Abstract
The low-density lipoprotein receptor–related protein 1 (LRP-1) binds and can internalize a diverse group of ligands, including members of the fibrinolytic pathway, urokinase plasminogen activator (uPA), and its receptor, uPAR. In this study, we characterized the role of LRP-1 in uPAR processing, collagen synthesis, proteolysis, and migration in pleural mesothelial cells (PMCs). When PMCs were treated with the proinflammatory cytokines TNF-α and IL-1β, LRP-1 significantly decreased at the mRNA and protein levels (70 and 90%, respectively; P < 0.05). Consequently, uPA-mediated uPAR internalization was reduced by 80% in the presence of TNF-α or IL-1β (P < 0.05). In parallel studies, LRP-1 neutralization with receptor-associated protein (RAP) significantly reduced uPA-dependent uPAR internalization and increased uPAR stability in PMCs. LRP-1–deficient cells demonstrated increased uPAR t1/2 versus LRP-1–expressing PMCs. uPA enzymatic activity was also increased in LRP-1–deficient and neutralized cells, and RAP potentiated uPA-dependent migration in PMCs. Collagen expression in PMCs was also induced by uPA, and the effect was potentiated in RAP-treated cells. These studies indicate that TNF-α and IL-1β regulate LRP-1 in PMCs and that LRP-1 thereby contributes to a range of pathophysiologically relevant responses of these cells.
Keywords: pleural mesothelial cells, uPAR, LRP-1, internalization, half-life
Clinical Relevance
Our observations link lipoprotein receptor–related protein (LRP)-1 expression to human pleural mesothelial cell proteolysis, migration, and collagen 1 expression. Further, we discuss the novel findings that the inflammatory cytokines TNF and IL-1 can modulate uPAR expression by down-regulating LRP-1 and that uPA can stimulate collagen 1 expression.
The low-density lipoprotein receptor–related protein 1 (LRP-1) is a 600-kD surface receptor composed of a 515-kD α-ligand binding domain that is noncovalently associated with an 85-kD β-transmembrane domain (1, 2). LRP-1 is a member of the low-density lipoprotein receptor (LDLR) family, which includes the very low-density lipoprotein receptor and gp330/megalin. Members of the LDLR family share structural homology. They also play an important role in the internalization of diverse surface receptors and ligands, including α-2 macroglobulin, Pseudomonas exotoxin, urokinase plasminogen activator (uPA), plasminogen activator inhibitor (PAI)-1, and the uPA cognate receptor (uPAR) (3–5). Members of the LDLR family bind ligands with different affinities. The ability of LRP-1 in particular to bind such a diverse group of ligands suggests that it could play an important role in tissue remodeling, protein metabolism, and proteolytic activity. The receptor-associated protein (RAP) is a cytosolic chaperone for LRP-1; however, it also blocks the binding of natural ligands for members of the LDLR family, thus neutralizing their endocytotic function (6–8). We found that LRP-1 is expressed by PMCs in normalcy and disease, leading us to infer that it might influence a range of pathophysiologically relevant responses of these cells.
LRP-1 regulates cell motility (9) that involves members of the fibrinolytic pathway, specifically uPA and uPAR (9–11). LRP-1 has also been shown to regulate cellular fibrinolytic activity by rapidly internalizing single-chain uPA and the uPA/PAI-1/uPAR complex (12–14). Further, a motif on D3 of uPAR is believed to be responsible for a direct interaction between uPAR and LRP-1, which has been reported to facilitate uPAR internalization (13). Although the effects of the LDLRs on uPA/PAI-1 clearance and uPAR internalization have been examined in several systems (4, 7, 13, 14), we are unaware of any prior studies in which the contribution of LRP-1 to PMC functionality, including collagen expression, has been examined.
Proinflammatory cytokines such as TNF-α and TGF-β enhance uPAR expression in PMCs and malignant pleural mesothelioma (MPM) cells through increased transcription and stabilization of uPAR mRNA (15–17). We recently demonstrated that enhanced uPAR expression contributes to the increased migration and invasiveness of MPM in vivo (18). In a related vein, we report here that TNF-α and IL-1β enhance uPAR expression at the cell surface of PMC by down-regulating LRP-1. We find that LRP-1 directs internalization of uPAR and thereby regulates collagen expression, proteolysis, and migration of PMCs, responses germane to pleural remodeling after injury.
Materials and Methods
Additional information is provided in online Supplement.
Cell Culture
Cell lines used in these studies include MeT5A human PMCs; MPM cell lines REN, MS-1, and M9K; and primary human pleural mesothelial cells (HPMCs). Cells were grown at 37°C in a humidified 5% CO2 environment as previously described (18). Rabbit pleural mesothelial cells (RPMCs) were isolated from the visceral and parietal pleura of the rabbit thoracic cavity as previously described (19). Deidentified HPMCs were isolated from pleural fluids of patients with congestive heart failure (CHF) under a protocol approved by The University of Texas Health Science Center Institutional Review Board and cultured as previously described (20). Characterization of the cells used in this study is shown in Table 1.
TABLE 1.
CHARACTERIZATION OF PLEURAL MESOTHELIAL CELLS
| Cell Line | MeT5A | REN | HPMC | RPMC |
| uPAR | + | ++ | + | + |
| LRP-1 | + | − | ++ | ++ |
| Primary cell | − | − | + | + |
Definition of abbreviations: HPMC = human pleural mesothelial cell; LRP-1 = lipoprotein receptor–related protein 1; RPMC = rabbit pleural mesothelial cell; uPAR = urokinase plasminogen activator receptor.
Total Protein Extraction and Western Blotting
MeT5A, REN, MS-1, and M9K cells and HPMCs were serum starved for 18 hours. The cells were then lysed using PBX-100 (PBS [pH 7.4], a 1% Triton X-100 protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) for 30 minutes on ice. The lysates were resolved on SDS-PAGE and probed for LRP-1, uPAR, and β-actin.
Fibrin Enzymography
To detect cell-associated uPA activity, fibrin gel enzymography was performed as previously described (21). Cells were then washed with glycine buffer (pH 3.0) and incubated in the presence of PBS, ATN-617 (an antibody that blocks binding of uPA to uPAR [22]), or isotype-matched IgG-treated cells. Cells were incubated in the presence or absence of 10 or 20 nM uPA on ice for 20 minutes, and 50 μg of cleared lysate was resolved on a 10% SDS-PAGE and assayed via enzymography, as previously described (21).
Cell Migration
Migration analyses were performed as previously described (18, 23). Briefly, the apical and basolateral surfaces of 6.5-mm, 8-μm pore Transwell filter inserts (Corning Inc., Corning, NY) were vitronectin coated (23). MeT5A cells in suspension were treated with RAP (200 nM) for 15 minutes at RT. A total of 2 × 104 cells were then seeded in the apical chamber of the filter inserts in serum-free media (SFM) in the presence or absence of 10 nM uPA and/or 200 nM RAP. The basolateral chambers contained RPMI media supplemented with 2.5% FBS in the presence or absence of 10 nM uPA. Cells were allowed to incubate for 6 hours at 37°C and were fixed, stained, and counted (18).
Hydroxyproline Analysis
Hydroxyproline analyses were performed as previously described (24, 25). Conditioned media were collected from serum-starved RPMCs and HPMCs treated with PBS or TGF-β in the presence or absence of uPA and/or RAP at 37°C for 48 hours. The samples were assayed for hydroxyproline content and standardized against a collagen control.
Statistics
For studies evaluating the statistical significance of protein expression, uPAR t1/2, and internalization, paired two-tail t tests were performed, and a P < 0.05 was considered statistically significant.
