Abstract
Fibroblasts are the major mesenchymal cells present within the interstitium of the lung and are a major source of vascular endothelial growth factor (VEGF), which modulates the maintenance of pulmonary microvasculature. Prostaglandin E2 (PGE2) acts on a set of E-prostanoid (EP) receptors that activate multiple signal transduction pathways leading to downstream responses. We investigated the modulation by PGE2 of VEGF release by human lung fibroblasts. Human lung fibroblasts were cultured until reaching 90% confluence in tissue culture plates, after which the culture media were changed to serum-free Dulbecco's modified Eagle's medium, with or without PGE2, and with specific agonists or antagonists for each EP receptor. After 2 days, culture media were assayed for VEGF by ELISA. The results demonstrated that PGE2 and the EP2 agonist ONO-AE1-259-01 significantly stimulated the release of VEGF in a concentration-dependent manner. Agonists for other EP receptors did not stimulate the release of VEGF. The stimulatory effect of PGE2 was blocked by the EP2 antagonist AH6809, but was not blocked by antagonists for other EP receptors. The protein kinase–A (PKA) inhibitor KT-5720 also blocked the stimulatory effect of PGE2. The increased release of VEGF induced by PGE2 was accompanied by a transient increase in the concentration of VEGF mRNA. These findings demonstrate that PGE2 can modulate the release of VEGF by human lung fibroblasts through its actions in the EP2 receptor/PKA pathway. This activity may contribute to the maintenance of pulmonary microvasculature in the alveolar wall.
Keywords: prostaglandin E2, vascular endothelial growth factor, human lung fibroblasts, tissue repair
Vascular endothelial growth factor (VEGF) was first identified as a vascular permeability factor in the supernatant of a tumor cell line (1), and was independently reported as a growth factor with mitogenic effects on vascular endothelial cells (2). Recent studies showed that many physiological effects of VEGF are crucial to normal functioning of the lung, which is one of the organs with the greatest expression of VEGF. In the fetal lung, VEGF plays a key role in organ development, and in the adult lung, VEGF is required for pulmonary capillary endothelial cell survival and for the maintenance of the normal alveolar wall. Consequently, the inhibition of VEGF signaling causes alveolar cell apoptosis–dependent emphysema (3).
Alveolar fibroblasts are a major component of the alveolar interstitial space. They comprise approximately 40% of all alveolar cells (4), and are a rich source of growth factors and intercellular mediators. The production of VEGF by interstitial fibroblasts may contribute to the maintenance of alveolar endothelial cell populations. In this context, prostacyclin analogues stimulate the production of VEGF in human lung fibroblasts, an effect mediated by the I-prostanoid receptor acting through the cyclic adenosine monophosphate (cAMP)/protein kinase–A (PKA) pathway (5).
Prostaglandin E2 (PGE2) is a metabolite of arachidonic acid derived via the cyclooxygenase pathway, and is the major eicosanoid produced by lung fibroblasts and many other lung cells (6). Therefore, PGE2 has the potential to function as both an autocrine and a paracrine mediator. PGE2 was reported to be a potent inhibitor of fibroblast-mediated tissue repair, including chemotactic recruitment (7, 8), proliferation (6), matrix production (9, 10), and matrix remodeling (11, 12). PGE2 exerts its actions by acting on four distinct G protein–coupled E-prostanoid (EP) receptors that produce distinctly different effects (13–16). The EP1 receptor regulates Ca2+ channel gating via an unidentified G protein. The EP2 and EP4 receptors couple to Gs and mediate increases in concentrations of cAMP. The EP3 receptor is more complex, but one of its major pathways is involved with coupling to Gi and decreasing concentrations of cAMP. Thus many of the actions of PGE2 on fibroblasts are mediated by cAMP. Because prostacyclin analogues act through cAMP/PKA to stimulate the release of VEGF from fibroblasts, PGE2 is hypothesized to exert a similar effect, and may not be entirely inhibitory of all functions in fibroblasts.
Accordingly, the present study evaluates the effects of PGE2 on the production of VEGF by human lung fibroblasts and the participation of specific EP receptors and signal transduction pathways.
