Abstract
The males of some species of moths possess elaborate feathery antennae. It is widely assumed that these striking morphological features have evolved through selection for males with greater sensitivity to the female sex pheromone, which is typically released in minute quantities. Accordingly, females of species in which males have elaborate (i.e., pectinate, bipectinate, or quadripectinate) antennae should produce the smallest quantities of pheromone. Alternatively, antennal morphology may be associated with the chemical properties of the pheromone components, with elaborate antennae being associated with pheromones that diffuse more quickly (i.e., have lower molecular weights). Finally, antennal morphology may reflect population structure, with low population abundance selecting for higher sensitivity and hence more elaborate antennae. We conducted a phylogenetic comparative analysis to test these explanations using pheromone chemical data and trapping data for 152 moth species. Elaborate antennae are associated with larger body size (longer forewing length), which suggests a biological cost that smaller moth species cannot bear. Body size is also positively correlated with pheromone titre and negatively correlated with population abundance (estimated by male abundance). Removing the effects of body size revealed no association between the shape of antennae and either pheromone titre, male abundance, or mean molecular weight of the pheromone components. However, among species with elaborate antennae, longer antennae were typically associated with lower male abundances and pheromone compounds with lower molecular weight, suggesting that male distribution and a more rapidly diffusing female sex pheromone may influence the size but not the general shape of male antennae.
Keywords: Antennal morphology, forewing length, Lepidoptera, phylogenetic generalized least squares, sex pheromone
Introduction
Moths are popularly characterized by two remarkable traits associated with chemical communication in a sexual context. First is the apparent ability of males to detect and respond to female sex pheromones over impressively long distances, including one anecdotal report of 11 km in an emperor moth, Pavonia pavonia (Regnier and Law 1968), even though females typically produce very small quantities of sex pheromone in the order of nanograms or even picograms (Greenfield 1981). Second, males of many species have beautiful and conspicuous feathery (i.e., “bipectinate” or “quadripectinate” comb-like—e.g., Fig. 1a) antennae (hereafter referred to as elaborate antennae), of which the most impressive examples are the Luna (Actias luna) and Hercules (Coscinocera hercules) moths.
Figure 1.

Antennal types: (a) elaborate bipectinate antennae of a male Hemileuca eglanterina, (b) simple filiform antennae of a male Cydia pomonella. Photos reproduced by kind permission: (a) Nicky Davis, http://www.wildutah.us, (b) Len Willan, CSIRO Entomology, http://www.csiro.au/resources/Australian-Moths.html
These impressive biological receptor organs are usually found on males only, encouraging the view that elaborate antennae increase olfactory sensitivity to detect miniscule amounts of female pheromone in the atmosphere (Greenfield 1981; Birch and Haynes 1982; Cardé and Baker 1984; Rutowski 1984; Phelan 1992; Steinbrecht 1996; Svensson 1996). In insects, larger antennae are typically associated with a greater number of olfactory receptors (Chapman 1982) and a corresponding higher sensitivity to chemical signals (Birch and Haynes 1982; Chapman 1982; Spaethe et al. 2007). Such enhanced sensitivity to small amounts of sex pheromone detected over a long distance would be especially advantageous to males when there is strong competition for access to females, or if females are indirectly sexually selecting their mates by producing minute quantities in order to attract high-quality “sensitive” males (Lloyd 1979; Greenfield 1981). Additionally, elaborate antennae slow down and trap air-flow over the sensilla (e.g., Loudon and Koehl 2000), thereby potentially increasing the ability to detect scarce pheromone components in the atmosphere. Nevertheless, while many species of moths use long-distance sex pheromones (Greenfield 1981; Tamaki 1985; Cardé and Haynes 2004; El–Sayed 2011), relatively few moth species have elaborate antennae (Mankin and Mayer 1984), and most have simple filiform antennae (e.g., Fig. 1b) suggesting that the link between these characteristics is not necessarily straightforward. Accordingly, we test whether the long-held assumption that the evolution of elaborate antennae is linked to pheromone dynamics is supported by evidence from comparative data.
Elaborate antennae may provide an increased sensitivity to the pheromone by increasing the “active space” (Elkinton and Cardé 1984) of the signal: the area in which the concentration of the pheromone is above a threshold of detection and thus causes a behavioral response. Initial estimations of the size of this area were based on Bossert and Wilson's (1963) models of steady pheromone diffusion over time (see also Wyatt 2003, pp. 210–212). More recent research reveals that males detect pheromones by tracking wind-borne odor plumes that vary in concentration in space (Vickers 2006; Cardé and Willis 2008). Further, the turbulent nature of the environment (wind speed, temperature, local habitat structure, etc.) plays a critical role in shaping the response to pheromones (Elkinton and Cardé 1984; Byers 2008). While these environmental factors are vital to the immediate response of individual moths to pheromones, they cannot provide meaningful data from the perspective of the species, simply because there is no such thing as the average wind-speed for a species, for example. Nevertheless, the amount produced and composition of the sex pheromone, as well as population density, also determine the active space for detection, and are amenable to examination in a cross-species context. Thus, it is possible to derive means of testing whether elaborate antennae in male moths have evolved in response to the need for greater sensitivity to female sex pheromone.
We assessed the association between antennal morphology and aspects of their sexual chemical communication system using comparative data from 152 moth species. We started with two assumptions: (1) that the female sex pheromone signaling system will have evolved to attract at least one (but possibly more than one) suitable mate, driven by various processes of sexual selection; and (2) that the larger the active space of the pheromone plume then the greater the likelihood that there will be a male present that detects the pheromone. From these assumptions, we made the general prediction that elaborate antennae will have evolved to compensate against a reduction in the active space caused by some other property of the signaling system.
The first possibility is that the active space becomes smaller because females produce smaller quantities of pheromone. Thus, we predicted that females of species in which males have elaborate antennae should produce, on average, smaller amounts of pheromone than females of species in which males have more simple filiform antennae.
Second, we predict that active space becomes smaller if the pheromone components diffuse more quickly. Diffusion rates relate to both the generic type of compounds used (alkanes, alcohols, aldehydes, etc.), and to the molecular weight of the components of the pheromone blend (Butler and McDonough 1981; Ryan 1992). Moths typically use moderately volatile compounds, although there is variation in their diffusion rates, with molecular weights in the range of 200–300 (Wyatt 2003). We predict that elaborate male antennae will be more likely in species in which females use lower molecular weight compounds, which produce more rapidly dissipating signals.
The final prediction relates to population abundance. We predict that species with elaborate male antennae are more likely to be those characterized by low population abundance (hence a male is less likely to be within the active space of the female sex pheromone). In an analysis comparing antennal structure in two species of mantids (Holwell et al. 2007), the differences were explained using a similar argument that males require greater sensitivity to the volatile, long-distance mate attraction signals if the population exists at lower abundance. Here, we perform a wider comparative test of that prediction.
We also considered two other factors that may be important: body size and phylogeny. Body size is closely correlated with the length of antennal structures for many insects (e.g., Strauss 1990; Wcislo 1995; Kawano 2006), and so larger bodied species should similarly have larger (and perhaps more elaborate) antennae. This might particularly be so if there are aerodynamic costs associated with have larger, more unwieldy antennae at small body size (Ellington 1991). The relationship between pheromone titre and body size has only occasionally been investigated at the individual level, and then with varying results (e.g., Delisle and Vincent 2002; Ruther et al. 2009; Harari et al. 2011). Body size has well-documented negative relationships with population abundance (e.g., Currie 1993; White et al. 2007): smaller bodied species tend to have lower energetic demands and thus more individuals in a population can be sustained per unit area (Damuth 1981; Cotgreave 1993). Thus, in testing our principal hypotheses, we controlled for the possible confounding effect of body size.