Results
LRP-1 and uPAR Are Expressed by PMCs
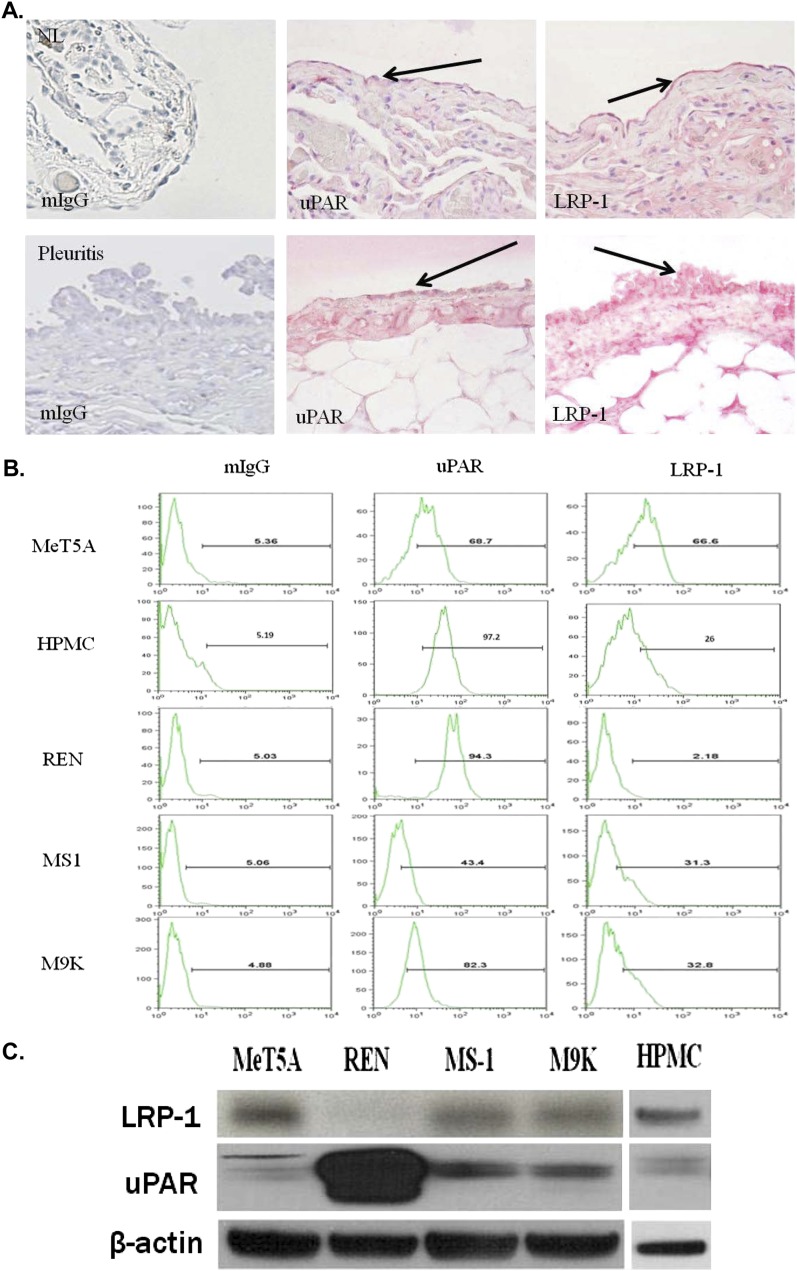
LRP-1 has been shown to regulate the expression of numerous cell surface receptors, including uPAR, suggesting it could regulate uPAR-mediated responses in PMCs. To determine whether LRP-1 was expressed by PMCs in health and disease, the pleural lining from normal human lung tissues or from a patient with pleuritis were analyzed. A pathologist examined the pleural surfaces on each slide at 40× for mesothelial cell immunopositivity. LRP-1 and uPAR were detected in the pleural mesothelium of the injured (pleuritis) and noninjured lung (Figure 1A). Staining of the relatively flat visceral normal mesothelium for LRP-1 and uPAR was detectable and was readily apparent in the more reactive cuboidal mesothelial cells found in injured pleural tissue. MeT5A cells, MPM cells, and HPMCs were next assayed for uPAR and LRP-1 at the cell surface via FACS analysis. HPMCs from patients with CHF were used to mitigate the potential for in situ activation by proinflammatory mediators. Figure 1B shows that MeT5A cells, MPM cells, and HPMCs express uPAR and LRP-1 at the cell surface. REN MPM cells, which had no detectable surface LRP-1 expression, exhibited the highest level of uPAR expression. Western blotting confirmed that LRP-1 and uPAR antigen were present in the HPMCs, MeT5A cells, and MPM cells, with the exception of REN (Figure 1C). Cell lysates were also tested for expression of very low density lipoprotein receptor which has also been reported to internalize uPAR (11) and megalin, but neither was detected in these cells (data not shown). These data confirm that LRP-1 and uPAR are expressed by HPMCs as well as MeT5A and MPM cell lines (Table 1).
Figure 1.
Analysis of urokinase plasminogen activator receptor (uPAR) and lipoprotein receptor–related protein (LRP) expression in human lung tissues, in pleural mesothelial cells (PMCs), and malignant pleural mesothelioma (MPM) cells. (A) Tissue sections were prepared from the lungs of patient without lung disease and a patients with pleuritis. The sections were probed for the expression of uPAR and LRP-1 by the pleural mesothelial cell lining using immunohistochemical analysis. The sectioned pleural mesothelium in its entirety was analyzed for uPAR and LRP-1 expression. The arrows indicate Fast-red staining of uPAR and LRP-1 in the pleural mesothelium. NL indicates the findings in representative normal lung visceral pleura, which shows underlying compressed lung parenchyma. The parietal pleural surface from the patient with pleuritis best illustrated reactivity for each antigen and is shown accordingly. Original magnification, ×40. (B) Serum-starved MeT5A cells, human pleural mesothelial cells (HPMCs) and REN, MS-1, and M9K MPM cells were probed with mouse monoclonal antibodies against uPAR and LRP-1 and analyzed via FACS analysis. The data are presented as a representative histogram of three independent experiments. (C) Serum-starved MeT5A, REN, MS-1, M9K, and HPMC cell lysates were probed for LRP-1 light chain expression (85 kD) and uPAR or β-actin loading controls via Western blot analysis.
Proinflammatory Cytokines Decrease LRP-1 mRNA and Protein in PMCs
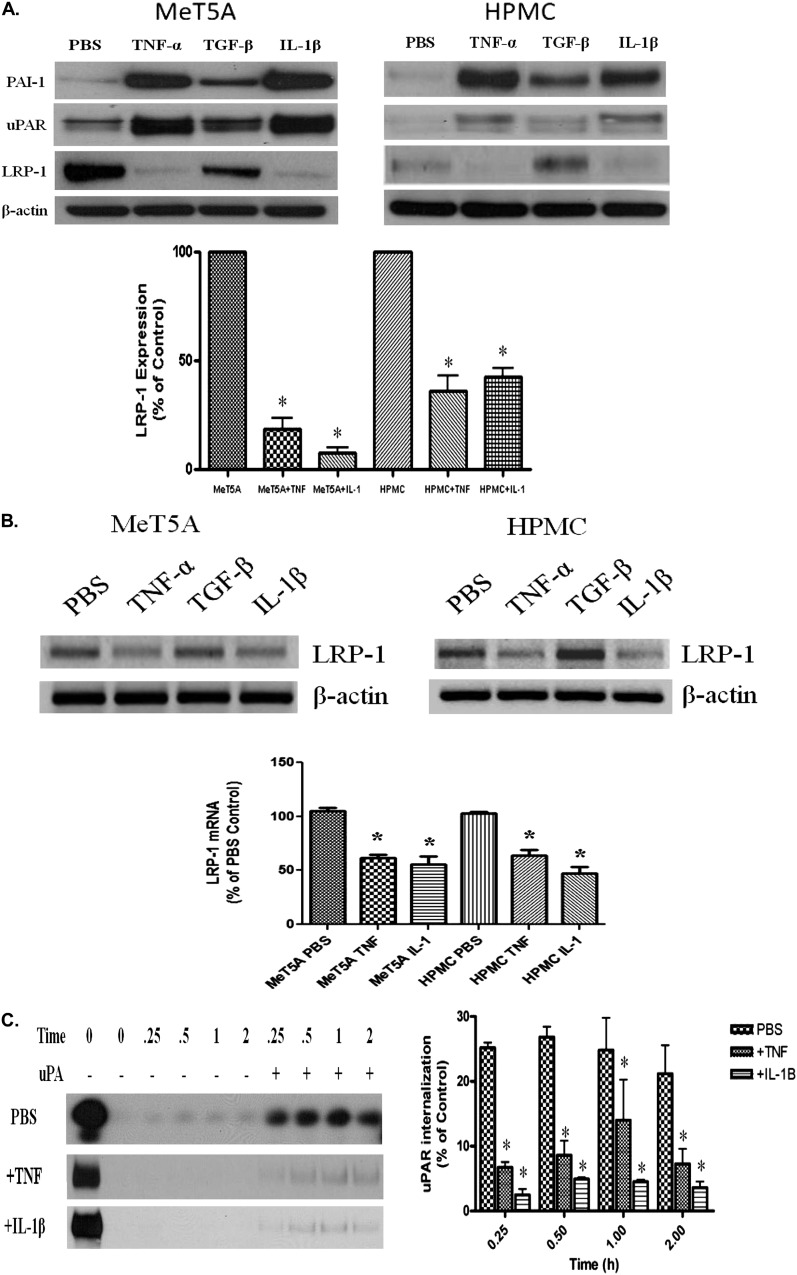
Proinflammatory cytokines such as IL-1β and TNF-α have long been associated with inflammation associated with diverse lung and pleural diseases and can induce uPAR (26, 27). Because TNF-α and other inflammatory cytokines induce LRP-1 mRNA and protein expression in other cell types (23, 28, 29), we inferred that LRP-1 could contribute to the regulation of uPAR by PMCs. To determine if inflammatory cytokines affect LRP-1 expression in PMCs, serum-starved MeT5A cells and HPMCs were incubated in the presence and absence of TNF-α, TGF-β, or IL-1β for 12 to 18 hours. The cells were then lysed and assayed for LRP-1, uPAR, and PAI-1 protein. Figure shows that MeT5A cells and HPMCs incubated with TNF-α, TGF-β, and IL-1β increased PAI-1 and uPAR protein expression by Western blotting. TNF-α and IL-1β enhanced uPAR expression to the greatest extent. Conversely, LRP-1 protein expression was reduced by TNF-α and IL-1β in MeT5A cells and HPMCs. TNF-α also down-regulated cell surface expression of LRP-1 via FACS analysis (data not shown). LRP-1 mRNA levels were also significantly reduced by TNF-α and IL-1β in MeT5A cells and HPMCs (Figure 2B). LPS had no effect on uPAR or LRP-1 expression in PMCs (data not shown). Thus, TNF-α and IL-1β decrease LRP-1 in PMCs.
