eosinophil surface. The entire cell surface is highly positive for CD9. Intracellular labeling is also detected in association with secretory granules (Gr). (Bii) The boxed area of (Bi) showing CD9 clusters (arrowheads) at the plasma membrane. (C) Eosinophils stained with isotype control antibody did not show immunonanogold labeling. Unstimulated eosinophils were processed for immunoEM as described in Materials and Methods. Scale bars, 1.2 μm (Bi, C); 500 nm (Bii). Gr = specific granules; N = nucleus.
Disruption of HLA-DR–Containing DRMs of Eosinophils Reduces Their Ability to Stimulate CD69 Expression, Proliferation, and Cytokine Production by CD4+ T Cells in a Superantigen-Mediated Fashion
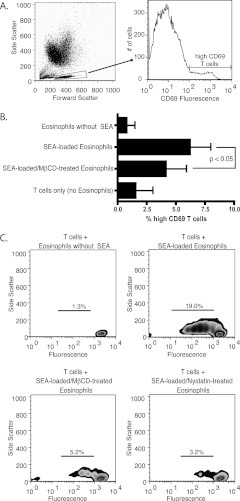
To determine if disrupting HLA-DR–containing DRMs in human eosinophils has functional significance, we followed an in vitro experimental protocol in which we used SEA as a superantigen to mediate stimulation of CD4+ T cells by eosinophils via MHC Class II (26). GM-CSF–stimulated eosinophils loaded with SEA were cocultured with CD4+ T cells from the same donor. After overnight coculture, cells were analyzed by flow cytometry with gating on live T cells by forward- and side- scatter characteristics compared with the high side-scatter eosinophil population (see Figure E1 in the online supplement), and T cells with high expression of CD69 were identified (Figure 5A). Compared with control T cells incubated with SEA-loaded, non–MβCD-treated eosinophils, T cells that were incubated with SEA-loaded eosinophils treated with MβCD had a decreased percentage of high CD69 expression (Figure 5B). Because CD69 is a marker of early T-cell activation (27), our findings are consistent with decreased SEA-mediated T-cell activation via MHC Class II when DRMs are disrupted. The viability of eosinophils was similar in MβCD-treated cells (90%) compared with non–MβCD-treated cells (95%) as measured by trypan blue exclusion (data not shown).
Figure 5.
Disruption of HLA-DR–containing DRMs reduces the ability of human eosinophils to stimulate CD69 expression and proliferation by CD4+ T cells in a superantigen-mediated fashion. (A) T cells were identified by their distinct forward- and side-scatter characteristics compared with eosinophils. For results shown in Figure 4B, high CD69-expressing cells were identified from this population. (B) DRM disruption of eosinophils reduces superantigen-mediated stimulation of CD69 expression on CD4+ T cells. T cells were analyzed after overnight coculture with eosinophils as described above. Data are from three separate experiments from three different donors. Mean percentages of high CD69-expressing T cells are displayed. A decreased percentage of T cells exhibited high CD69 expression when cocultured with DRM-disrupted, Staphylococcal Enterotoxin A (SEA)-loaded eosinophils (4.1%) than when cocultured with DRM-intact, SEA-loaded eosinophils (6.2%) (P < 0.05, two-tailed paired t test; bars represent SEM). High CD69 expression was also measured in T cells cultured with DRM-intact, non–SEA-loaded eosinophils (0.8%) and in T cells cultured without eosinophils (1.5%). (C) DRM disruption of eosinophils reduces superantigen-mediated proliferation of CD4+ T cells. Carboxyfluorescein diacetate succinimidyl ester–loaded T cells were analyzed after 4 days of coculture with eosinophils. Proliferation is depicted with zebra density plots. Percentages of divided cells are displayed. T cells had decreased proliferation when cocultured with DRM-disrupted (MβCD and nystatin), SEA-loaded eosinophils than when cocultured with DRM-intact, SEA-loaded eosinophils. Proliferation is minimal in T cells cultured with non–SEA-loaded eosinophils. Data are representative of three separate experiments from three different donors.