Materials And Methods
Materials
We used several commercially available reagents. Dulbecco's modified Eagle's medium (DMEM) and FCS were obtained from Invitrogen (Grand Island, NY). PGE2, forskolin, indomethacin, and 3,3,5,5-tetramethylbenzidine (TMB) were obtained from Sigma (St. Louis, MO). KT-5720 was obtained from Calbiochem (San Diego, CA). AH6809 was obtained from Cayman Chemical (Ann Arbor, MI). Anti-human VEGF antibody and biotinylated anti-human VEGF antibody were obtained from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)–streptavidin conjugate was obtained from Zymed Laboratories (South San Francisco, CA). ONO-DI-004, ONO-AE1-259-01, ONO-AE-248, ONO-AE1-329, ONO-8713, ONO-AE3–240, and ONO-AE3-208 were kindly donated by the Ono Pharmaceutical Co., Ltd. (Osaka, Japan).
Cell Culture
Human fetal lung fibroblasts (HFL-1; lung, diploid, human; CCL-153) were obtained from the American Type Culture Collection (Rockville, MD). Primary adult lung fibroblasts were obtained from patients undergoing thoracic surgery for the removal of tumors, under a protocol approved by the Human Studies Committee of Grosshansdorf Hospital (Grosshansdorf, Germany). All subjects were current or former smokers, and had undergone lung function tests that excluded a diagnosis of chronic obstructive pulmonary disease (COPD). Fibroblast cultures were initiated from normal-appearing areas of the pulmonary parenchyma, in a region as far as possible from the tumor, that were free of pleura or large airways, as described previously (17). After primary culture, cells were aliquoted, frozen, and shipped to the University of Nebraska Medical Center, where all in vitro experiments were performed. Cells were cultured in 100-mm tissue culture dishes (BD Falcon; Becton Dickinson Labware, Lincoln Park, NJ) with DMEM supplemented with 10% FCS, 50 units/ml penicillin, 50 μg/ml streptomycin sulfate (penicillin streptomycin; Invitrogen), and 1 μg/ml amphotericin B (Geneva Pharmaceuticals, Dayton, NJ). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2, and were passaged every 3–5 days at a 1:4 ratio. In all experiments, HFL-1 cells were used between passages 14 and 18, and adult lung fibroblasts were used between passages 3 and 7. To evaluate the production of VEGF in monolayer culture, 1.0 ml of cells at a cell density of 1 × 105/ml was seeded in 12-well tissue culture plates. At 90% confluence, the medium was changed to DMEM without FCS for 2 hours, and then cells were treated with various concentrations of PGE2 and the EP agonists in serum-free DMEM. The supernatants of monolayer cultures were harvested 48 hours later and stored at −80°C until assayed. The EP antagonists (1 × 10−5 M), KT-5720 (1 × 10−6 M), and indomethacin (2 × 10−6 M) were added 1 hour before the addition of PGE2, and remained present during treatment with PGE2. Cells were counted to normalize the concentration of VEGF in the supernatants.
Measurement of VEGF by ELISA
VEGF in the supernatants of monolayer cultures was quantified by ELISA. Ninety-six–well plates were coated with 50 ng/ml monoclonal anti-human VEGF antibody at 4°C overnight. After washing three times, standards and samples were added and incubated at room temperature for 2 hours. After washing three times, bound antigen was detected with 100 ng/ml biotinylated anti-human VEGF antibody for 1 hour at room temperature, followed by washing and the application of HRP–streptavidin conjugate (1:20,000 dilution) for 1 hour at room temperature. After a final wash, bound HRP was detected with TMB, as described elsewhere (18). The reaction was stopped with 1 M H2SO4, and the product was quantified at 450 nm with a microplate reader (Bio-Rad, Hercules, CA).
EP2 and EP4 Receptor Silencing
HFL-1 cells were seeded in 12-well tissue culture plates in DMEM with 10% FCS. At 50% confluence, transfection with the EP2 and EP4 receptors’ small, interfering RNA (siRNA; SMART Pool; Dharmacon, Lafayette, CO) was performed, as we described previously (18). Briefly, Lipofectamine 2000 and Opti-MEM (Invitrogen, Carlsbad, CA) were used as transfection reagents, according to the manufacturer's instructions. Cells were incubated with the siRNA (200 nM) or nontargeting RNA as the control in DMEM without FCS and antibiotics at 37° for 6 hours. The medium was then changed to DMEM with 10% FCS, and cells were cultured for an additional 42 hours. The siRNA-treated cells were then used to assess the role of PGE2 on the release of VEGF.