Figure 2.
TNF-α and Il-1β decrease LRP-1 expression in PMCs. (A) Serum-starved MeT5A and primary HPMCs were treated with TNF-α, TGF-β, and IL-1β for 12 hours at 37°C in serum-free RPMI. The cells were then lysed and probed for LRP-1, uPAR, PAI-1, and β-actin. Images are representative of three independent experiments. *P < 0.01. (B) Serum-starved MeT5A and HPMCs were treated with TNF-α, TGF-β, and IL-1β as described in Methods. RNA was isolated from the cells, transcribed into cDNA, and probed for LRP-1 cDNA. β-actin cDNA was used as a loading control. (C) Serum-starved MeT5A cells were treated with PBS, TNF-α, or IL-1β for 12 hours. The cells were then biotinylated and incubated in the presence or absence of uPA for 0, 0.25, 0.50, 1, and 2 hours. The cells were subjected to glutathione (GSH) washes, lysed, and probed for uPAR internalization. Images are representative of three independent experiments.
TNF-α and IL-1β Decrease uPA-Mediated uPAR Internalization
Because TNF-α and IL-1β were found to down-regulate LRP-1 mRNA and protein levels in MeT5A cells and HPMCs, we sought to determine the role of these cytokines on uPA-mediated uPAR internalization. Serum-starved MeT5A cells were treated with TNF-α or IL-1β in SFM for 12 hours before assaying uPAR internalization in MeT5A cells. TNF-α– and IL-1β–treated PMCs internalized less uPAR than PBS-treated controls at all points (Figure 2C). These results suggest that TNF-α and IL-1β can influence cell surface stability of uPAR in human PMCs through down-regulation of LRP-1.
LRP-1 Neutralization Retards uPA-Mediated Internalization
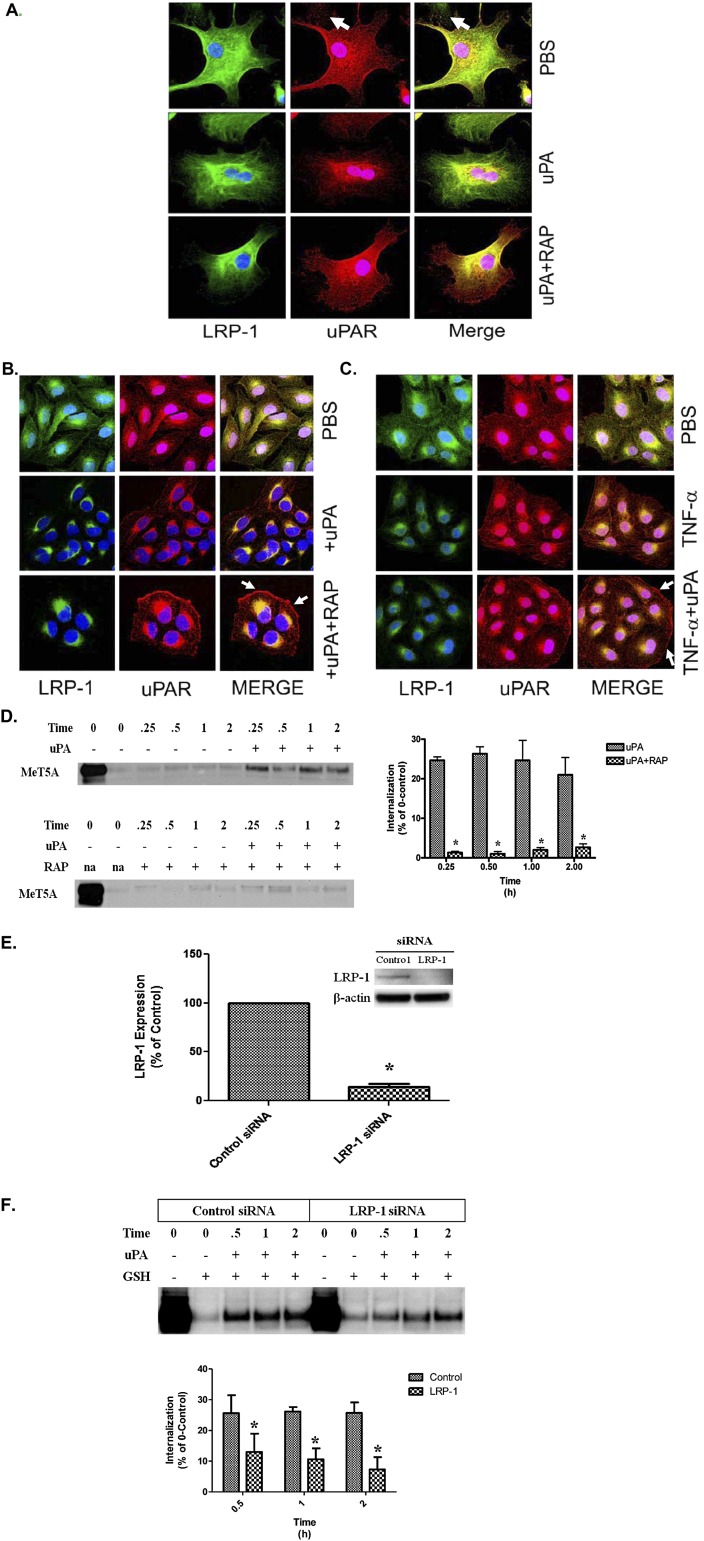
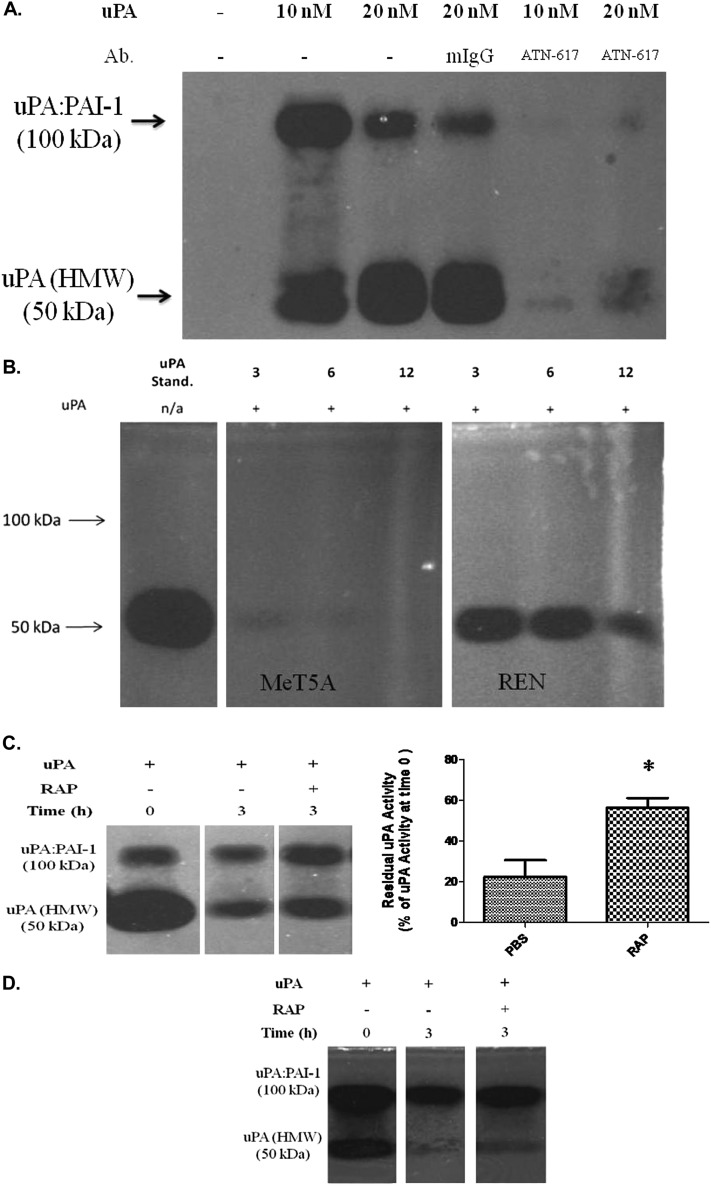
Because TNF-α and IL-1β reduced uPA-mediated uPAR internalization, we sought to confirm the role of LRP-1 on uPAR processing in PMCs. In these analyses, we used RAP, which has been reported to neutralize cell surface LDLRs (4, 7, 30). Immunofluorescence confocal microscopy showed that uPAR and LRP were diffusely distributed in primary HPMCs (Figure 3A). The addition of uPA down-regulated uPAR and LRP-1 expression and shifted their localization from the cell surface to a perinuclear distribution pattern. When cells were treated with RAP before uPA treatment, uPAR clusters were retained at the cell surface, whereas LRP-1 assumed a primarily perinuclear distribution. Figure 3B shows that RAP pretreatment down-regulated LRP-1 expression while stabilizing uPAR expression at the cell surface in the presence of uPA in MeT5A cells, which are more readily available and recapitulate the induction of uPAR in primary PMCs by TNF-α. TNF-α down-regulated LRP-1 expression while up-regulating uPAR in MeT5A cells (Figure 3C). These findings are best appreciated in the merged images. Further, TNF-α treatment prevented uPA-dependent down-regulation of uPAR in MeT5A cells. In uPAR internalization assays (Figure 3D), RAP treatment significantly decreased internalization of surface-labeled uPAR in the presence of uPA in MeT5A cells. uPA internalized approximately 20 to 25% of surface-labeled uPAR within 15 minutes. The addition of RAP abrogated most uPA-mediated internalization in MeT5A cells. In parallel internalization studies, LRP-1–deficient REN cells demonstrated little detectable uPAR internalization in the presence of uPA (data not shown). To further dissect the role of LRP-1 in uPA-dependent processing of uPAR in PMCs, LRP-1 siRNA analyses were performed. MeT5A cells transfected with LRP-1 siRNA were found to express approximately 70% less LRP-1 than the control siRNA-treated cells (Figure 3E), which expressed slightly lower LRP-1 than naive cells (not shown). LRP-1 siRNA-treated cells exhibited approximately 50% less uPA-mediated uPAR internalization when compared with control siRNA-treated cells (Figure 3F). These studies confirm that LRP-1 regulates the processing of uPAR at the PMC surface.