The distribution of elaborate antennae among species is likely to be phylogenetically clumped. By mapping this trait onto a putative phylogeny, we estimated the number of evolutionary origins of this trait. More pertinently, closely related species may not necessarily provide independent data points in an analysis because they may share characteristics through common descent (Harvey and Pagel 1991). Therefore, we also control for phylogeny in our subsequent analyses.
Methods
Data collation
Information on antennal morphology of males was derived from published literature and field guides, as well as on-line lepidopterist resources. We categorized moth antennae unambiguously into two categories—simple and elaborate antennae. The former category covers moths whose antennae principally consist of a single shaft, which may be described as filiform, beadlike, ciliate, serrate, or dentate (e.g., Fig. 1b). Antennae were deemed elaborate if the antennal shaft branched off into side branches giving them a comb- or feather-like appearance, and may be described as pectinate, bipectinate, or quadripectinate (e.g., Fig. 1a). To provide a measure of body size, we collated information on the mean length of the forewing for males. This particular measure is the most commonly reported size parameter for Lepidoptera and regularly used in comparative analyses of the order (e.g., Gage 1994; Lindström et al. 1994; Summerville et al. 2006; Hambäck et al. 2007), and has been shown to act as a good proxy for body mass (R2≍ 80%; Miller 1977, 1997). Where the source gave a range for the length of the forewing (e.g., 14–20 mm), the midpoint of the range was taken as the value. We also collated data on antennal length, either directly from the literature, or by using length of forewing as a guide, and estimating length from pictures of male moths from the sources. Ideally, other measures of antennal area would also be employed (antennal width, overall surface area), but are not generally recorded in the literature and are difficult to estimate from pictures, therefore antennal length alone serves as our proxy for antennal size. However, in the analysis of antennal length, the moths were split into their two antennal morphology groups (simple or elaborate) and analyzed separately.
Information on pheromone titre was initially gathered from the Pherobase (El–Sayed 2011), cross-checking all information with the primary sources specified therein. Pheromone release rates have been identified for very few species, so pheromone titre, which is typically reported in most papers that analyze the pheromone composition, was used. We did, though, find a good association (R2 = 54%, P = 0.01) between pheromone release rate and pheromone titre in 10 species for which we had information on both measures (M. Symonds, unpubl. data). Pheromone titre is here described as the total quantity in nanograms of pheromone components that are functionally active (i.e., cause male attraction) present in the female gland. This value is averaged across the females chemically sampled, generating a measure of nanogram per female. In addition to the total quantity of components, we also carried out the analysis using amount of the major and most minor components as our measure of pheromone titre, but it had no effect on our conclusions (data not shown).
Data on the molecular weights of the functionally active compounds in the pheromone blend was also taken from the Pherobase. We calculated a mean molecular weight of these components in cases where more than one compound was identified as active. Analyses using maximum and minimum molecular weights produced qualitatively the same results and are not shown.
Estimating population densities or abundance is challenging, especially from pheromone trap data because many factors (climatic conditions, seasonality, number of traps, trap spacing, concentration and quality of pheromone, host plants, use of insecticides, etc. see McNeil 1991 for a review) can affect male responses. Light-trapping data are similarly problematic because they provide poor estimates at low populations densities and light-traps do not necessarily attract moths at the time (seasonally or daily) at which females are calling (Delisle et al. 1998). In addition, fewer data on population density from light trapping exist for the species in our analysis. Accordingly, we use the pheromone trapping information taken primarily from the same sources that described pheromone composition and titre—this having the added advantage of abundance estimates being directly taken from the same populations as our pheromone data. We noted the highest recorded trapping intake observed for that population of moths (standardized across papers as the number of males caught per trap per night). We considered this a better measure of male abundance than mean values from these papers since not all of the trapping experiments reported in a study use optimal pheromone compositions. Also male abundance can vary greatly even during the course of a season, making mean abundance a less reliable measure. Maximum trapping intake should therefore more closely reflect the actual number of males within the “active space” of the pheromone at the time when females are most likely to be calling. In order to account for differences in trapping protocol across papers, we also calculated a second measure of abundance that took into account the number and distance apart of the traps: specifically we calculated the residuals from the model predicting the log number of individuals trapped per trap night using the predictors of log number of traps and log minimum distance between traps. In practice, the absolute and relative measures of abundance gave qualitatively the same results, so we report results using the absolute measure only. The dataset is presented in Table 1.