Preparation of RNA and Synthesis of cDNA
For quantitative real-time PCR, cells were cultured until confluent. The medium was then changed to DMEM without serum for 2 hours. Cells were exposed to 1 × 10−7 M PGE2 for 0.5, 1, 2, 4, 6, and 24 hours. Total RNA was isolated by the single-step guanidinium–thiocyanate–phenol chloroform extraction procedure (19), and the total amount was quantified spectrophotometrically (Shimadzu Scientific Instruments, Columbia, MD). One microgram of total RNA was treated with RNase-free DNase I according to the manufacturer's instructions (Invitrogen) for 15 minutes at room temperature, to remove possible contaminating genomic DNA. The reaction was then stopped with 25 mM EDTA by heating at 65°C for 10 minutes, followed by 95°C for 5 minutes. For the synthesis of cDNA, approximately 400 ng of DNase-treated RNA were transcribed with cDNA transcription reagents (Applied Biosystems, Foster City, CA) through the use of random hexamers, and the cDNA was used for quantitative real-time PCR.
Quantitative Real-Time PCR
Gene expression was measured with the use of an ABI Prism 7500 Sequence Detection System (Applied Biosystems), as described previously (20). TaqMan Gene Expression Assays, which include predesigned primers and probes for the detection of VEGF mRNA (Hs00173626_m1), were purchased from Applied Biosystems. Probes were labeled at the 5′ end with the reporter dye 6-carboxy–fluorescein, and at the 3′ end with the quencher dye 6-carboxytetramethyl–rhodamine. Ribosomal RNA (rRNA) was simultaneously analyzed using TaqMan Ribosomal RNA Control Reagents (Applied Biosystems). The quantitative real-time PCR of cDNA samples was performed in a total volume of 50 μl with 1× TaqMan Universal PCR Master Mix (Applied Biosystems), with primers at 900 nm and probes at 250 nm. Thermal cycler parameters included 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles involving denaturation at 95°C for 15 seconds, with annealing/extension at 60°C for 1 minute. Data were normalized to the amount of rRNA from the same preparations, and expressed as fold of control value.
Statistical Analysis
Data are expressed as means ± SE. Experiments with multiple comparisons were evaluated by one-way ANOVA, followed by the Tukey post hoc test to adjust for multiple comparisons. For the evaluation of experiments within a group where paired samples were available, the Wilcoxon test was used. P < 0.05 was considered significant.
Results
Effect of PGE2 on the Release of VEGF by HFL-1 Cells
To determine whether PGE2 could alter the production of VEGF, the release of VEGF into monolayer culture medium was evaluated by ELISA. After 2 days, culture with various concentrations of PGE2 (1 × 10−10 to 1 × 10−5 M) increased the production of VEGF significantly and in a concentration-dependent manner (Figure 1A). After stimulation with PGE2, the production of VEGF increased almost linearly from the first time point assessed (i.e., 12 hours) to the last time assessed (i.e., 72 hours) (Figure 1B).
Figure 1.
Effect of prostaglandin E2 (PGE2) on the release of vascular endothelial growth factor (VEGF) by human fetal lung fibroblasts (HFL-1). HFL-1 cells were cultured until they reached 90% confluence in 12-well tissue culture plates, after which medium was changed to serum-free Dulbecco's modified Eagle's medium (SF-DMEM), with or without PGE2. (A) Concentration dependence. After 2 days, the monolayer culture medium of HFL-1 stimulated by varying concentrations of PGE2 was harvested and assayed for VEGF by ELISA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, concentration of PGE2 (molar). (B) Time course. Monolayer cultures were stimulated with PGE2 (1 × 10−7 M), and the medium was harvested at varying time points and assayed for VEGF by ELISA. Vertical axis, release of VEGF (picograms per 105 cells). Horizontal axis, time (hours). Open circles, control sample; solid circles, PGE2. The data represent the means ± SE of three separate experiments. *P < 0.05 and **P < 0.01, compared with values of control sample.
Identification of EP Receptors Involved in the Effect of PGE2 on the Release of VEGF
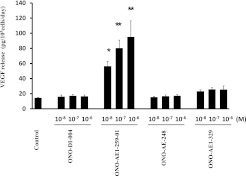
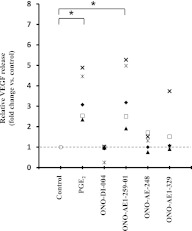
Two approaches were used. First, we used specific EP receptor agonists, and quantified the release of VEGF into monolayer culture medium by ELISA. After a 2-day exposure, the EP2 receptor agonist ONO-AE1-259-01 (1 × 10−8 to 1 × 10−6 M) increased the production of VEGF significantly and in a concentration-dependent manner (Figure 2). The EP1 receptor agonist ONO-DI-004 and the EP3 receptor agonist ONO-AE-248 did not affect the release of VEGF. The EP4 receptor agonist ONO-AE1-329 slightly increased the production of VEGF, but the difference was not statistically significant, and no concentration dependence was evident.