Figure 3.
Immunofluorescence labeling of uPAR and LRP-1 in uPA-treated PMCs. (A) Serum-starved HPMCs were treated in the presence or absence of 200 nM RAP for 15 minutes at 37°C. The cells were then incubated in the presence or absence of 20 nM uPA for 30 minutes at 37°C. The cells were fixed, permeabilized, and immunofluorescence labeled for uPAR and LRP-1 expression. (B) Serum-starved MeT5A cells were treated with 20 nM uPA in the presence or absence of RAP for 30 minutes at 37°C. The cells were then fixed, permeabilized, and labeled by immunofluorescence for uPAR and LRP-1 expression. Solid arrows indicate the presence of uPAR at the cell surface in uPA/RAP-treated cells. (C) Serum-starved MeT5A cells were treated with TNF-α for 15 to 18 hours in serum-free medium (SFM). The cells were then incubated in the presence or absence of 20 nM uPA for 30 minutes at 37°C. The cells were fixed, permeabilized, and labeled by immunofluorescence for uPAR and LRP-1 expression. Solid arrows indicate the presence of uPAR at the cell surface in uPA/TNF-α–treated cells. (D) Serum-starved MeT5A cells were treated with uPA in the presence and absence of 200 nM RAP. After incubation for 0.25, 0.50, 1, and 2 hours, the cells were subjected to GSH washes, lysed, and probed for uPAR internalization. Serum-starved MeT5A cells were surface biotinylated and incubated in the presence and absence of 20 nM uPA for 0, 0.25, 0.5, 1, and 2 hours. At the completion of the incubation periods, the cells were treated with GSH to remove remaining cell surface biotin. The designated 0 time point represents the total amount of uPAR on the cell surface at the time of biotinylation and is assigned a value of 100%. The graphs illustrate the percentage of the initial 100% of uPAR that was internalized. Internalized protected biotinylated protein was then isolated from lysates, and Western blot was performed to detect uPAR. (E) MeT5A cells were transfected with control or LRP-1 siRNA. Cells were then cultured in complete RPMI media for 24 hours and placed in SFM for 15 to 18 hours. Cells were then probed for LRP-1 expression by Western blot. β-actin was used as a loading control. The data represent the average of three independent experiments. *P < 0.05. (F) MeT5A cells were transfected with control or LRP-1 siRNA. Cells were then cultured in complete RPMI media for 24 hours and placed in SFM for 15 to 18 hours. Cells were surface biotinylated and incubated in the presence and absence of 20 nM uPA for 0, 0.5, 1, and 2 hours. At the completion of the incubation periods, the cells were treated with GSH to remove remaining cell surface biotin. The 0 time point indicates the total amount of uPAR on the cell surface at the time of biotinylation and is assigned a value of 100%. The bar graphs represent the percent of the total 100% of uPAR that was internalized into the cells from the cell surface in response to uPA treatment. *P < 0.05. The images are representative of at least three independent experiments.
LRP-1 Neutralization Increases uPAR Half-life in PMCs
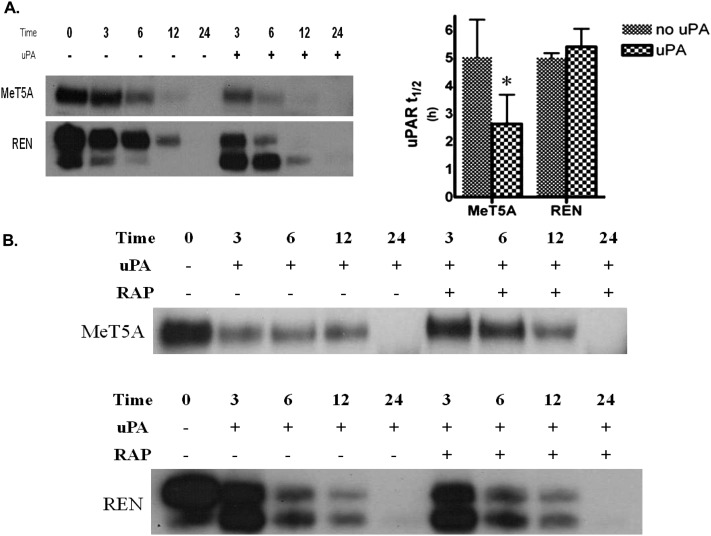
To determine the role of LRP-1 on surface-labeled uPAR stability in PMCs, surface biotinylation analyses were performed (Figure 4A). Cells were first biotinylated at the cell surface and then incubated in the presence or absence of uPA for 0, 3, 6, 12, and 24 hours to determine uPAR half-life (t1/2). The uPAR t1/2 in MeT5A cells was approximately 5 hours in the absence of uPA and was significantly reduced to 2.5 hours in the presence of uPA (P < 0.05). The uPAR t1/2 of MS-1 and M9K cells was approximately 4.3 hours in the absence of uPA and was reduced to 2.3 hours in the presence of uPA (data not shown). However, the uPAR t1/2 in REN cells was relatively unchanged by the presence or absence of uPA (∼ 5 h). An interesting finding in the REN line was that the D2-D3 moiety of uPAR became the dominant detectable species in the presence of uPA over time (Figure 4A). To evaluate the role of uPA fibrinolytic activity on uPAR stability, preformed uPA/PAI-1 complexes were used. These complexes decreased uPAR t1/2 to a similar extent as uPA alone (data not shown). RAP extended the uPAR t1/2 from 2.5 to 6 hours in the presence of uPA in MeT5A cells (Figure 4B). Predictably, RAP had no effect on uPA-mediated uPAR internalization or t1/2 in the LRP-1–deficient REN cells. These data support the concept that enhanced uPAR expression in REN cells occurs in part due to increased uPAR t1/2 as a consequence of the absence of LRP-1. The findings further indicate that LRP-1 regulates the uPAR t1/2 in PMCs. HPMCs could not be tested in these analyses because these cells were not viable after biotinylation at 4°C.
Figure 4.
uPAR t1/2 measurements in PMC and LRP-1–deficient MPM cells. (A) Serum-starved MeT5A and REN cells were surface biotinylated and treated with 20 nM uPA for 0, 3, 6, 12, and 24 hours in SFM. Biotinylated proteins were then isolated from lysates, resolved on SDS-PAGE, and probed for uPAR. The images are representative of at least three independent experiments. (B) Serum-starved MeT5A and REN cells were treated with uPA in the presence and absence of 200 nM RAP. The t1/2 of biotinylated uPAR protein was calculated via densitometric analysis, and the calculated t1/2 for the control and uPA treated samples were statistically analyzed. The data presented represent the average of three independent experiments. *P < 0.05.