Table 1.
The dataset used in the analyses. ANT, antenna type; AL, antenna length (mm); FL, forewing length (mm); Q, pheromone titre (ng); MW, mean molecular weight of pheromone components; kmales, abundance of males (maximum number of males caught per trap per night). Pheromone data were taken from El–Sayed (2011) and references therein, as were male abundance (trapping) data, except where indicated in the references (*). References for additional natural history data on antennal morphology and forewing length are also given
| Family | Species | ANT | AL | FL | Q | MW | kmales | Refs. |
|---|---|---|---|---|---|---|---|---|
| Acrolepiidae | Acrolepiosis assectella | Simple | 3.54 | 5.90 | 1.00 | 238.41 | 10.70 | 6, 93* |
| Arctiidae | Holomelina lamae | Simple | 2.61 | 9.00 | 7498.0 | 259.18 | 58 | |
| Arctiidae | Panaxia quadripunctaria | Simple | 10.34 | 23.50 | 10100 | 285.54 | 9 | |
| Arctiidae | Pyrrharctia isabella | Simple | 7.15 | 27.50 | 254.50 | 80 | ||
| Arctiidae | Utetheisa ornatrix | Simple | 9.02 | 22.00 | 32.00 | 290.53 | 4.70 | 80 |
| Argyresthiidae | Argyresthia conjugella | Simple | 2.34 | 6.00 | 1.10 | 282.47 | 10.10 | 9 |
| Bombycidae | Bombyx mori | Elaborate | 6.80 | 20.00 | 210.90 | 237.41 | 21 | |
| Carposinidae | Carposina sasakii | Simple | 4.93 | 8.50 | 1.67 | 287.51 | 3.75 | 26 |
| Carposinidae | Coscinoptycha improbana | Simple | 3.75 | 7.50 | 69.80 | 301.54 | 0.45 | 36 |
| Cossidae | Cossus cossus | Elaborate | 17.21 | 46.50 | 151.50 | 240.39 | 2.43 | 8 |
| Cossidae | Holcocerus insularis | Simple | 9.69 | 19.00 | 14.40 | 226.38 | 18 | |
| Crambidae | Deanolis sublimbalis | Simple | 6.80 | 10.00 | 0.48 | 278.50 | 4.24 | 40 |
| Crambidae | Eoreuma loftini | Simple | 6.10 | 10.00 | 56.30 | 286.49 | 6.10 | 5 |
| Crambidae | Glyphodes perspectalis | Simple | 13.80 | 20.00 | 86.00 | 238.41 | 0.80 | 18, 52 |
| Crambidae | Glyphodes pyloalis | Simple | 5.40 | 10.00 | 2.00 | 278.43 | 24.64 | 5 |
| Cramgbidae | Ostrinia furnacalis | Simple | 8.53 | 13.75 | 12.40 | 254.41 | 6.07 | 23, 50 |
| Crambidae | Ostrinia latipennis | Simple | 7.53 | 14.20 | 3.80 | 212.37 | 1.70 | 18, 72 |
| Crambidae | Ostrinia palustralis | Simple | 7.88 | 17.50 | 37.50 | 254.41 | 0.96 | 35 |
| Crambidae | Ostrinia zaguliaevi | Simple | 8.24 | 13.50 | 22.80 | 254.41 | 18 | |
| Eriocranidae | Eriocrania cicatricella | Simple | 2.21 | 4.90 | 159.00 | 115.20 | 29.60 | 47 |
| Gelechiidae | Anarsia lineatella | Simple | 4.25 | 6.25 | 283.40 | 177.29 | 8 | |
| Gelechiidae | Keiferia lycopersicella | Simple | 2.57 | 5.25 | 10.10 | 240.39 | 29.40 | 5, 49 |
| Gelechiidae | Pectinophora gossypiella | Simple | 6.13 | 8.75 | 10.00 | 280.45 | 76 | |
| Gelechiidae | Phthorimaea operculella | Simple | 4.81 | 7.75 | 12.50 | 237.36 | 61.00 | 24, 49 |
| Gelechiidae | Scrobipalpa ocellatella | Simple | 4.02 | 5.75 | 70.00 | 226.36 | 8 | |
| Gelechiidae | Tuta absoluta | Simple | 2.88 | 4.50 | 5.44 | 251.39 | 810.00 | 91, 102* |
| Geometridae | Abraxas grossulariata | Simple | 7.49 | 20.25 | 0.55 | 242.41 | 2.82 | 1 |
| Geometridae | Alsophila pometaria | Simple | 6.45 | 15.00 | 46.20 | 261.13 | 10.40 | 10, 11 |
| Geometridae | Ascotis selenaria | Elaborate | 7.96 | 21.50 | 42.00 | 278.48 | 18, 19 | |
| Geometridae | Ascotis selenaria cretacea | Elaborate | 21.50 | 35.00 | 270.48 | 16.00 | 18, 19 | |
| Geometridae | Erannis defoliaria | Elaborate | 7.30 | 18.25 | 2.00 | 270.48 | 0.15 | 42, 46 |
| Geometridae | Eupithecia assimilata | Simple | 8.93 | 19.00 | 23.00 | 306.53 | 0.17 | 1 |
| Geometridae | Idaea aversata | Simple | 7.41 | 14.25 | 238.37 | 0.07 | 1 | |
| Geometridae | Lambdina athasaria | Elaborate | 4.05 | 13.50 | 0.06 | 261.52 | 10.00 | 5 |
| Geometridae | Milionia basalis pryeri | Simple | 15.82 | 28.25 | 7.00 | 278.48 | 29.00 | 67 |
| Geometridae | Mnesampela privata | Simple | 11.80 | 20.00 | 110.00 | 262.48 | 1.32 | 24, 68 |
| Geometridae | Operophtera bruceata | Simple | 6.09 | 14.50 | 1.00 | 260.46 | 28.78 | 69 |
| Geometridae | Operophtera brumata | Simple | 4.73 | 11.25 | 1.00 | 260.46 | 11.84 | 63 |
| Geometridae | Peribatodes rhomboidaria | Elaborate | 9.20 | 20.00 | 1.25 | 270.48 | 1.63 | 42 |
| Geometridae | Sabulodes caberata | Simple | 12.00 | 25.00 | 37.50 | 264.49 | 2.06 | 5, 21 |
| Geometridae | Tephrina arenacearia | Elaborate | 6.38 | 12.50 | 4.00 | 250.40 | 87 | |
| Gracilariidae | Caloptilia porphyretica | Simple | 7.50 | 6.00 | 238.41 | 37.10 | 8, 25 | |
| Gracilariidae | Conopomorpha cramerella | Simple | 6.24 | 6.00 | 0.10 | 254.42 | 12.93 | 32, 33 |
| Gracillariidae | Phyllonorycter mespilella | Simple | 2.80 | 3.50 | 1.00 | 224.34 | 190.00 | 77, 78 |
| Gracillariidae | Phyllonorycter ulmifoliella | Simple | 2.80 | 4.00 | 1.00 | 254.41 | 39.30 | 9, 79 |
| Incurvariidae | Lampronia capitella | Simple | 4.03 | 7.75 | 15.00 | 223.70 | 12.73 | 9, 62 |
| Lasiocampidae | Malacosoma neustrium | Elaborate | 6.67 | 14.50 | 143.10 | 181.30 | 1.21 | 8, 18 |
| Limacodiidae | Parasa lepida | Elaborate | 10.40 | 20.00 | 20.00 | 154.25 | 2.00 | 75 |
| Lymantriidae | Artaxa subflava | Elaborate | 4.21 | 14.50 | 10.00 | 326.56 | 0.63 | 18 |
| Lymantriidae | Euproctis pseudoconspersa | Elaborate | 2.64 | 12.00 | 10.00 | 326.56 | 19.60 | 50 |
| Lymantriidae | Euproctis pulverea | Elaborate | 4.11 | 13.25 | 270.00 | 376.61 | 0.21 | 51 |
| Lymantriidae | Orgyia leucostigma | Elaborate | 5.85 | 15.00 | 5.00 | 306.53 | 1.03 | 13, 70 |
| Lymantriidae | Orgyia postica | Elaborate | 4.46 | 13.50 | 30.70 | 306.53 | 2.40 | 71 |
| Lymantriidae | Perina nuda | Elaborate | 4.55 | 17.50 | 263.00 | 317.20 | 7.60 | 18, 71 |
| Lymantriidae | Teia anartoides | Elaborate | 4.00 | 10.00 | 57.29 | 300.55 | 0.90 | 23, 24 |
| Lyonetiidae | Lyonetia clerkella | Simple | 3.38 | 4.50 | 100.00 | 266.51 | 116.00 | 8, 41 |