Figure 2.
Effect of E-prostanoid (EP) receptor agonists on release of VEGF by HFL-1. HFL-1 cells were cultured until they reached 90% confluence in 12-well tissue culture plates, after which the medium was changed to SF-DMEM, with varying concentrations of EP receptor agonists. After 2 days, the medium was harvested and assayed for VEGF by ELISA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, concentrations of EP receptor agonists. The data represent the means ± SE of three separate experiments. *P < 0.05 and **P < 0.01, compared with values of control sample.
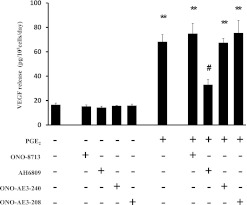
After ascertaining that the EP2 receptor agonist stimulated VEGF, we confirmed the importance of the EP2 receptor by using receptor-specific antagonists to block the effect of PGE2. The antagonists (1 × 10−5 M) were added to the medium 1 hour before the addition of PGE2 (1 × 10−7 M). None of the antagonists exerted an effect when added alone. The EP2 receptor antagonist AH6809 significantly inhibited the stimulatory effect of PGE2 (Figure 3). In contrast, the EP1 receptor antagonist ONO-8713, the EP3 receptor antagonist ONO-AE3-240, and the EP4 receptor antagonist ONO-AE3-208 were without effect on PGE2-stimulated cells.
Figure 3.
Effects of EP receptor–specific antagonists on the release of VEGF by HFL-1. HFL-1 cells cultured in monolayer were incubated with or without the EP receptor–specific antagonists (1 × 10−5 M) for 1 hour before treatment with PGE2 (1 × 10−7 M) or with control medium. After 2 days, the culture medium was harvested and assayed for VEGF by ELISA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, conditions. The data represent the means ± SE of three separate experiments. **P < 0.01, compared with values of control sample. #P < 0.05, compared with nontreated groups.
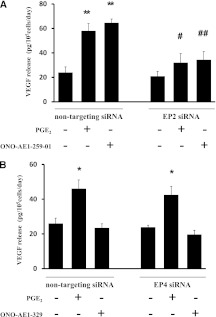
Finally, to confirm further that the release of VEGF by PGE2-stimulated cells was mediated by the EP2 receptor, we used EP2 receptor–specific siRNA. Although PGE2 (1 × 10−7 M) and the EP2 receptor agonist ONO-AE1-259-01 (1 × 10−7 M) stimulated the release of VEGF in nontargeting siRNA–treated cells, cells treated with the EP2 receptor siRNA showed a significant inhibition of the VEGF release stimulated by PGE2 as well as by the EP2 receptor agonist (Figure 4A). PGE2 (1 × 10−7 M), but not EP4 receptor agonist ONO-AE1-329 (1 × 10−7 M), stimulated the release of VEGF in nontargeting siRNA–treated cells (Figure 4B). The EP4 receptor siRNA–treated cells did not inhibit the release of VEGF stimulated by PGE2. Taken together, these data indicate that PGE2 stimulates the production of VEGF through the EP2 receptor in HFL-1 cells.
Figure 4.
Effect of EP2 and EP4 receptor silencing by short, interfering RNA (siRNA) on stimulation by PGE2 of VEGF release by HFL-1 cells. HFL-1 cells cultured in monolayer were incubated with siRNA for EP2 and EP4 or with control transfection reagent, as described in Materials and Methods. At 90% confluence, the medium was changed to SF-DMEM, with or without PGE2 (1 × 10−7 M) or the corresponding receptor agonist (1 × 10−7 M). After 2 days, the culture medium was harvested and assayed for VEGF by ELISA. (A) EP2 receptor silencing by siRNA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, conditions. (B) EP4 receptor silencing by siRNA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, conditions. The data represent the means ± SE of three separate experiments. *P < 0.05 and **P < 0.01, compared with values of control sample. #P < 0.05 and ##P < 0.01, compared with nontreated groups.