LRP-1 Regulates Cell-Associated Fibrinolytic Activity in PMCs
To evaluate the ability of LRP-1 to control PMC-mediated proteolysis, cellular uPA activity was monitored via fibrin gel enzymography. We first sought to confirm that the primary interaction of uPA at the PMC cell surface involves uPAR. Figure 5A demonstrates that uPA activity is retained by PMCs treated with uPA and that the interaction is uPAR dependent because ATN-617, an anti-uPAR antibody, blocks detection of cell-associated uPA.
Figure 5.
Enzymographic analyses of uPA activity in PMCs. (A) Serum-starved MeT5A cells were acid washed and incubated with ATN-617 or mIgG for 15 minutes on ice, followed by incubation with uPA on ice. Cells were washed, lysed, and analyzed for the presence of cell-associated uPA activity by fibrin gel enzymography. (B) Serum-starved, acid-washed MeT5A and REN cells were incubated in the presence of uPA for 0, 3, 6, and 12 hours. The cells were washed and lysed, and 50 μg of protein from isolated lysates were analyzed for the presence of cell-associated uPA activity by fibrin gel enzymography. LRP-1 neutralization increases the duration of uPA activity in PMCs. Images were taken after a 4-hour incubation at 37°C. (C) Serum-starved, acid-washed MeT5A cells were pulse labeled with uPA in the presence or absence of RAP for 20 minutes on ice. The cells were then incubated at 37°C for 0 and 3 hours. Cells were lysed and analyzed for protected proteolysis using fibrin gel enzymography. Images were taken after a 12-hour incubation at 37°C. *P < 0.05. (D) Serum-starved, acid-washed HPMCs cells were pulse labeled with uPA in the presence or absence of RAP for 20 minutes on ice. The cells were incubated at 37°C for 0 and 3 hours, lysed, and analyzed for protected proteolysis using fibrin gel enzymography. Images were taken after a 12-hour incubation at 37°C. These experiments were performed twice.
To assess cell-associated uPA activity over time, serum-starved MeT5A and REN cells were incubated in the presence and absence of uPA for 0, 3, 6, and 12 hours. Residual uPA activity was monitored in the lysates of these treated cells by fibrin gel enzymography (Figure 5B). MeT5A and REN cells were chosen for these analyses because they were devoid of endogenous uPA activity in this assay, consistent with our previous report (18). After treatment with exogenous uPA, cell-associated uPA activity was detectable in MeT5A lysates at 3 hours but quickly decreased by 6 hours (Figure 5B). REN cells retained activity of exogenous uPA, and uPA activity was maintained in the LRP-1–deficient REN line compared with MeT5A cells. These data indicate that LRP-1 can regulate the expression of uPA activity in PMCs.
In an alternative approach to assess the ability of LRP-1 to regulate PMC-associated fibrinolytic activity, the enzymographic activity of uPA was examined in the presence and absence of RAP in MeT5A cells. Figure 5C demonstrates that uPA binds the MeT5A cell surface and that cellular expression rapidly declines over 3 hours. However, when pretreated with RAP, cellular uPA activity significantly was increased at 3 hours (P < 0.05). Further, uPA/PAI-1 complexes accumulated in RAP-treated cells. The same responses were observed in HPMCs (Figure 5D).
LRP-1 Regulates PMC Migration
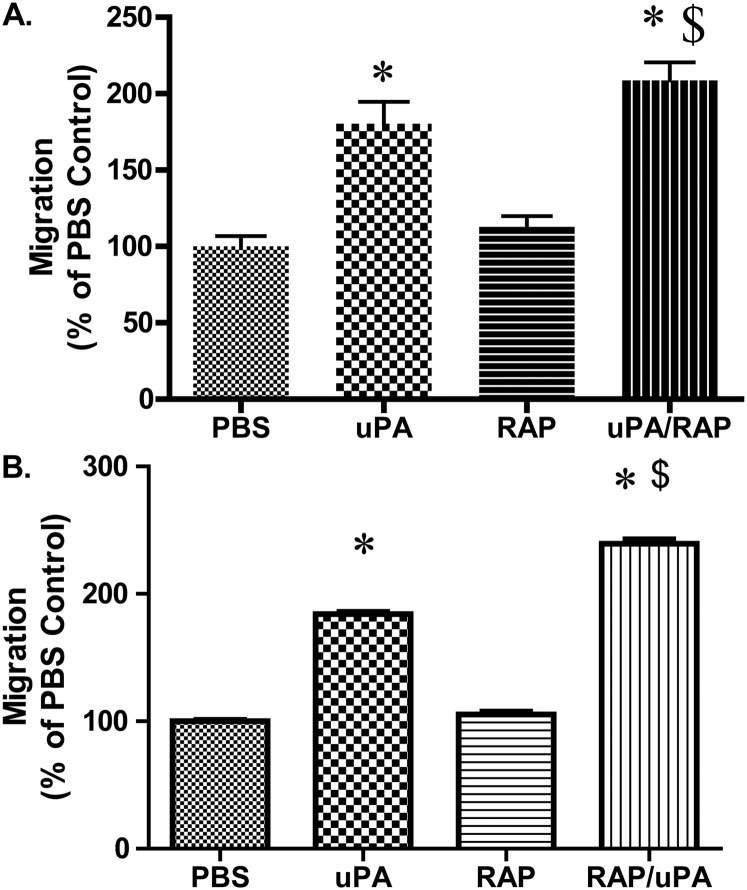
We next examined the consequences of LRP-1–mediated changes in cellular migration. PMCs migrate across vitronectin-coated filter inserts toward a FBS gradient (Figure 6A). Because uPA enhances migration of PMCs, uPA was included as a chemotactic agent in these assays. The addition of uPA was found, as expected, to potentiate MeT5A cell migration. Although RAP pretreatment had no effect on FBS-mediated migration, it enhanced uPA-mediated migration. The same responses were observed in parallel experiments in which HPMCs were tested (Figure 6B), indicating that LRP-1 regulates PMC migration in a uPA-dependent manner.
Figure 6.
LRP-1 regulates uPA-dependent migration in PMCs. (A) Serum-starved MeT5A cells were incubated in the presence or absence of RAP for 15 minutes at room temperature. Cells were placed on vitronectin-coated filter inserts in the presence or absence of uPA and allowed to migrate at 37°C for 6 hours. Cells were then fixed, stained, and counted. This experiment was repeated twice (n = 3 determinations per group in each experiment). Data from a representative experiment are illustrated. (B) Serum-starved HPMCs cells were incubated in the presence or absence of RAP for 15 minutes at room temperature. Cells were then placed on vitronectin-coated filter inserts in the presence or absence of uPA and allowed to migrate at 37°C for 6 hours. Cells were fixed, stained, and counted (n = 3 determinations per group in each experiment). Data from a representative experiment are illustrated. *P < 0.05 when compared with PBS controls. $P < 0.05 when compared with uPA.