The ability of eosinophils to stimulate CD4+ T cells in vitro was then measured by T cell proliferation, assayed by CFSE dilution, after 4 days of eosinophil and T-cell coculture. SEA-loaded eosinophils that were not treated with DRM-disrupting agents were able to robustly stimulate T-cell proliferation (Figure 5C). MβCD-treated eosinophils had a substantially decreased ability to stimulate T-cell proliferation. We also assayed the effect of disrupting DRMs of SEA-loaded eosinophils with an alternative cholesterol-binding agent, nystatin. Nystatin-treated eosinophils showed a similar reduction to MβCD eosinophils in their ability to stimulate T-cell proliferation. In the experiment shown, which is representative of three separate experiments, there was a lower percentage of dividing T cells with coculture with MβCD-treated eosinophils (5.2%) and with nystatin-treated eosinophils (3.2%) than with non-treated eosinophils (19.0%). Therefore, by T-cell activation and proliferation assays, disruption of HLA-DR–containing DRMs decreases the ability of superantigen-loaded human eosinophils to stimulate T cells.
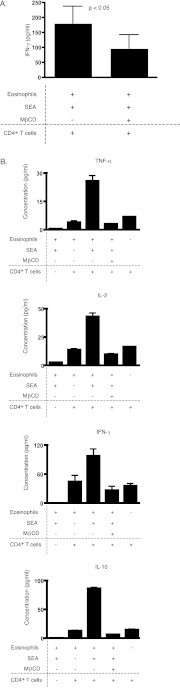
Using a similar experimental system, we observed that disruption of HLA-DR–containing DRMs of SEA-loaded eosinophils impairs their ability to stimulate cytokine production by T cells. To minimize the possibility of eosinophil contribution to coculture supernatant cytokine content, eosinophils were fixed in 2% paraformaldehyde and washed before initiating cculture with T cells. Eosinophils and CD4+ T cells remained in coculture for 48 hours before collection of supernatants for cytokine measurements. Five separate experiments from five different donors were performed. Mean supernatant IFN-γ concentrations were lower when T cells were cocultured with SEA-loaded, MβCD-treated eosinophils compared with SEA-loaded, untreated eosinophils (P < 0.05) (Figure 6A). TNF-α, IL-2, IFN-γ, and IL-10 measurements from a single donor representative of four out of the five donors tested showed that SEA-loaded eosinophils stimulated the production of each of these cytokines and that disruption of eosinophil DRMs with MβCD decreased their production by CD4+ T cells (Figure 6B). Mean TNF-α, IL-2, and IL-10 concentrations from the five donors all showed a similar trend to that seen with mean IFN-γ but were not statistically significant (data not shown). Cytokine concentrations of culture supernatant from fixed SEA-loaded eosinophils without T cells were minimal in all five donors, indicating that the coculture supernatant concentrations for all experimental conditions were representative of T-cell cytokine production (Figure 6B). IL-4 and IL-12 were also measured, but the concentrations measured were undetectable or close to the lower limits of the assay (<1 pg/ml for cocultures, data not shown).
Figure 6.
DRM disruption of eosinophils reduces superantigen-mediated cytokine production by CD4+ T cells. Eosinophils were fixed in 2% paraformaldehyde before coculturing with T cells. Culture supernatants were collected after 48 hours of incubation. Cytokine concentrations were measured by BioPlex multiplex assay. Experiments from five separate donors were performed. (A) Mean supernatant IFN-γ concentrations were lower with MβCD treatment of eosinophils than without MβCD treatment (P < 0.05, two tailed paired t test; bars represent SEM). (B) TNF-α, IL-2, IFN-γ, and IL-10 concentrations from a single donor representative of four of five donors tested were lower with MβCD treatment of eosinophils than without MβCD treatment (bars represent SEM for duplicate measurements). There was minimal cytokine production by eosinophils cultured alone. Data are representative of all five donors.
Discussion
Our findings demonstrate that MHC Class II can migrate and localize to DRMs in human eosinophils in a functionally significant manner. The association of MHC Class II and the tetraspanin CD9 with DRMs in eosinophils adds to the evidence that eosinophils have important professional APC functions. Although there is accumulating evidence supporting eosinophils as important and potent APCs, a gap exists in the understanding of the structural and organizational aspects of the antigen presentation complex in eosinophils. Distinct parallels can be drawn between the present study of human eosinophils and prior studies of DCs and other professional APCs, in which both tetraspanin molecules and DRMs likely play a pivotal role in the organization of the antigen presentation complex.