Dependence of PGE2 Stimulation of VEGF Release on PKA
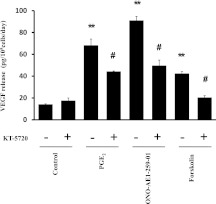
We evaluated the signal transduction pathway for the increased production of VEGF. The EP2 receptor couples to Gs and mediates increases in concentrations of cAMP, and cAMP can activate the classic PKA pathway, which was reported to stimulate the release of VEGF in response to prostacyclin (5). Thus, we determined whether the cAMP/PKA pathway mediated the effect of PGE2 in stimulating the release of VEGF. To accomplish this, the PKA inhibitor KT-5720 (1 × 10−6 M) was added 1 hour before the addition of PGE2 (1 × 10−7 M) or the EP2 receptor agonist ONO-AE1-259-01 (1 × 10−7 M). KT-5720 significantly reduced the stimulatory effect of PGE2 and the EP2 receptor agonist on the release of VEGF (Figure 5), whereas it exerted no effect on the release of VEGF when added alone. At the same time, we used forskolin (1 × 10−6 M) as a control to stimulate PKA. Forskolin stimulated the release of VEGF significantly, and the effect was almost completely blocked by KT-5720.
Figure 5.
Effect of KT-5720 on stimulation by PGE2 of VEGF release by HFL-1 cells. HFL-1 cells cultured in monolayer were incubated with or without KT-5720 (1 × 10−6 M) for 1 hour before treatment with PGE2 (1 × 10−7 M). After 2 days, the culture medium was harvested and assayed for VEGF by ELISA. Vertical axis, release of VEGF (picograms per 105 cells per day). Horizontal axis, conditions. The data represent the means ± SE of three separate experiments. **P < 0.01, compared with values of control sample. #P < 0.05, compared with nontreated groups.
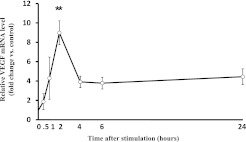
Effect of PGE2 on the Expression of VEGF mRNA
To determine whether the stimulation of VEGF release was associated with a change in gene expression, quantitative real-time PCR assays of VEGF mRNA were performed. PGE2 (1 × 10−7 M) stimulated the expression of VEGF mRNA (Figure 6). The peak increase in VEGF mRNA was observed at 2 hours, and measured 9.0 ± 1.2 fold. Afterward it decreased, but remained elevated at 24 hours, the last time point assessed. However, the difference was not significant.
Figure 6.
Effect of PGE2 on the expression of VEGF mRNA. HFL-1 cells were cultured in 60-mm dishes until confluence. The medium was changed to DMEM without serum for 2 hours, after which the cells were incubated with PGE2 (1 × 10−7 M) for 0.5, 1, 2, 4, 6, and 24 hours. Extracted RNA was quantified by TaqMan real-time PCR, as described in Materials and Methods. Vertical axis, level of VEGF mRNA expression, expressed as fold of control values. Horizontal axis, time (hours). The data represent the means ± SE of three separate experiments. **P < 0.01, compared with control sample.
Effect of PGE2 and EP Receptor Agonists on of VEGF by Primary Adult Lung Fibroblasts
To determine if the stimulation of VEGF release by PGE2 observed in HFL-1 cells extends to adult lung cells, fibroblasts obtained from the lungs of five smokers without COPD were assessed. As with the HFL-1 cells, PGE2 and the EP2 agonist stimulated the release of VEGF in fibroblasts from all five subjects (Figure 7). Agonists for EP1, EP3, and EP4 were without effect for the group as a whole, although one individual demonstrated a response to the EP4 agonist.
Figure 7.
Effects of PGE2 and EP receptor agonists on the release of VEGF by primary adult lung fibroblasts. Primary adult lung fibroblasts were cultured until they reached 90% confluence in 12-well tissue culture plates, after which the medium was changed to SF-DMEM with PGE2 or EP receptor agonists (all at 1 × 10−7 M). After 2 days, the monolayer culture medium was harvested and assayed for VEGF by ELISA. Vertical axis, level of VEGF release expressed as fold of control values. Horizontal axis, conditions. Control sample, without addition, is indicated by open circles. Each normal adult strain is indicated by a single type of symbol. *P < 0.05, compared with values of control sample.