uPA Increases Collagen 1 Expression in Primary PMCs
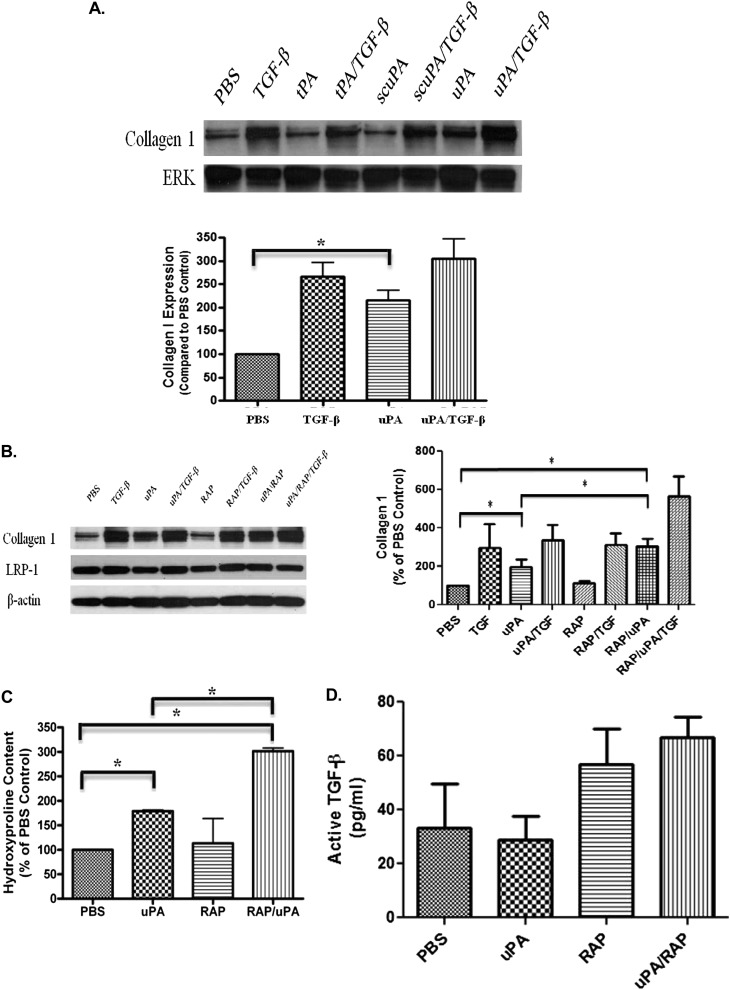
We assessed the role of LRP-1 in the control of collagen synthesis by PMC. TGF-β has been shown to induce collagen 1 in PMCs (24) and induced collagen expression in HPMCs and RPMCs (Figure 7). In RPMCs, uPA also enhanced collagen 1 expression, whereas tPA and scuPA showed no effect (Figure 7A). This represents a newly recognized uPA-mediated response in these cells. In HPMCs, an additive effect was demonstrated when uPA and TGF-β were used in combination. Although RAP had no effect on TGF-β–mediated collagen 1 expression, it potentiated uPA-mediated induction of collagen 1 expression in HPMCs (Figure 7B). uPA itself had little effect on LRP-1 expression in HPMCs (data not shown). Hydroxyproline analyses were also performed to assess total collagen expression by HPMCs. These analyses confirmed that uPA enhanced collagen expression by HPMCs and that RAP potentiated the effect (Figure 7C). The effect of uPA with or without RAP treatment was also assessed in RPMCs. uPA significantly increased collagen 1 expression and cellular hydroxyproline in RPMCS, with a trend toward potentiation with the addition of RAP (data not shown). Because TGF-β potently induces collagen expression by PMCs, we next sought to determine whether induction of collagen in these cells by uPA involved cross-talk with this growth factor. To address this possibility, we next assessed the ability of uPA or RAP to induce total and active TGF-β expression in HPMCs. HPMCs were treated with uPA in the presence and absence of RAP, and the conditioned media was assayed for total and active TGF-β by sandwich ELISA (BioLegend, San Diego CA). uPA did not induce active TGF-β in the HPMCs (Figure 7D). RAP alone or in combination with uPA did not induce significant increments in active TGF-β in the HPMCs, although there was a trend toward increased levels. In addition uPA did not affect the amount of active TGF-β present in TGF-β–treated (5 ng/ml) HPMCs (data not shown). Although total TGF-β in HPMCs demonstrated similar trends, increased levels that reached statistical significance were found in uPA-, RAP-, and uPA/RAP-treated cells (data not shown). These data show that uPA can induce the expression of collagen 1 in primary cultured PMCs, that the effect does not involve a detectable induction of active TGF-β, and that this newly recognized effect is subject to regulation by LRP-1.
Figure 7.
uPA increases collagen 1 expression in primary PMCs. (A) Primary RPMCs were treated with tPA (tissue type plasminogen activator; 20 nM), scuPA (single chain or proenzyme uPA; 20 nM), and uPA (two chain uPA; 20 nM) in the presence and absence of TGF-β (5 ng/ml). Conditioned media and lysates were collected, resolved on SDS-PAGE, and probed for collagen 1 and ERK. The images are representative of three independent experiments. (B) HPMCs were treated with uPA and/or TGF-β in the presence or absence of RAP (200 nM) for 48 hours in SFM. Conditioned media and cell lysates were collected, resolved by SDS-PAGE, and subjected to Western blotting for Collagen 1 and LRP-1. β-actin was used in loading controls. The images are representative of three independent experiments. (C) HPMCs were treated with uPA and/or TGF-β in the presence or absence of RAP for 48 hours in SFM. Conditioned media were collected and assayed for hydroxyproline content. This experiment was repeated twice (n = 3 determinations per group in each experiment). *P < 0.05. A representative experiment is shown. (D) HPMCs were treated with uPA in the presence or absence of RAP for 48 hours in SFM. Conditioned media were collected and assayed for active TGF-β via ELISA.
Discussion
The literature strongly supports the participation of LRP-1 in numerous cellular processes, including the regulation of cellular fibrinolytic activity and migration (3, 13, 31, 32). In this study, we investigated the contribution of LRP-1 to a range of responses of PMCs germane to pleural inflammation and remodeling. We found that uPAR and LRP-1 are expressed by the human pleural mesothelium in normalcy and after injury. We also found that LRP-1 is expressed by PMCs and determined, for the first time, that LRP-1 is suppressed in these cells by TNF-α and IL-1β. Lastly, we discovered that LRP-1 is the only member of the LDL superfamily represented in HPMCs, in which it regulates collagen expression induced by uPA, uPAR stability at the cell surface, PMC proteolysis, and cellular migration.
We and others showed that TGF-β and TNF-α increase expression of uPAR through transcriptional and posttranscriptional mechanisms (15, 26, 16, 33). Because these cytokines have also been reported to regulate LRP-1 expression in other cell types (28, 29, 34), we sought to determine the role of TNF-α, TGF-β, and IL-1β on LRP-1 expression in PMCs. As expected, TNF-α, TGF-β, and IL-1β increased uPAR expression in MeT5A cells and HPMCs. However, TNF-α and IL-1β increased uPAR expression to a greater extent while reducing LRP-1. Further, TNF-α and IL-1β reduced uPA-mediated uPAR internalization by 80%. These changes were not observed in peritoneal mononuclear cells in a previous study (35), perhaps relating to topologic heterogeneity between PMCs and peritoneal mononuclear cells or to the enhanced ability of relatively unstimulated PMCs from patients with CHF to respond to proinflammatory mediators. Because we cannot harvest normal HPMCs from subjects without pleural disease, HPMCs from patients with hydrostatic, relatively noninflammatory pleural effusions represent a relatively quiescent source of human cells. The findings recapitulate the responses of LRP-1 to TNF-α and IL-1β in MeT5A cells and, in the aggregate, represent a newly recognized mechanism by which the selected proinflammatory mediators can regulate uPAR and its functional repertoire in HPMCs.
The role of LRP-1 in uPAR processing in PMCs was confirmed by neutralizing cell surface LRP-1 in MeT5A cells and HPMCs with RAP in immunofluorescence localizations. uPA drives LRP-1 and uPAR to a primarily perinuclear distribution in these cells. RAP promotes uPAR clustering at the cell surface, suggesting that LRP-1 contributes to uPAR localization in PMCs. uPAR clustering was once believed to primarily localize cellular proteolytic activity (16, 36, 37). However, recent studies have shown that uPAR clustering at the cell surface occurs at the leading edge of migrating cells and influences cellular signaling (36, 38). Our immunofluorescence results are supported by parallel studies showing that uPA-mediated uPAR internalization decreased by 80% (from 25% to < 5%; P < 0.05) in the presence of RAP. Down-regulation of LRP-1 with siRNA reduced uPA-dependent uPAR internalization by approximately 50% but did not reach the inhibition seen with RAP. This effect may have been due to the relatively incomplete down-regulation of LRP-1 by siRNA (70%). Further, RAP treatment extended the t1/2 of uPAR in PMCs from 2.5 to 6 hours in the presence of uPA. The findings indicate that complexes of uPAR with exogenous uPA and PAI-1 expressed by the PMC are internalized via interaction with LRP-1. We attempted to stably express the complete LRP-1 construct in the deficient REN cells (data not shown) to test how gain of LRP functionality affects uPAR processing; however, this approach was problematic given the size of the cDNA (> 15 kb) and our inability to confirm cell surface localization of the construct.
Flow cytometry studies indicated that LRP-1 expression correlated with uPAR surface expression in PMCs. Previous studies have shown that LRP-1 function directly affects expression of uPA, PAI-1, and uPAR (39). In this study, LRP-1–expressing MeT5A PMCs and LRP-1–deficient REN were used in internalization assays. uPA enhances uPAR internalization in MeT5A but not in REN cells. Because these cells express PAI-1, we postulate that endogenously produced PAI-1 forms an inhibitory complex with exogenously added uPA. This newly formed complex binds uPAR and drives internalization of uPAR through interaction with LRP-1 in MeT5A cells. To determine the role of uPA fibrinolytic activity in uPAR catabolism and internalization, preformed uPA/PAI-1 complexes were also used in t1/2 and internalization assays. uPA activity was not required to reduce uPAR t1/2 or to drive uPAR internalization in MeT5A cells (data not shown). However, uPA activity was required to cleave uPAR in the REN line (data not shown). In REN internalization assays, a small but quantifiable amount of uPAR was detected in the glutathione treatment control. This is most likely due to the inability to efficiently remove all biotin from the REN cell surface as a consequence of robust uPAR expression. It is conceivable that basal internalization of biotinylated surface uPAR could contribute to this result.