The tetraspanins are a group of proteins belonging to the transmembrane-4 superfamily that have major function in the organization of protein complexes in cell membranes (28). Unternaehrer and colleagues demonstrated in mouse DCs that the tetraspanin CD9 associates with MHC Class II molecules in the plasma membrane (18). Their data showed that CD9 mediates the association of heterologous MHC Class II molecules, suggesting that CD9 plays a key role in organizing antigen-presenting molecules in a manner that allows for more efficient APC function. They also found that the CD9 expression on the plasma membrane of murine DCs was high, whereas it was low on “non-professional” APCs such as B blasts. We have demonstrated here that surface expression of CD9 is comparable between human eosinophils and monocyte-derived DCs, further supporting eosinophils as professional APCs. As seen in previous studies, CD9 is abundant on the cell surface of eosinophils to such an extent as to be considered a marker for eosinophils (13). Here, we extend these studies showing, for the first time, the ultrastructural immunolocalization of CD9 in human eosinophils. Our EM data confirm the substantial localization of CD9 at the cell surface and the presence of CD9 clusters at areas of the plasma membrane (Figure 4Bii). Our findings strongly suggest that a subset of CD9 and HLA-DR are associated in DRMs on the surface of human eosinophils. We have found that HLA-DR and CD9 not only colocalize with one another but also colocalize with the DRM marker CTB. In addition, disruption of DRMs leads to marked decrease in expression of HLA-DR and CD9 on the surface of human eosinophils. Cholesterol depletion with MβCD does not cause a reduction in detectable GM1 by flow cytometry on the surface of eosinophils, consistent with preferential removal of DRM-associated proteins compared with gangliosides. Although patterns of GM1 expression with DRM disruption have not been studied in eosinophils, changes in GM1 distribution rather than a decrease in total GM1 content may explain this differential effect of MβCD. A prior report has demonstrated changes in GM1 distribution with MβCD treatment in murine fibroblasts (29).
We have biochemically demonstrated that HLA-DR and CD9 are present in DRM fractions of human eosinophil lysates subjected to sucrose density gradient fractionation. Particularly noteworthy from a biochemical perspective are our findings regarding CD9. CD9 and other tetraspanins have been shown to associate with cholesterol and other membrane lipids in DRMs distinct from lipid rafts known as tetraspanin-enriched microdomains (TEMs) (30). However, unlike lipid rafts, TEMs generally appear to be soluble in stronger detergents, such as Triton X-100 (28, 30). Our data suggest that CD9 in human eosinophils is present in lipid rafts, rather than only in TEMs, given the abundant multimeric CD9 found to have floated upward into the detergent-resistant area with sucrose density gradient fractionation of Triton X-100 eosinophil lysates (Figure 3).
The present study investigated the necessity of HLA-DR–containing DRM integrity to superantigen-mediated eosinophil APC function. The role of DRMs in the organization of antigen-presenting molecules in eosinophils has been unknown to this point. In fact, the relevance of DRMs in eosinophil biology in general has not been well characterized. To our knowledge, only one study has examined DRMs in human eosinophils (12). DRMs have previously been demonstrated to have an important role in the organization and activity of MHC Class II molecules in a variety of other APCs (9–11, 31–36). MHC Class II in murine B cells has been observed in DRMs; in these cells, disruption of DRMs with MβCD inhibited their ability to present antigen (10). In human monocyte–derived DCs, HLA-DR coaggregates with the DRM markers CD59 and GM1-ganglioside when cross-linked, and disruption of DRMs biochemically eliminates DRMs from the DC-T cell synapse and inhibits T-cell activation by the DCs (11). We functionally demonstrate that eosinophils loaded with the superantigen SEA are able to stimulate CD4+ T cells in a DRM-dependent fashion. We did so by measuring three different indicators of the activation state of T cells: expression of the early activation marker CD69 at 24 hours, production of cytokines by T cells at 48 hours, and proliferation of T cells by CFSE dilution at 96 hours. The ability of human eosinophils to stimulate T cells by these metrics is a novel finding.