| Tineidae | Tineola bisselliella | Simple | 5.06 | 6.25 | 0.53 | 265.46 | 0.82 | 9, 90, 101* |
| Torticidae | Cnephasia longana | Simple | 5.30 | 10.00 | 0.10 | 226.36 | 0.69 | 8, 14 |
| Noctuidae | Agrotis ipsilon | Elaborate | 14.63 | 22.50 | 0.21 | 254.41 | 2.70 | 8, 13 |
| Noctuidae | Autographa gamma | Simple | 15.00 | 20.00 | 2.20 | 205.34 | 0.55 | 8, 20, 94* |
| Noctuidae | Brithys crini | Simple | 9.60 | 20.00 | 600.00 | 238.41 | 23, 24 | |
| Noctuidae | Copitarsia decolora | Simple | 8.69 | 16.10 | 7.55 | 233.39 | 3.97 | 34 |
| Noctuidae | Cornutiplusia circumflexa | Simple | 9.03 | 21.00 | 2.82 | 205.34 | 3.27 | 9, 35 |
| Noctuidae | Earias insulana | Simple | 5.52 | 12.00 | 1.00 | 236.40 | 3.14 | 43, 44 |
| Noctuidae | Earias vittella | Simple | 5.20 | 10.00 | 40.00 | 247.09 | 176.50 | 9 |
| Noctuidae | Epiglaea apiata | Simple | 11.70 | 19.50 | 2.00 | 264.44 | 6.13 | 5 |
| Noctuidae | Euxoa messoria | Simple | 12.38 | 18.75 | 10.40 | 268.46 | 26.00 | 21 |
| Noctuidae | Euxoa ochrogaster | Simple | 9.68 | 19.75 | 12.49 | 226.36 | 4.78 | 21 |
| Noctuidae | Graphania mutans | Elaborate | 23.60 | 40.00 | 4.63 | 233.39 | 1.97 | 53 |
| Noctuidae | Helicoverpa armigera | Simple | 10.40 | 20.00 | 46.72 | 238.41 | 8.40 | 8, 23 |
| Noctuidae | Helicoverpa assulta—Korea | Simple | 7.38 | 12.50 | 421.00 | 260.44 | 18.75 | 23, 24 |
| Noctuidae | Helicoverpa assulta—Thailand | Simple | 7.38 | 12.50 | 161.28 | 238.41 | 2.09 | 23, 24 |
| Noctuidae | Helicoverpa peltigera | Simple | 11.02 | 19.00 | 58.30 | 229.73 | 10.20 | 56 |
| Noctuidae | Helicoverpa punctigera | Simple | 16.40 | 20.00 | 10.00 | 253.77 | 20.50 | 24, 40 |
| Noctuidae | Helicoverpa virescens | Simple | 9.14 | 15.75 | 169.31 | 229.73 | 1.56 | 10, 57, 97* |
| Noctuidae | Helicoverpa zea | Simple | 10.40 | 20.00 | 23.53 | 238.41 | 12.00 | 8 |
| Noctuidae | Lacinipolia renigera | Simple | 7.27 | 12.75 | 8.10 | 253.41 | 5.65 | 37, 61 |
| Noctuidae | Mocis latipes | Simple | 7.80 | 20.00 | 20.06 | 291.54 | 5 | |
| Noctuidae | Nephelodes minians | Elaborate | 10.63 | 21.25 | 134.30 | 260.44 | 1.30 | 5, 21 |
| Noctuidae | Oraesia excavata | Elaborate | 7.27 | 24.25 | 130.00 | 307.54 | 18 | |
| Noctuidae | Panolis flammea | Simple | 9.18 | 17.00 | 53.00 | 263.76 | 8, 73 | |
| Noctuidae | Peridroma saucia | Simple | 14.10 | 23.50 | 65.00 | 268.44 | 3.20 | 8 |
| Noctuidae | Sesamia grisescens | Simple | 5.95 | 17.50 | 85.93 | 261.45 | 2.00 | 40 |
| Noctuidae | Sesamia nonagrioides | Elaborate | 6.97 | 17.00 | 33.79 | 253.77 | 44.10 | 8, 81 |
| Noctuidae | Spodoptera eridania | Simple | 21.60 | 36.00 | 3.50 | 258.82 | 14.30 | 82 |
| Noctuidae | Spodoptera littoralis | Simple | 11.34 | 18.00 | 13.37 | 252.40 | 345.00 | 8, 99* |
| Noctuidae | Thysanoplusia orichalcea | Simple | 13.00 | 20.00 | 22.20 | 225.35 | 1.23 | 9, 89 |
| Noctuidae | Trichoplusia ni | Simple | 11.03 | 17.50 | 69.19 | 236.05 | 2.66 | 9 |
| Noctuidae | Tyta luctuosa | Simple | 6.76 | 13.00 | 139.00 | 224.39 | 84 | |
| Nolidae | Uraba lugens | Elaborate | 5.91 | 13.75 | 19.50 | 259.43 | 0.41 | 23, 92 |
| Oecophoridae | Cheimophila salicella | Simple | 5.70 | 9.50 | 9.50 | 232.39 | 29.70 | 27, 95* |
| Plutellidae | Homadaula anisocentra | Simple | 3.43 | 7.00 | 10.00 | 254.41 | 59 | |
| Plutellidae | Plutella xylostella | Simple | 2.87 | 7.00 | 0.37 | 253.77 | 56.00 | 8, 23 |
| Psychidae | Thridopteryx ephemeraeformis | Elaborate | 4.37 | 13.25 | 375.00 | 242.40 | 27.30 | 5, 15 |
| Pyralidae | Acrobasis nuxvorella | Simple | 7.75 | 12.50 | 0.002 | 258.43 | 1.23 | 2, 3 |
| Pyralidae | Acrobasis vaccinii | Simple | 5.27 | 8.50 | 0.52 | 267.43 | 11.25 | 4, 5 |
| Pyralidae | Etiella behrii | Simple | 4.73 | 10.50 | 0.23 | 240.89 | 1.10 | 48 |
| Pyralidae | Etiella zinckenella (Europe) | Simple | 6.75 | 11.25 | 14.90 | 254.92 | 0.91 | 23, 49 |
| Pyralidae | Etiella zinckenella (Japan) | Simple | 6.75 | 11.25 | 6.80 | 245.06 | 1.90 | 23, 49 |
| Pyralidae | Homoeosoma nebulellum | Simple | 6.70 | 11.75 | 9.52 | 237.74 | 3.10 | 9 |
| Pyralidae | Plodia interpunctella | Simple | 6.12 | 9.00 | 26.30 | 231.38 | 3.93 | 8, 23, 98* |
| Saturniidae | Coloradia velda | Elaborate | 14.62 | 39.50 | 2.79 | 266.44 | 1.80 | 5, 30, 31 |
| Saturniidae | Hemileuca eglanterina | Elaborate | 10.64 | 38.00 | 84.99 | 251.75 | 2.11 | 5, 30, 31 |
| Saturniidae | Hemileuca maia | Elaborate | 9.38 | 31.25 | 22.74 | 251.75 | 1.46 | 5, 30, 31 |
| Sesiidae | Macroscelesia japona | Elaborate | 5.78 | 10.50 | 4.50 | 265.46 | 1.81 | 18 |
| Sesiidae | Macroscelesia longipes | Elaborate | 5.57 | 10.50 | 17.20 | 265.46 | 3.35 | 18 |
| Sesiidae | Paradoxecia pieli | Elaborate | 5.27 | 13.50 | 250.00 | 308.50 | 5.50 | 74 |
| Sesiidae | Synanthedon exitiosa | Simple | 9.75 | 16.25 | 100.00 | 308.50 | 85 | |
| Sesiidae | Synanthedon pictipes | Simple | 6.66 | 10.25 | 4.00 | 308.50 | 111.25 | 28, 86 |
| Sphingidae | Agrius convolvuli | Simple | 22.50 | 50.00 | 7.00 | 236.40 | 12 | |
| Sphingidae | Manduca sexta | Simple | 25.80 | 53.75 | 15.20 | 235.39 | 16.00 | 64, 65 |
| Stathmopodidae | Stathmopoda masinissa | Simple | 5.11 | 7.00 | 0.05 | 280.45 | 5.71 | 83, 84 |
| Thaumetopoeidae | Thaumetopoea pityocampa | Elaborate | 7.82 | 17.00 | 1.00 | 278.43 | 2.39 | 8, 100* |
| Tortricidae | Adoxophyes orana | Simple | 4.08 | 8.50 | 186.40 | 233.39 | 4.95 | 7, 8 |
| Tortricidae | Agapeta zoegana | Simple | 5.40 | 10.00 | 8.00 | 254.41 | 9 | |
| Tortricidae | Archips breviplicanus | Simple | 4.50 | 10.00 | 28.90 | 254.41 | 15.95 | 14 |
| Tortricidae | Archips semiferana | Simple | 5.46 | 9.75 | 33.33 | 254.41 | 62.10 | 5, 15 |
| Tortricidae | Argyrotaenia pomililiana | Simple | 3.65 | 7.30 | 3.16 | 232.39 | 1.77 | 16 |
| Tortricidae | Argyrotaenia velutinana | Simple | 2.80 | 6.50 | 115.00 | 245.73 | 2.45 | 17 |
| Tortricidae | Bonagota salubricola | Simple | 2.85 | 7.50 | 2.71 | 246.39 | 0.50 | 22 |