Because uPAR internalization was found to depend on the expression and function of LRP-1, uPAR half-life studies were performed in LRP-1–expressing and LRP-1–deficient cells. In surface biotinylation assays, we found that uPA treatment reduced uPAR t1/2 in MeT5A, MS-1, and M9K cells by almost 50%, which is consistent with previously published observations in other cell types (11). The basal uPAR t1/2, in the absence of supplemental uPA, in MS-1 and M9K was found to be around 4.5 hours (data not shown), similar to that found in the REN and MeT5A cells (5 h). Only REN cells exhibited the cleaved form of uPAR (D2D3) in the presence of uPA throughout the 24-hour time course. The lack of LRP-1 in REN cells may explain the increment of D2D3-uPAR in the presence of uPA. The D2D3 domain of uPAR is believed to play a role in enhanced cell migration and mesenchymal transition (40–42). The increased uPAR t1/2 and the abundance of D2D3-uPAR found in the REN cells may contribute to their greater aggressiveness and invasiveness in vivo, as we previously reported (18). This aspect of uPAR processing is being evaluated in ongoing independent analyses.
Although LRP-1 neutralization blocked uPAR internalization and extended uPAR t1/2 in PMCs, uPAR was steadily catabolized throughout the time course in the presence or absence of uPA. Further, LRP-1 neutralization did not foster accumulation of cleaved D2D3-uPAR in MeT5A cells, as found in REN cells. These data suggest that LRP-1 mediates uPA-dependent uPAR down-regulation and internalization but that selective cleavage of uPAR by uPA in REN cells may involve alternative form of receptor–protease interaction.
Due to the stabilized expression of uPAR in LRP-deficient REN cells, we hypothesized that RAP treatment would extend the activity of exogenous uPA over a 12-hour time course. MeT5A and REN cells were selected for these analyses because they do not express levels of endogenous uPA that could confound the findings using fibrin enzymography (18). Because uPA has been previously reported to bind cells through receptors other than uPAR (43, 44), we first confirmed that uPA-associated with the PMC surface through interactions with uPAR. Because uPA activity was not detected in ATN-617–treated MeT5A cells, we infer that most, but likely not all, of the binding of uPA was to uPAR at the cell surface. In uPA-treated REN cells, the durability of uPA activity is most likely attributable to the lack of LRP-1 and overexpression of uPAR. This conclusion is buttressed by the ability of RAP to sustain uPA activity associated with MeT5A cells, most likely through the stabilization of uPAR at the cell surface. Comparable responses were observed in HPMCs. These data show that LRP-1 regulates cell-associated uPA activity in MeT5A cells and in primary HPMCs.
Previous studies have shown that uPA can also potentiate migration in a uPA/uPAR-dependent manner (23, 45). Our studies demonstrate that the addition of uPA as a chemo-attractant potentiates PMC migration across a vitronectin-coated filter insert. Further, LRP-1 neutralization with RAP potentiates the promigratory effect of uPA. Although the FBS used in these assays may contain bovine uPA, the species-specific nature of the uPA and uPAR interaction makes a confounding effect unlikely (46). These studies show that neutralization of LRP-1 potentiates uPA-mediated migration in MeT5A cells and HPMCs.
We also report the novel observation that uPA and LRP-1 can regulate PMC collagen 1 expression. Because the MeT5A line does not produce collagen 1 in response to TGF-β1, primary PMCs were used in these analyses. TGF-β has been reported to stimulate collagen 1 expression in murine and rat PMCs in vitro and in vivo (24, 47–49). We confirmed that TGF-β induced collagen 1 in RPMCs and HPMCs. We also found that uPA stimulates collagen 1 expression in both these primary cell types. Further, the combination of uPA and TGF-β enhanced collagen 1 expression. Although RAP did not enhance the TGF-β effect, the combination of RAP with uPA increased collagen 1 expression. Hydroxyproline analysis confirmed that total collagen expression was also increased by uPA in RPMCs and HPMCs and that these effects were potentiated by RAP. The data show that LRP-1 can influence uPA-mediated collagen 1 expression.
Because previous studies have shown that plasmin can convert latent TGF-β to its active form (50, 51), we assayed the potential of uPA or RAP to activate latent TGF-β. uPA did not increase the levels of active TGF-β in HPMCs, indicating that uPA-mediated induction of collagen by HPMCs under the conditions we used may involve alternative intermediaries. RAP alone or with uPA did not change active TGF-β levels in HPMCs, although there was a trend toward increased levels. However, total levels of TGF-β were increased by these conditions as well as by uPA alone. We therefore posit that blockade of LRP-1 by RAP may hinder the internalization and degradation of TGF-β in PMCs and facilitate its accumulation, as reported previously in other cell systems (52). On the other hand, our data clearly show that RAP alone did not induce collagen in HPMCs, strongly suggesting that levels of induction of activated TGF-β were likely insufficient to recapitulate the effects of the exogenous TGF-β we used. Alternatively, it is possible that the processing of activated TGF-β may be accelerated in the presence of RAP, consistent with its induction of total TGF-β in HPMCs. Although the data do not exclude a potential contribution of TGF-β in uPA-mediated induction of HPMC collagen, its processing by PMCs stimulated by uPA with or without RAP may be complex. Full elucidation of the mechanism by which uPA induces collagen expression by HPMCs requires comprehensive studies that extend this work. Although the precise mechanism is unknown, we posit that uPA may cleave uPAR and thereby initiate mesenchymal transition, as reported by other groups (42) and will pursue this possibility in an extension of this work.
In summary, the ability of inflammatory cytokines to alter expression of LRP-1 in HPMCs is a novel observation, as is the induction of collagen 1 in primary HPMCs by uPA and potentiation of the effect by RAP. Our results show that TNF-α and IL-1β down-regulate LRP-1 expression at the mRNA and protein levels. Exposure of PMCs to these agents or uPA can thereby influence a broad functional repertoire subject to regulation via LRP-1, including cellular proteolysis, migration, and collagen expression.
Supplementary Material
Footnotes
Supported by the NIH Postdoctoral Diversity Supplement (T.T.) and by NIH grant PO-1 HL076406 (S.I., K.K., A.K., G.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2011-0071OC on September 1, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Herz J, Strickland DK. Lrp: a multifunctional scavenger and signaling receptor. J Clin Invest 2001;108:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kounnas MZ, Henkin J, Argraves WS, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates cellular uptake of pro-urokinase. J Biol Chem 1993;268:21862–21867 [PubMed] [Google Scholar]
- 3.Conese M, Blasi F. Urokinase/urokinase receptor system: internalization/degradation of urokinase-serpin complexes: mechanism and regulation. Biol Chem Hoppe Seyler 1995;376:143–155 [PubMed] [Google Scholar]
- 4.Cortese K, Sahores M, Madsen CD, Tacchetti C, Blasi F. Clathrin and lrp-1-independent constitutive endocytosis and recycling of upar. PLoS ONE 2008;3:e3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi AA. Binding of urokinase to low density lipoprotein-related receptor (lrp) regulates vascular smooth muscle cell contraction. J Biol Chem 2002;277:40499–40504 [DOI] [PubMed] [Google Scholar]
- 6.Teesalu T, Blasi F, Talarico D. Embryo implantation in mouse: fetomaternal coordination in the pattern of expression of upa, upar, pai-1 and alpha 2mr/lrp genes. Mech Dev 1996;56:103–116 [DOI] [PubMed] [Google Scholar]
- 7.Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. Alpha-2 macroglobulin receptor/ldl receptor-related protein(lrp)-dependent internalization of the urokinase receptor. J Cell Biol 1995;131:1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nykjaer A, Kjoller L, Cohen RL, Lawrence DA, Garni-Wagner BA, Todd RF, III, van Zonneveld AJ, Gliemann J, Andreasen PA. Regions involved in binding of urokinase-type-1 inhibitor complex and pro-urokinase to the endocytic alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein: evidence that the urokinase receptor protects pro-urokinase against binding to the endocytic receptor. J Biol Chem 1994;269:25668–25676 [PubMed] [Google Scholar]
- 9.Weaver AM, Hussaini IM, Mazar A, Henkin J, Gonias SL. Embryonic fibroblasts that are genetically deficient in low density lipoprotein receptor-related protein demonstrate increased activity of the urokinase receptor system and accelerated migration on vitronectin. J Biol Chem 1997;272:14372–14379 [DOI] [PubMed] [Google Scholar]
- 10.Blasi F. The urokinase receptor and cell migration. Semin Thromb Hemost 1996;22:513–516 [DOI] [PubMed] [Google Scholar]