Up until this point, the structure of the antigen-presentation complex on eosinophils has been an unexplored topic, particularly given that eosinophils acting as professional APCs is a relatively recently described phenomenon. It is known that APCs only require a small number of MHC-peptide complexes to activate T cells (37, 38). Given that human eosinophils are efficient at antigen-presentation despite lower cell surface expression of HLA-DR than other professional APCs (6), one could speculate that the potential organization of MHC Class II into DRMs on eosinophils may help explain their relative efficiency.
Also currently unresolved is the true contribution of eosinophil APC function in vivo. Our group has previously demonstrated that eosinophils harvested from IL-5 transgenic mice and loaded with OVA traffic to paratracheal lymph nodes after intratracheal instillation into wild-type mice, where they are able to stimulate OVA-specific CD4+ T cells to express CD69, proliferate, and secrete IL-4 (7). This observation, in concert with the present findings that human eosinophils have functionally relevant grouping of MHC Class II with CD9 in DRMs, suggests that eosinophil APC function has in vivo physiologic, nonredundant significance.
Characterization of important immunoregulatory roles of eosinophils, including antigen presentation, has lagged behind that of other immune cell types. This lag may be in part responsible for an incomplete understanding of the pathobiology of asthma and other allergic inflammatory processes. Further investigation of the immunobiology of eosinophils has taken on increased concrete clinical importance given recent findings regarding the efficacy of eosinophil-depletion by anti-IL5 mAb in selected patients with asthma as well as their conspicuous presence in eosinophilic pulmonary disorders such as Churg-Strauss syndrome and eosinophilic pneumonia (39–41). This study adds to the ever-increasing store of evidence that eosinophils are significantly more complex and multifaceted than previously appreciated.
Supplementary Material
Acknowledgments
The authors thank Amy Radke, Kristen Young, and Jason Xenakis for technical assistance in isolating human eosinophils and Dr. Ionita Ghiran for providing expert guidance with light microscopy.
Footnotes
This work was supported by National Institutes of Health grants R01 AI051645 and R01/R37 AI020241 (P.F.W.), by grant T32 HL007633, by grant F32 AI081513 (P.A.), and by FAPEMIG and CNPq (Brazil) (R.C.N.M.).
Originally Published in Press as DOI: 10.1165/rcmb.2010-0335OC on September 1, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol 2007;119:1313–1320 [DOI] [PubMed] [Google Scholar]
- 2.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci USA 1989;86:1348–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura N, Ishii N, Nakazawa M, Nagoya M, Yoshinari M, Amano T, Nakazima H, Minami M. Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol 1996;44:229–238 [DOI] [PubMed] [Google Scholar]
- 4.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest 2000;105:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis 2007;196:1844–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun 2006;74:3232–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol 2007;179:7585–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol 2003;21:457–481 [DOI] [PubMed] [Google Scholar]
- 9.Huby RD, Dearman RJ, Kimber I. Intracellular phosphotyrosine induction by major histocompatibility complex Class II requires co-aggregation with membrane rafts. J Biol Chem 1999;274:22591–22596 [DOI] [PubMed] [Google Scholar]
- 10.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC Class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol 2000;1:156–162 [DOI] [PubMed] [Google Scholar]
- 11.Eren E, Yates J, Cwynarski K, Preston S, Dong R, Germain C, Lechler R, Huby R, Ritter M, Lombardi G. Location of major histocompatibility complex Class II molecules in rafts on dendritic cells enhances the efficiency of T-cell activation and proliferation. Scand J Immunol 2006;63:7–16 [DOI] [PubMed] [Google Scholar]
- 12.Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol 2007;179:8454–8462 [DOI] [PubMed] [Google Scholar]
- 13.Fernvik E, Hallden G, Hed J, Lundahl J. Intracellular and surface distribution of CD9 in human eosinophils. APMIS 1995;103:699–706 [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Bochner BS, Wakiguchi H, Kurashige T. Functional expression of transmembrane 4 superfamily molecules on human eosinophils. Int Arch Allergy Immunol 1999;120:38–44 [DOI] [PubMed] [Google Scholar]