| Tortricidae | Choristoneura conflictana | Simple | 5.85 | 15.00 | 18.00 | 210.36 | 11.00 | 5, 28 |
| Tortricidae | Choristoneura retiniana | Simple | 5.58 | 11.63 | 23.00 | 233.39 | 43.00 | 5 |
| Tortricidae | Cnephasia jactatana | Simple | 4.96 | 8.70 | 1.80 | 254.41 | 1.88 | 29 |
| Tortricidae | Croesia curvalana | Simple | 4.05 | 7.50 | 0.16 | 232.39 | 91.80 | 37, 38 |
| Tortricidae | Cryptophlebia amamiana | Simple | 3.30 | 7.50 | 6.00 | 226.36 | 39 | |
| Tortricidae | Cryptophlebia horii | Simple | 4.29 | 8.75 | 4.00 | 184.32 | 9.05 | 39 |
| Tortricidae | Ctenopseustis herana | Simple | 4.44 | 12.00 | 3.30 | 254.41 | 0.75 | 40 |
| Tortricidae | Ctenopseustis obliquana | Simple | 6.00 | 12.00 | 1.25 | 254.41 | 40 | |
| Tortricidae | Cydia caryana | Simple | 2.64 | 5.50 | 0.03 | 224.34 | 13.81 | 5, 21 |
| Tortricidae | Cydia pomonella | Simple | 6.82 | 11.00 | 3.39 | 197.94 | 18.00 | 8, 41 |
| Tortricidae | Cydia pyrivora | Simple | 4.31 | 10.50 | 0.50 | 224.34 | 0.26 | 41, 42 |
| Tortricidae | Cydia splendana | Simple | 5.13 | 9.00 | 0.09 | 224.34 | 8, 14 | |
| Tortricidae | Endopiza viteana | Simple | 2.05 | 5.00 | 1.44 | 240.39 | 0.68 | 5, 45 |
| Tortricidae | Epinotia tedella | Simple | 1.56 | 6.50 | 0.40 | 224.34 | 4.39 | 8, 14 |
| Tortricidae | Eupoecilia ambiguella | Simple | 2.31 | 7.00 | 2103.0 | 255.75 | 4.67 | 8, 14, 96* |
| Tortricidae | Grapholita dimorpha | Simple | 2.53 | 5.38 | 3.40 | 226.36 | 0.62 | 54, 55 |
| Tortricidae | Grapholita funebrana | Simple | 2.69 | 6.25 | 0.51 | 226.36 | 1.23 | 9, 41 |
| Tortricidae | Homona magnanima | Simple | 6.44 | 11.50 | 160.00 | 235.71 | 62.40 | 14, 18 |
| Tortricidae | Homona spargotis | Simple | 2.35 | 8.38 | 8.90 | 215.15 | 1.10 | 60 |
| Tortricidae | Lobesia botrana | Simple | 2.82 | 6.00 | 0.36 | 219.20 | 2.00 | 7, 8, 63 |
| Tortricidae | Melissopus latiferreanus | Simple | 4.50 | 9.00 | 2.00 | 224.34 | 2.18 | 15, 66 |
| Tortricidae | Platynota idaeusalis | Simple | 4.16 | 9.25 | 60.00 | 233.39 | 15 | |
| Tortricidae | Rhopobota naevana | Simple | 3.85 | 7.00 | 0.13 | 212.37 | 14.04 | 7 |
| Tortricidae | Sparganothis pilleriana | Simple | 3.71 | 9.50 | 0.22 | 245.06 | 3.11 | 5, 8, 14 |
| Tortricidae | Thaumatotibia batrachopa | Simple | 8.25 | 9.95 | 226.36 | 2.03 | 40, 88 | |
| Tortricidae | Thaumatotibia leucotreta | Simple | 4.29 | 8.25 | 296.00 | 226.36 | 0.93 | 40 |
| Tortricidae | Tortrix viridana | Simple | 5.61 | 11.00 | 4.00 | 254.41 | 8 | |
| Yponomeutidae | Yponomeuta cagnagellus | Simple | 6.89 | 11.30 | 5.32 | 255.08 | 4.76 | 7, 8, 103* |
| Yponomeutidae | Yponomeuta evonymellus | Simple | 6.39 | 10.30 | 3.92 | 240.40 | 1.10 | 8 |
| Yponomeutidae | Yponomeuta padellus | Simple | 7.04 | 11.00 | 13.56 | 263.76 | 2.02 | 7, 8, 103* |
| Yponomeutidae | Yponomeuta plumbellus | Simple | 6.39 | 9.00 | 1.24 | 254.41 | 2.62 | 7, 8, 103* |
| Yponomeutidae | Yponomeuta rorellus | Simple | 6.59 | 10.80 | 5.00 | 256.43 | 0.13 | 8, 103* |
1Skou (1986), 2United States Department of Agriculture (2011), 3Mulder and Grantham (2003), 4IPM North Carolina (1997a), 5North American Moth Photographers Group (2007), 6Landry (2007), 7Gustaffson (2003), 8Carter (1984), 9UK,Moths (2011), 10Canadian Biodiversity Information Facility (2011), 11Hoover and Haydt (2001), 12Pittaway and Kitching (2000–2011), 13Tumlison and Benjamin (2011), 14Meijerman and Ulenberg (2000), 15BugGuide.Net (2011), 16Trematerra and Brown (2004), 17The Virginia Fruit Page (2011), 18Jpmoth.org (2011), 19Forbes (1925), 20Venette et al. (2003), 21Arnett (2000), 22Brown and Razowski (2003), 23Australian Moths Online (1994–2011), 24Herbison–Evans and Crossley (2011), 25Zhang and Polavarapu (2004), 26Anonymous (2011), 27Medvedev (1990), 28E. H. Strickland Entomological Museum (2001–2011), 29Jiménez–Pérez and Wang (2004), 30Tuskes et al. (1996), 31Butterflies and Moths of North America (2011), 32Menzel and Waite (2005), 33Rauf (2008), 34Simmons and Pogue (2004), 35Jonko (2011), 36Hoare et al. (2011), 37Line (2007), 38Crozier (1996), 39Komai and Nasu (2003), 40Pest and Diseases Image Library (2011), 41Afonin et al. (2008), 42Alford (2007), 43BioLib (1999–2011),44Melifronides et al. (1978), 45Williams et al. (2011), 46Ramel (2011), 47Kurz and Kurz (2000–2011), 48Brier (2010), 49King and Saunders (1984), 50Korean Natural History Research Information System (2011), 51Insects of Japan (2011), 52Korycinska and Eyre (2011), 53Dugdale (1971), 54Bae and Park (1997), 55Komai (1979), 56National Museums Northern Ireland (2009–2011), 57Featured Creatures (1996–2011), 58Cardé (1965), 59Heppner and Dekle (1975), 60Whittle et al. (1987), 61Hants Moths Group (2011), 62Stichting TINEA (2011), 63Fraval (2011), 64Oehlke (2011), 65Schneider et al. (1997), 66Scott (2001–2011), 67National Taiwan University Insect Museum Digital Archives Project (2011), 68Elliott and Bashford (1978), 69Miller and Hammond (2000), 70Natural Resources Canada (2009), 71Mohn (1993–2005), 72Ohno et al. (2003), 73Savela (2011), 74Gorbunov and Arita (1997), 75Waller et al. (2007), 76Hill (2008), 77Papillons de Poitou–Charentes (2011), 78Norfolk Moths (2011), 79Association Lepiforum (2011), 80Conner (2008), 81Israeli Ministry of Agriculture and Rural Development (2011), 82IPM North Carolina (1997b), 83Naka et al. (1998), 84Watson and Dallwitz (2003–2011), 85Duckworth and Eichlin (1977), 86Hogmire (1995), 87Berlov and Berlov (1999–2011), 88United States Department of Agriculture (2010), 89Holloway (1985), 90Western Australian Department of Agriculture (2011), 91Russell IPM (2011), 92Phillips (1992), 93Renou et al. (1981), 94Tóth et al. (1983), 95Salas–Reyes (1985), 96Rauscher et al. (1984), 97Dickens et al. (1993), 98Doud and Phillips (2000), 99Kehat et al. (1976), 100Quero et al. (2003), 101Cox et al. (1996), 102Ferrara (2001), 103Löfstedt and Herrebout (1988).