Discussion
The present study demonstrates that PGE2 stimulates the production of VEGF by human lung fibroblasts. The specific EP2 receptor agonist ONO-AE1-259-01 showed an effect similar to that of PGE2, whereas other EP receptor agonists were ineffective. Consistent with the mechanism of EP2 receptor–mediated stimulation of VEGF production by PGE2 were confirmatory experiments using the specific EP2 receptor antagonist AH6809 and cells treated with EP2 receptor siRNA together with PGE2. Both of these experimental conditions significantly inhibited the stimulatory effect of PGE2. Li and colleagues confirmed that both EP2 and EP4 siRNA selectively reduced the expression of their corresponding receptor, as assayed by immunoblotting (8). The PKA inhibitor KT-5720 also significantly antagonized the stimulatory effect of PGE2. Taken together, these results support the concept that a variety of stimuli can modulate the production of PGE2 in the lung and, in turn, modulate the production of VEGF by fibroblasts, thus potentially affecting the pulmonary vasculature.
Our primary aims involved determining whether PGE2 modulated the release of VEGF by lung fibroblasts, and to determine the EP receptors mediating this effect. HFL-1 cells are widely used normal human fetal lung fibroblasts in which the expression of all EP receptors has been observed via immunoblotting (8). HFL-1 cells have several advantages, including reliable growth and in vitro characteristics, and their widespread use allows for comparisons of results among investigators. Nevertheless, not all responses observed within this cell strain are manifest in normal adult cells. Accordingly, we confirmed the presence of HFL-1 cells in five strains of fibroblasts obtained from adult smokers without COPD. Thus, the ability of PGE2 to stimulate the release of VEGF can be generalized to lung fibroblasts. The adult cells evaluated here were obtained from smokers undergoing surgery for suspected lung cancer. Interestingly, differences in the magnitude of the EP2 response were evident, and one subject demonstrated a response to the EP4 agonist. This suggests the possibility of interindividual variability, which could constitute a factor contributing to susceptibility to diseases affecting the lung vasculature.
Several studies evaluated PGE2 and EP receptors in the context of VEGF release in cells other than lung fibroblasts. PGE2 was reported to stimulate the release of VEGF in rat gastric fibroblasts through the EP4 receptor (21), and in human synovial fibroblasts through the EP2 and EP4 receptors (22). The present study extends these findings in several ways. First, it demonstrated that PGE2 stimulates the production of VEGF by human lung fibroblasts. Second, the EP2 receptor appears to mediate a great deal of this effect, indicating that fibroblast populations are somewhat heterogeneous with regard to the pathways by which PGE2 stimulates the release of VEGF. Lung fibroblasts express all four EP receptors (18). However, the results of this study did not demonstrate a role for the EP4 receptor, which also elevates concentrations of cAMP, in mediating the stimulatory effect of PGE2, but a role for EP4 cannot be excluded. A numerical, but not significant, increase in the release of VEGF in response to the EP4 agonist in HFL-1 cells was observed. Similarly, the EP2 antagonist significantly inhibited the PGE2-induced release of VEGF, but did not do so completely. This result may be attributed either to incomplete antagonism at the EP2 receptor, or to an action through an alternate pathway. Finally, one adult donor's fibroblasts showed a strong response to the EP4 agonist. Thus, the EP4 receptor may play a minor role overall or in specific patients.
The stimulation by PGE2 of VEGF release is associated with a change in VEGF gene expression. The peak increase in VEGF mRNA was observed at 2 hours. Consistent with this result, the prostacyclin analogues, iloprost and beraprost, were previously demonstrated to stimulate the expression of VEGF mRNA by HFL-1, with a peak increase at 1 hour (5), in a kinetic pattern very similar to that in the present results. The transient stimulation of mRNA concentrations was followed by a more prolonged increase in the release of VEGF protein. This finding suggests that PGE2 may increase VEGF by both transcriptional and translational or posttranslational effects.
For our mechanistic studies, we used the HFL-1 strain of fetal lung fibroblasts (catalogue number CCL-153; American Type Culture Collection). These cells are karyotypically normal and were used by a large number of investigators, making comparisons among laboratories and between experiments easier. However, we also demonstrated that PGE2 stimulates the release of VEGF from fibroblasts cultured from the lungs of adults. This finding demonstrates the generalizability of our results. Interestingly, the magnitude of the VEGF response appeared to differ among individuals. The basis for this difference remains to be determined. It does raise the interesting possibility that variability in the release of fibroblast VEGF could account for variability in susceptibilities to develop emphysema.