- 11.Webb DJ, Nguyen DH, Sankovic M, Gonias SL. The very low density lipoprotein receptor regulates urokinase receptor catabolism and breast cancer cell motility in vitro. J Biol Chem 1999;274:7412–7420 [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Strickland DK, Cines DB, Higazi AA. Regulation of single chain urokinase binding, internalization, and degradation by a plasminogen activator inhibitor 1-derived peptide. J Biol Chem 1997;272:27053–27057 [DOI] [PubMed] [Google Scholar]
- 13.Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (upar) to ldl receptor-related protein is required for endocytosis of upar and regulation of cell surface urokinase activity. Mol Biol Cell 2001;12:1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the upa:Serpin complexes. EMBO J 1997;16:2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty S, Idell S. Posttranscriptional regulation of urokinase receptor gene expression in human lung carcinoma and mesothelioma cells in vitro. Mol Cell Biochem 1999;199:189–200 [DOI] [PubMed] [Google Scholar]
- 16.Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G, Idell S. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol 1996;15:78–87 [DOI] [PubMed] [Google Scholar]
- 17.Shetty S, Velusamy T, Shetty RS, Marudamuthu AS, Shetty SK, Florova G, Tucker T, Koenig K, Shetty P, Bhandary YP, et al. Posttranscriptional regulation of plasminogen activator inhibitor-1 expression in human pleural mesothelial cells. Am J Respir Cell Mol Biol 2010;43:358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker TA, Dean C, Komissarov A, Koenig K, Mazar A, Allen T, Pendurthi U, Idell S. The urokinase receptor supports tumorigenesis of human malignant pleural mesothelioma cells. Am J Respir Cell Mol Biol 2010;42:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antony VB, Owen CL, Hadley KJ. Pleural mesothelial cells stimulated by asbestos release chemotactic activity for neutrophils in vitro. Am Rev Respir Dis 1989;139:199–206 [DOI] [PubMed] [Google Scholar]
- 20.Bajaj MS, Pendurthi U, Koenig K, Pueblitz S, Idell S. Tissue factor pathway inhibitor expression by human pleural mesothelial and mesothelioma cells. Eur Respir J 2000;15:1069–1078 [DOI] [PubMed] [Google Scholar]
- 21.Idell S, Allen T, Chen S, Koenig K, Mazar A, Azghani A. Intrapleural activation, processing, efficacy, and duration of protection of single-chain urokinase in evolving tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 2007;292:L25–L32 [DOI] [PubMed] [Google Scholar]
- 22.Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci 2008;121:3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in mcf-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem 1998;273:8502–8507 [DOI] [PubMed] [Google Scholar]
- 24.Owens MW, Grimes SR. Pleural mesothelial cell response to inflammation: tumor necrosis factor-induced mitogenesis and collagen synthesis. Am J Physiol 1993;265:L382–L388 [DOI] [PubMed] [Google Scholar]
- 25.Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erectile dysfunction in the type ii diabetic db/db mouse: impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol 2008;294:H2204–H2211 [DOI] [PubMed] [Google Scholar]
- 26.Shetty S, Kumar A, Johnson A, Pueblitz S, Idell S. Urokinase receptor in human malignant mesothelioma cells: role in tumor cell mitogenesis and proteolysis. Am J Physiol 1995;268:L972–L982 [DOI] [PubMed] [Google Scholar]
- 27.Schwab W, Schulze-Tanzil G, Mobasheri A, Dressler J, Kotzsch M, Shakibaei M. Interleukin-1beta-induced expression of the urokinase-type plasminogen activator receptor and its co-localization with mmps in human articular chondrocytes. Histol Histopathol 2004;19:105–112 [DOI] [PubMed] [Google Scholar]
- 28.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in schwann cells: possible implications in peripheral nerve injury. J Neurosci 2006;26:11197–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi T, Noguchi M, Masubuchi H, Seki T, Ariga T. Il-1beta down-regulates tissue-type plasminogen activator by up-regulating low-density lipoprotein receptor-related protein in aml 12 cells. Biochem Biophys Res Commun 2001;288:42–48 [DOI] [PubMed] [Google Scholar]
- 30.Goedde MF, Grimbergen JM, Toet KH, Sitter T, Quax PH, Kooistra T. Adenovirus-mediated transfer of the 39 kd receptor-associated protein increases fibrinolytic capacity. Kidney Int 2001;60:117–125 [DOI] [PubMed] [Google Scholar]
- 31.Dedieu S, Langlois B, Devy J, Sid B, Henriet P, Sartelet H, Bellon G, Emonard H, Martiny L. Lrp-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol Cell Biol 2008;28:2980–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol 2002;159:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Jones CJ, Dang J, Liang X, Olsen JE, Doe WF. Human urokinase receptor expression is inhibited by amiloride and induced by tumor necrosis factor and phorbol ester in colon cancer cells. FEBS Lett 1994;353:138–142 [DOI] [PubMed] [Google Scholar]
- 34.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:602–611 [DOI] [PubMed] [Google Scholar]
- 35.Sitter T, Toet K, Quax P, Kooistra T. Fibrinolytic activity of human mesothelial cells is counteracted by rapid uptake of tissue-type plasminogen activator. Kidney Int 1999;55:120–129 [DOI] [PubMed] [Google Scholar]
- 36.Mohanam S, Chandrasekar N, Yanamandra N, Khawar S, Mirza F, Dinh DH, Olivero WC, Rao JS. Modulation of invasive properties of human glioblastoma cells stably expressing amino-terminal fragment of urokinase-type plasminogen activator. Oncogene 2002;21:7824–7830 [DOI] [PubMed] [Google Scholar]
- 37.Schnaper HW, Barnathan ES, Mazar A, Maheshwari S, Ellis S, Cortez SL, Baricos WH, Kleinman HK. Plasminogen activators augment endothelial cell organization in vitro by two distinct pathways. J Cell Physiol 1995;165:107–118 [DOI] [PubMed] [Google Scholar]
- 38.Sitrin RG, Pan PM, Harper HA, Blackwood RA, Todd RF., III Urokinase receptor (cd87) aggregation triggers phosphoinositide hydrolysis and intracellular calcium mobilization in mononuclear phagocytes. J Immunol 1999;163:6193–6200 [PubMed] [Google Scholar]
- 39.Li Y, Knisely JM, Lu W, McCormick LM, Wang J, Henkin J, Schwartz AL, Bu G. Low density lipoprotein (ldl) receptor-related protein 1b impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J Biol Chem 2002;277:42366–42371 [DOI] [PubMed] [Google Scholar]
- 40.Montuori N, Carriero MV, Salzano S, Rossi G, Ragno P. The cleavage of the urokinase receptor regulates its multiple functions. J Biol Chem 2002;277:46932–46939 [DOI] [PubMed] [Google Scholar]
- 41.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the g protein-coupled chemotactic receptor fprl1/lxa4r. Proc Natl Acad Sci USA 2002;99:1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell 2007;18:2716–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, et al. Direct interaction of the kringle domain of urokinase-type plasminogen activator (upa) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost 2006;95:524–534 [DOI] [PubMed] [Google Scholar]
- 44.Kanse SM, Benzakour O, Kanthou C, Kost C, Lijnen HR, Preissner KT. Induction of vascular smc proliferation by urokinase indicates a novel mechanism of action in vasoproliferative disorders. Arterioscler Thromb Vasc Biol 1997;17:2848–2854 [DOI] [PubMed] [Google Scholar]
- 45.Nieves EC, Manchanda N. A cleavage-resistant urokinase plasminogen activator receptor exhibits dysregulated cell-surface clearance. J Biol Chem 2010;285:12595–12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quax PH, Grimbergen JM, Lansink M, Bakker AH, Blatter MC, Belin D, van Hinsbergh VW, Verheijen JH. Binding of human urokinase-type plasminogen activator to its receptor: residues involved in species specificity and binding. Arterioscler Thromb Vasc Biol 1998;18:693–701 [DOI] [PubMed] [Google Scholar]
- 47.Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. Tgf-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 2007;179:6043–6051 [DOI] [PubMed] [Google Scholar]
- 48.Liu Q, Mao H, Nie J, Chen W, Yang Q, Dong X, Yu X. Transforming growth factor {beta}1 induces epithelial-mesenchymal transition by activating the jnk-smad3 pathway in rat peritoneal mesothelial cells. Perit Dial Int 2008;28:S88–S95 [PubMed] [Google Scholar]
- 49.Nasreen N, Mohammed KA, Mubarak KK, Baz MA, Akindipe OA, Fernandez-Bussy S, Antony VB. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to tgf-beta1 in vitro. Am J Physiol Lung Cell Mol Physiol 2009;297:L115–L124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalil N, Corne S, Whitman C, Yacyshyn H. Plasmin regulates the activation of cell-associated latent tgf-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 1996;15:252–259 [DOI] [PubMed] [Google Scholar]
- 51.Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ. Plasminogen-dependent activation of latent transforming growth factor beta (tgf beta) by growing cultures of osteoblast-like cells. J Cell Physiol 1993;157:528–534 [DOI] [PubMed] [Google Scholar]
- 52.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RG, Herz J. Lrp1 functions as an atheroprotective integrator of tgfbeta and pdfg signals in the vascular wall: implications for marfan syndrome. PLoS ONE 2007;2:e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.