Phylogenetic information
We constructed a composite phylogeny (see Fig. 2) combining phylogenetic information from a number of sources as follows: The species were initially split based on taxonomy down to the generic level. Relationships between superfamilies were derived from Kristensen et al. (2007) with further resolution of relationships from Minet (1991) (Gelechoidea, Yponomeutoidea, Cossoidea, Sessoidea, and Zygaenoidea) and Regier et al. (2008) (Geometroidea, Noctuoidea, Lasiocampoidea, and Bombycoidea). Relationships within the Geometroidea were taken from Yamamoto and Sota (2007), with further resolution of the position of Peribatodes and Ascotis from Hunter (1995), and Tephrina from Young (2008). Bombycoidea internal relationships were derived from Regier et al. (2008), while Gelechioidea phylogeny was derived from Kaila (2004) with additional resolution for the Gelechiidae from Lee et al. (2009). Solis (2007) provided the phylogeny for the Pyraloidea, with additional resolution within the Pyralidae from Simonsen (2008) and within Ostrinia from Ishikawa et al. (1999). The phylogeny of the Noctuoidea was taken from Mitchell et al. (2006) with further resolution within the Lymantriidae and the Hadeninae from Lafontaine and Fibiger (2006) and within the Heliothinae from Cho et al. (2008). Relationships between genera in the Yponomeutoidea were derived from the systematic arrangement proposed by Dugdale et al. (1999), and further resolution within the genus Yponomeuta was taken from Löfstedt et al. (1991). Finally, family and subfamily relationships within the Tortricoidea were taken from Roelofs and Brown (1982), with resolution within the Archipini derived from Pashley (1983), Lee et al. (2005), Hulcr et al. (2007), and Safonkin and Triseleva (2008), and relationships within the Grapholitini from Pashley (1983) and Komai (1999).
Figure 2.
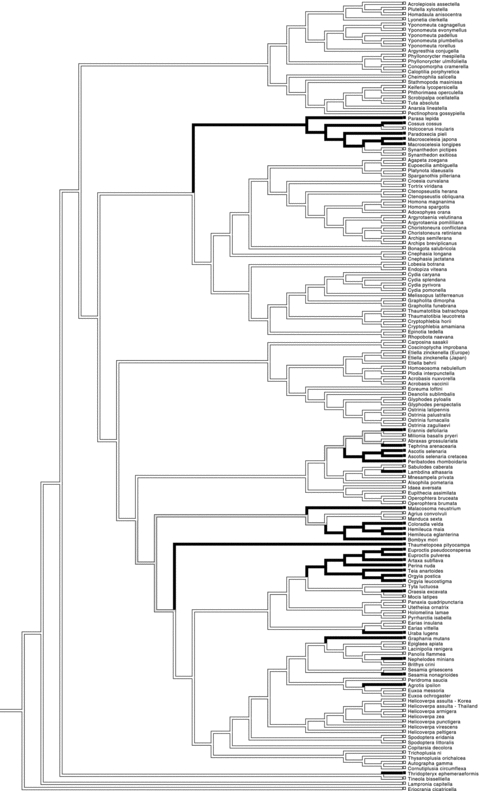
Phylogeny used in the analysis. Lineages leading to species with elaborate antennae are marked on in black. Putative reconstruction of evolutionary transitions is based on maximum parsimony analysis in Mesquite (Maddison and Maddison 2010).
The composite nature of the phylogeny means that branch length information was not available, and so all branch lengths were set to the same length (= 1).
Data analysis
To meet assumptions of normality, all continuous variables except mean molecular weight were log-transformed before inclusion in the analysis. Relationships between aspects of antennal morphology, body size (forewing length), and the main variables of interest (pheromone titre, mean molecular weight, and population abundance) were determined controlling for phylogenetic relatedness. This was achieved using phylogenetic generalized least squares (PGLS) (Martins 1996; Martins and Hansen 1997), implemented through the package COMPARE (Martins 2004). PGLS is a statistical method that allows one to investigate the correlation between continuous variables and a limited number of categorical variables (as predictor variables) across species. By comparing the observed covariance in traits with that expected under a specified model of evolution (in this case a Brownian motion model) it can calculate this correlation controlling for the phylogenetic signal in the traits being analyzed (expressed in terms of the variable α, where low values tending to 0 indicate a strong phylogenetic signal, and high values > 15 indicate effectively no signal).
We initially investigated the relationship of body size with our other variables. Log forewing length was therefore entered into COMPARE as the independent (X) variable, with the other variable as the dependent (Y) variable. When it became apparent that body size was correlated with our other traits (see results), we subsequently controlled for its potential confounding effects on our analysis in the manner advocated by Freckleton (2009)—that is, by including it as a covariate in our other calculations where we related aspects of pheromone titre, molecular weight, and population abundance to antennal morphology (either presence/absence of elaborate antennae or antennal length). The reported PGLS correlations between these characteristics are therefore partial correlations controlling for body size.
Finally, for the species for which we had complete information on pheromone titre, male abundance, and molecular weight, we examined which combination of factors served as the best approximating model of antennal morphology including the model with body size only as predictor. Comparison of models was performed using Akaike's information criterion (AICc) correcting for small sample size (Burnham and Anderson 2002). From the AICc values, we calculated Akaike weight (Wi) for each model in the candidate set as well as the evidence ratio (ER). The latter provides a means of expressing the relative likelihood of one model over another.
Results
Relationship with body size
Body size (measured by forewing length) is significantly linked with antennal morphology. On average, species with elaborate antennae are larger than those with simple antennae (PGLS: α = 9.48, t151 = 3.339, P = 0.001: Fig. 3a). Elaborate antennae are typically shorter, relative to forewing length, than simple antennae (Fig. 3b; average length = 39.9% of forewing length for elaborate antennae, 53.9% of forewing length for simple antennae, t151 = 5.936, P < 0.001). With both types of antennae there is a strong correlation between antennal length and forewing length (Fig. 3b; simple antennae: α = 2.79, r = 0.839, n = 117, P < 0.001, elaborate antennae: α = 3.94, r = 0.891, n = 30, P < 0.001).
Figure 3.