VEGF is believed to be an important mediator of tissue maintenance and repair. VEGF is required for pulmonary capillary endothelial cell survival, which is required for the structural maintenance of the alveolar wall. In the context of lung disease, Koyama and colleagues (23) demonstrated a decrease in VEGF concentrations in bronchoalveolar lavage fluid from patients with pulmonary fibrosis, compared with nonsmoking volunteers. Moreover, Kasahara and colleagues (3) documented that the inhibition of VEGF signaling causes alveolar cell apoptosis–dependent emphysema. The release of VEGF into induced sputum is significantly increased in patients with chronic bronchitis, whereas it is significantly decreased in patients with emphysema (24), and the expression of VEGF in lung tissue was reduced in severe emphysema (25). Because alveolar fibroblasts are believed to be a rich source of VEGF, the present study suggests that PGE2, by stimulating the fibroblast production of VEGF, can contribute to the pathogenesis or maintenance of lung vasculature in health and in disease, including fibrosis and emphysema. Consistent with such a role, Tsuji and colleagues demonstrated that an EP2 receptor agonist increases both the concentration of VEGF and alveolar vascularization after the development of emphysema in a murine model (26). Conversely, the EP2-deficient mouse fails to develop lung tumors, which may be attributed to a deficiency in vascularization (27).
PGE2 was suggested to function as a potent inhibitor of fibroblast-mediated tissue repair of the lung. Kohyama and colleagues (7) previously demonstrated that PGE2 inhibited fibroblast chemotaxis through the cAMP/PKA pathway. Huang and colleagues (12) demonstrated that PGE2 also inhibited the expression and proliferation of collagen in lung fibroblasts through the EP2 receptor and cAMP signaling. The results of the present study demonstrate that PGE2 also stimulates the production of VEGF. Signaling through cAMP and PKA appears to mediate at least part of this effect. However, because the inhibition of PKA blocked the effect of forskolin (a direct activator of adenylyl cyclase) but only partly inhibited the action of PGE2 and the EP2 agonist, signaling through a cAMP-independent pathway is not excluded. By stimulating the release of VEGF, PGE2 may have the ability to inhibit lung fibrotic responses while stimulating vascular responses.
Within the lung, PGE2 is produced by fibroblasts and many other lung cells, and acts as an autocrine/paracrine mediator. Many factors stimulate the endogenous production of PGE2 by lung fibroblasts, including IL-1, TNF-α (28), and transforming growth factor–β (TGF-β) (29). However, IL-1 and TNF-α inhibit the fibroblast-mediated contraction of collagen matrix (30), which is an index of repair function. TGF-β, in contrast, stimulates the contraction of fibroblasts (31). This suggests the possibility that PGE2 can modulate fibroblast-mediated tissue repair functions in the lung in a variety of settings. The demonstration of a physiologic role for the PGE-driven fibroblast production of VEGF, however, will require in vivo experimentation.
In conclusion, we demonstrated that PGE2 stimulates the production of human lung fibroblast VEGF through the EP2 receptor and activation of the PKA pathway. This pathway could modulate the maintenance of the pulmonary microvasculature in the alveolar wall.
Supplementary Material
Acknowledgments
The authors acknowledge the kind gift of reagents from Ono Pharmaceutical Co. (Osaka, Japan) and thank Lillian Richards for excellent secretarial support.
Footnotes
This work was supported by National Institutes of Health grant 1R01 HL-640880 (S.I.R.), by the Larson Endowment (University of Nebraska Foundation, University of Nebraska Medical Center), and by the Chancellor's Office at the University of Nebraska Medical Center.