- 15.Kim JT, Gleich GJ, Kita H. Roles of CD9 molecules in survival and activation of human eosinophils. J Immunol 1997;159:926–933 [PubMed] [Google Scholar]
- 16.Sloma I, Zilber MT, Vasselon T, Setterblad N, Cavallari M, Mori L, De Libero G, Charron D, Mooney N, Gelin C. Regulation of CD1a surface expression and antigen presentation by invariant chain and lipid rafts. J Immunol 2008;180:980–987 [DOI] [PubMed] [Google Scholar]
- 17.Zilber MT, Setterblad N, Vasselon T, Doliger C, Charron D, Mooney N, Gelin C. MHC Class II/CD38/CD9: a lipid-raft-dependent signaling complex in human monocytes. Blood 2005;106:3074–3081 [DOI] [PubMed] [Google Scholar]
- 18.Unternaehrer JJ, Chow A, Pypaert M, Inaba K, Mellman I. The tetraspanin CD9 mediates lateral association of MHC Class II molecules on the dendritic cell surface. Proc Natl Acad Sci USA 2007;104:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akuthota P, Spencer LA, Radke AL, Ghiran I, Weller PF. Association of the tetraspanin CD9 with MHC Class II in human eosinophils. Am J Respir Crit Care Med 2008;177:A601 [Google Scholar]
- 20.Akuthota P, Spencer LA, Radke AL, Weller PF. MHC Class II and CD9 localize to lipid rafts in human eosinophils. Am J Respir Crit Care Med 2009;179:A3697 [Google Scholar]
- 21.Neves JS, Perez SA, Spencer LA, Melo RC, Weller PF. Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J Immunol Methods 2009;344:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt EA, Sarfaty S, van den Akker F, L'Hoir C, Martial JA, Hol WG. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci 1994;3:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000;1:31–39 [DOI] [PubMed] [Google Scholar]
- 24.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 1998;141:929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalenko OV, Yang X, Kolesnikova TV, Hemler ME. Evidence for specific tetraspanin homodimers: inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem J 2004;377:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mawhorter SD, Kazura JW, Boom WH. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology 1994;81:584–591 [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T, Jung LK, Bjorndahl JM, Fu SM. Human T cell activation: III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med 1986;164:1988–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J 2009;420:133–154 [DOI] [PubMed] [Google Scholar]
- 29.Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell 2007;18:2112–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005;6:801–811 [DOI] [PubMed] [Google Scholar]
- 31.Becart S, Setterblad N, Ostrand-Rosenberg S, Ono SJ, Charron D, Mooney N. Intracytoplasmic domains of MHC Class II molecules are essential for lipid-raft-dependent signaling. J Cell Sci 2003;116:2565–2575 [DOI] [PubMed] [Google Scholar]
- 32.Bouillon M, El Fakhry Y, Girouard J, Khalil H, Thibodeau J, Mourad W. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem 2003;278:7099–7107 [DOI] [PubMed] [Google Scholar]
- 33.Goebel J, Forrest K, Flynn D, Rao R, Roszman TL. Lipid rafts, major histocompatibility complex molecules, and immune regulation. Hum Immunol 2002;63:813–820 [DOI] [PubMed] [Google Scholar]
- 34.Poloso NJ, Roche PA. Association of MHC Class II-peptide complexes with plasma membrane lipid microdomains. Curr Opin Immunol 2004;16:103–107 [DOI] [PubMed] [Google Scholar]
- 35.Setterblad N, Becart S, Charron D, Mooney N. B cell lipid rafts regulate both peptide-dependent and peptide-independent APC-T cell interaction. J Immunol 2004;173:1876–1886 [DOI] [PubMed] [Google Scholar]
- 36.Setterblad N, Roucard C, Bocaccio C, Abastado JP, Charron D, Mooney N. Composition of MHC Class II-enriched lipid microdomains is modified during maturation of primary dendritic cells. J Leukoc Biol 2003;74:40–48 [DOI] [PubMed] [Google Scholar]
- 37.Demotz S, Grey HM, Sette A. The minimal number of Class II MHC-antigen complexes needed for T cell activation. Science 1990;249:1028–1030 [DOI] [PubMed] [Google Scholar]
- 38.Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC Class II/peptide complexes necessary for T-cell stimulation. Nature 1990;46:574–576 [DOI] [PubMed] [Google Scholar]
- 39.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009;360:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009;360:985–993 [DOI] [PubMed] [Google Scholar]
- 41.Wechsler ME. Pulmonary eosinophilic syndromes. Immunol Allergy Clin North Am 2007;27:477–492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.