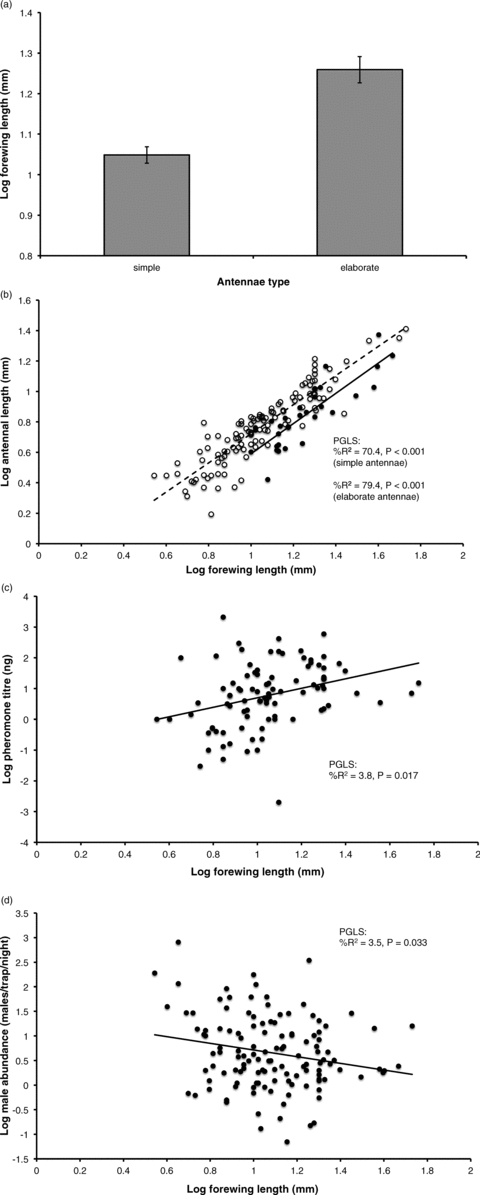
Relationships with body size (log forewing length) and (a) antennal shape (means and standard errors of forewing length shown); (b) log antennal length (open circles, dotted line: simple antennae, filled circles, solid line: elaborate antennae); (c) pheromone titre (log ng pheromone per female); and (d) male abundance (log number of males caught per trap per day).
Larger bodied species have significantly larger pheromone titres (α = 11.44, r = 0.194, n = 150, P = 0.017: Fig. 3c). However, there was no evolutionary association between mean molecular weight of pheromone components and body size (α = 3.63, r = 0.049, n = 152, P = 0.549). Male abundance (maximum number of individual males caught per trap per night) significantly declined with body size (α = 14.51, r = -0.188, n = 127, P = 0.033: Fig. 3d).
Relationship with pheromone titre
We found no significant association between pheromone titre and antennae type (i.e., simple or elaborate) after taking phylogeny and body size into account (α = 6.44, t148 = –0.175, P = 0.863). Nor was there an association between pheromone titre and antenna length (species with simple antennae: α = 6.99, r = –0.073, n = 117, P = 0.434; species with elaborate antennae: α = 2.82, r = –0.214, n = 30, P = 0.256).
Relationship with molecular weight
Initial examination of the data suggested an association between mean molecular weight of compounds and antennae type, although in the opposite direction to that predicted, with species with elaborate antennae using heavier compounds. However, the association was not significant after body size and phylogeny were taken into account (α = 7.21, t150 = 1.633, P = 0.105). There was no association between antennal length and molecular weight for species with simple antennae (α = 6.46, r = 0.073, n = 119, P = 0.430). However, in species with elaborate antennae, there was a significant negative relationship between molecular weight and antennal length (α = 4.46, r = –0.453, n = 30, P = 0.012: Fig. 4).
Figure 4.
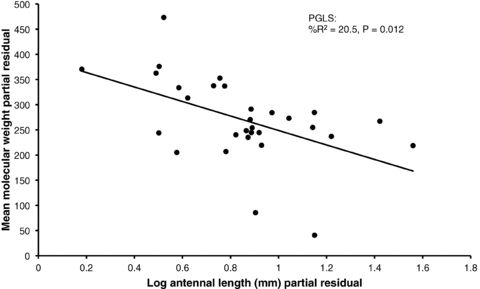
Partial residual plot of the relationship between antennal length and mean molecular weight of female sex pheromone components in moths species with elaborate male antennae.
Relationship with male population abundance
Although species with elaborate antennae tended to have lower abundance of males, as determined by trapping numbers, there was no significant association between abundance and the type of antenna after controlling for body size and phylogeny (α = 5.63, t125 = –1.563, P = 0.121). However, for species with elaborate antennae, there was a significant negative relationship (Fig. 5) between abundance and antennae length (α = 0.95, r = –0.561, n = 26, P = 0.003). No such pattern was evident in species with simple antennae (α = 8.91, r = 0.080, n = 97, P = 0.436).
Figure 5.
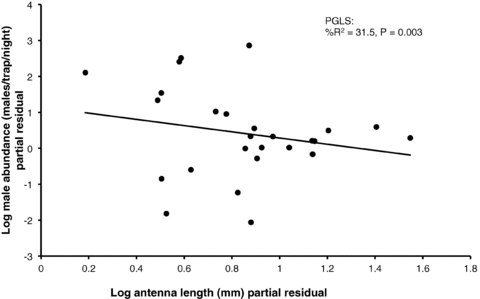
Partial residual plot of the relationship between antennal length and male abundance in moth species with elaborate male antennae.
Comparison of models
Evaluation of the AICc scores for models predicting variation in antennal morphology (simple or elaborate) revealed that the model including both body size and male population abundance was the best approximating model (Table 2a). However, the Akaike weight (0.41) for this model showed that there is only a 41% chance that it was correctly identified as the best approximating model. The ER indicated it was only 1.49 times more likely than the model that included body size alone. The full-factorial model was the least strongly supported model. This was also the case in the comparison of models predicting antennal length in species with simple antennae (Table 2b). In that case, the best approximating model contained only body size, and none of our putative predictors, although the Akaike weights indicated considerable uncertainty in the identity of the best approximating model.
Table 2.
Comparison of models predicting antennal morphology, and antennal length in species with simple and elaborate antennae, respectively. Models are compared using Akaike's Information Criterion corrected for small sample size (AICc). For other abbreviations see Methods
| Model | %R2 | AICc | ΔAICc | Wi | ER |
|---|---|---|---|---|---|
| (a) Antennal morphology (n = 126) | |||||
| Pheromone titre + male abundance + molecular weight + body size | 7.79 | –137.44 | 3.57 | 0.07 | 5.96 |
| Pheromone titre + body size | 3.82 | –138.11 | 2.90 | 0.10 | 4.26 |
| Male abundance + body size | 5.95 | –141.01 | 0 | 0.41 | 1 |
| Molecular weight + body size | 5.84 | –139.04 | 1.97 | 0.15 | 2.67 |
| Body size only | 3.78 | –140.21 | 0.79 | 0.27 | 1.49 |
| (b) Antennal length in species with simple antennae (n = 97) | |||||
| Pheromone titre + male abundance + molecular weight + body size | 75.57 | –342.98 | 3.21 | 0.07 | 4.98 |
| Pheromone titre + body size | 73.93 | –345.64 | 0.55 | 0.26 | 1.32 |
| Male abundance + body size | 73.98 | –344.66 | 1.53 | 0.16 | 2.15 |
| Molecular weight + body size | 74.38 | –344.92 | 1.27 | 0.18 | 1.84 |
| Body size only | 73.44 | –346.19 | 0 | 0.34 | 1 |
| (c) Antennal length in species with elaborate antennae (n = 26) | |||||
| Pheromone titre + male abundance + molecular weight + body size | 90.75 | –100.50 | 1.96 | 0.23 | 2.66 |
| Male abundance + molecular weight + body size | 90.22 | –102.46 | 0 | 0.62 | 1 |
| Pheromone titre + body size | 82.04 | –90.83 | 11.63 | 0.00 | 334.65 |
| Male abundance + body size | 87.86 | –99.27 | 3.19 | 0.13 | 4.92 |
| Molecular weight + body size | 84.80 | –95.31 | 7.15 | 0.02 | 35.63 |
| Body size only | 81.75 | –93.06 | 9.40 | 0.01 | 109.78 |
By contrast, comparison of models predicting variation in antennal length in species with elaborate antennae (Table 2c) revealed that the model including both male population abundance and molecular weight was estimated to be the best approximating model, with a fairly robust Akaike weight (0.62), although the full-factorial model and the model including only population abundance both stand as credible alternatives. By summing Akaike weights across models, we can estimate predictor weights, which indicate a 98% likelihood that male abundance features in the best approximating model, and an 85% likelihood that molecular weight does as well. Our estimated best approximating model is almost 110 times more likely to be the best model than the model including only body size.