Originally Published in Press as DOI: 10.1165/rcmb.2010-0115OC on September 15, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–985 [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989;161:851–858 [DOI] [PubMed] [Google Scholar]
- 3.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;126:332–337 [DOI] [PubMed] [Google Scholar]
- 5.Kamio K, Sato T, Liu X, Sugiura H, Togo S, Kobayashi T, Kawasaki S, Wang X, Mao L, Ahn Y, et al. Prostacyclin analogs stimulate VEGF production from human lung fibroblasts in culture. Am J Physiol Lung Cell Mol Physiol 2008;294:L1226–L1232 [DOI] [PubMed] [Google Scholar]
- 6.Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage–driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest 1986;77:700–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E2 inhibits fibroblast chemotaxis. Am J Physiol 2001;281:L1257–L1263 [DOI] [PubMed] [Google Scholar]
- 8.Li YJ, Wang XQ, Sato T, Kanaji N, Nakanishi M, Kim M, Michalski J, Nelson AJ, Sun JH, Farid M, et al. Prostaglandin E2 inhibits human lung fibroblast chemotaxis through disparate actions on different E-prostanoid receptors. Am J Respir Cell Mol Biol 2011;44:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltzman LE, Moss J, Berg RA, Hom B, Crystal RG. Modulation of collagen production by fibroblasts: effects of chronic exposure to agonists that increase intracellular cyclic AMP. Biochem J 1982;204:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamoto M, Romberger DJ, Nakamura Y, Tate L, Ertl RF, Spurzem JR, Rennard SI. Modulation of fibroblast Type I collagen and fibronectin production by bovine bronchial epithelial cells. Am J Respir Cell Mol Biol 1995;12:425–433 [DOI] [PubMed] [Google Scholar]
- 11.Mio T, Liu X, Adachi Y, Striz I, Skold CM, Romberger DJ, Spurzem JR, Illig MG, Ertl R, Rennard SI. Human bronchial epithelial cells modulate collagen gel contraction by fibroblasts. Am J Phys 1998;274:L119–L126 [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 2007;292:L405–L413 [DOI] [PubMed] [Google Scholar]
- 13.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 2004;36:1187–1205 [DOI] [PubMed] [Google Scholar]
- 14.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 2001;41:661–690 [DOI] [PubMed] [Google Scholar]
- 15.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999;79:1193–1226 [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 2007;282:11613–11617 [DOI] [PubMed] [Google Scholar]
- 17.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jorres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 2004;24:575–579 [DOI] [PubMed] [Google Scholar]
- 18.Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. Th2 cytokine–enhanced and TGF-beta–enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol 2003;111:1307–1318 [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidine thiocyanate–phenol–chloroform extraction. Anal Biochem 1987;162:156–159 [DOI] [PubMed] [Google Scholar]
- 20.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000;25:169–193 [DOI] [PubMed] [Google Scholar]
- 21.Hatazawa R, Tanigami M, Izumi N, Kamei K, Tanaka A, Takeuchi K. Prostaglandin E2 stimulates VEGF expression in primary rat gastric fibroblasts through EP4 receptors. Inflammopharmacology 2007;15:214–217 [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Takamori M, Shimoyama Y, Ishibashi H, Yamamoto S, Koshihara Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol 2002;136:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:382–385 [DOI] [PubMed] [Google Scholar]
- 24.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 2003;114:354–358 [DOI] [PubMed] [Google Scholar]
- 25.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1250–1256 [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T, Aoshiba K, Yokohori N, Nagai A. A systemically administered EP2 receptor agonist stimulates pulmonary angiogenesis in a murine model of emphysema. Prostaglandins Other Lipid Mediat 2009;90:85–88 [DOI] [PubMed] [Google Scholar]
- 27.Keith RL, Geraci MW, Nana-Sinkam SP, Breyer RM, Hudish TM, Meyer AM, Malkinson AM, Dwyer-Nield LD. Prostaglandin E2 receptor subtype 2 (EP2) null mice are protected against murine lung tumorigenesis. Anticancer Res 2006;26:2857–2861 [PubMed] [Google Scholar]
- 28.Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol 1987;138:3812–3816 [PubMed] [Google Scholar]
- 29.Wen FQ, Skold CM, Liu XD, Ertl RF, Zhu YK, Kohyama T, Wang H, Rennard SI. Glucocorticoids and TGF-beta1 synergize in augmenting fibroblast mediated contraction of collagen gels. Inflammation 2001;25:109–117 [DOI] [PubMed] [Google Scholar]
- 30.Zhu YK, Liu X-D, Skold CM, Umino T, Wang H, Romberger DJ, Spurzem JR, Kohyama T, Wen FQ, Rennard SI. Cytokine inhibition of fibroblast-induced gel contraction is mediated by PGE2 and NO acting through separate parallel pathways. Am J Respir Cell Mol Biol 2001;25:245–253 [DOI] [PubMed] [Google Scholar]
- 31.Tingstrom A, Heldin CH, Rubin K. Regulation of fibroblast-mediated collagen gel contraction by platelet-derived growth factor, interleukin-1 alpha and transforming growth factor–beta 1. J Cell Sci 1992;102:315–322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.