The R2 values of our full-factorial models predicting antennal shape are extremely poor (7.79%), when compared with the models predicting antennal length (75.57% for species with simple antennae, 90.75% for species with elaborate antennae).
Discussion
Elaborate antennae in male moths appear have to have evolved at least 13 times, as judged from our phylogeny and sample of species (Fig. 2). Major families in which the characteristic has appeared include the Lymantriidae, Saturniidae, Bombycidae, Geometridae, Cossidae, Sesiidae, and Limacodidae.
The presence of elaborate antennae in moths is closely associated with larger body size (longer forewing length). While antennal length predictably scales with body size in insects (e.g. Emlen and Allen 2004; Bonduriansky 2007), the fact that the gross shape of the antennae also appears to be linked to body size suggests a cost of bearing these antennae that is difficult to sustain for small-bodied species. Elaborate antennae tend to be shorter, relative to forewing length, than simple antennae, suggesting a constraint on antennal length imposed by shape. For example, one cost of elaborate antennae might be related to flight ability. On the one hand, in the large-bodied Manduca sexta hawkmoth, elaborate antennae act as mechanosensory gyroscopes, helping to stabilize the animal while hovering (Sane et al. 2007). However, at small body sizes, inertial drag forces associated with having elaborate antennae would be disproportionately higher (Ellington 1991).
These results are also complicated by the use of forewing length as a measure of body size. Typically, moths with longer wings tend to be better at flying over longer distances and for longer periods of time (e.g., Shirai 1993) and hence potentially face lower selection pressure for greater sensitivity to pheromones, because they can compensate for lower sensitivity by airborne searching for longer. In such case, we would be less likely to see a relationship between long forewings and elaborate antennae.
Body size is also linked to pheromone titre: larger species tend to produce greater quantities of pheromone. This relationship between pheromone titre and body size has been identified within several species of moths and may reflect female quality (Jaffe et al. 2007, Johansson and Jones 2007; Harari et al. 2011): females that produce greater quantities of pheromone tend to attract more males (Nagata et al. 1972; Turgeon et al. 1983; Evenden and Gries 2008). Body size is also negatively correlated with male abundance, consistent with other studies of many animal assemblages, including insects (Blackburn et al. 1993; White et al. 2007). The associations of body size with antennal shape and pheromone titre revealed in our analysis indirectly contradict our predictions. If females of small-bodied species produce absolutely smaller amounts of pheromone, then males of these smaller bodied species should be more likely to have elaborate antennae, yet we found the opposite pattern.
However, we found no clear associations between basic antennal shape, or antennal length and pheromone titre after controlling for body size. Female moths typically produce minute quantities of pheromone (Svensson 1996), and males typically possess a greater number of sensilla on their antennae than females (Chapman 1982), suggesting there is a selective advantage for greater sensitivity. However, we cannot find evidence that reduced pheromone titre has selected for increases in antennal size and elaboration. It is important to stress that pheromone titre should ideally be measured as the number of molecules released per unit time (i.e., the release rate) (Hölldobler and Wilson 1990, p. 244), rather than absolute amount in the gland, as we have used here. This was not feasible for the present study because comparative data on release rates for moth species are very sparse. Both pheromone titre and release rates vary within species and within individuals, relating to age, time of day, and proximity of other individuals (e.g., Sanders and Lucuik 1972; Raina et al. 1986; Foster et al. 1995; Lim et al. 2007). Although there is evidence that release rates are limited by pheromone gland titres (Schal et al. 1987), and that pheromone titre is reflective of pheromone release rate (see Methods), it is possible that using fixed quantities of pheromone as a substitute for the quantity released is simply too inaccurate. Additionally, although gas chromatography-mass spectrometry analytically techniques have become increasingly more powerful at detecting minute quantities of chemical components, it is possible that many of the species in our analysis represent the high end of pheromone production rates. Therefore, while we cannot find support for an association, we are reluctant to rule out a relationship between pheromone production and antennal morphology.
Antennal shape was not significantly associated with either the abundance of males or the diffusion rate of the female sex pheromone (as measured by the mean molecular weight of compounds). Nevertheless, the trend is in the direction predicted (more elaborate antennae in species with lower male abundance), and model selection with AICc suggested that the best model predicting antennal shape included both body size and male abundance. This model was only slightly better supported than the model that included body size alone, and adding male abundance to the model explained only an additional 2% of the variation. Evidently, any effect of male abundance on the evolution of elaborate antennae is difficult to disentangle from the effects of body size.
Nevertheless, variation in antennal length is significantly explained by male abundance and the molecular weight of the female sex pheromone. In species that possess elaborate antennae, there were clear relationships: longer antennae are found in species that have lower male abundances and where the females use pheromone compounds that have, on average, lower molecular weights (i.e., that diffuse and fade out more quickly). Model selection with AICc demonstrated much stronger support for models that included these predictors than the model that included body size alone (see Table 2c). This result is interesting because antennal length is only one component of antennal size and there is considerable variation in elaborate antennae area apart from length. Some species (e.g., Agrotis ipsilon) have only slightly ramified antennae compared to the broad width on others such as Coloradia velda. Additionally, most of the species in our analysis are agricultural pests whose population sizes are likely to have increased enormously since the introduction of intensive agricultural practices in the past few hundred years. Present measures of abundance through trapping data may therefore be unreflective of the population dynamics under which the species (and their antennae) evolved. Having said this, changes in abundance should apply to all species (i.e., they have all increased in abundance recently), so we doubt any systematic bias in our analysis. More pertinently, despite all these potential inaccuracies and the possible confounding effects of forewing length and flight ability (see earlier), the strong pattern (model R2 > 90%) observed here relating antennal length to abundance and molecular weight strikes us as being biologically significant.
It is noteworthy that these patterns apply to species with elaborate antennae only and not to species with simple antennae. Given the approximately one-dimensional nature of a filiform antenna, an increase in length of antenna would have less effect on the number of sensilla than it would on a more two- or three-dimensional feathery antenna. Specifically, in the latter cases, an increase in the length of antenna would result in either a squaring or cubing of the antennal surface area, rather than the simple isometric increase that would occur in species with filiform antennae. In species with filiform antennae, greater sensitivity might be achieved by developing longer sensilla (rather than longer antennae per se), as noted in the cabbage looper moth, Trichoplusia ni (O’Connell et al. 1983).
Our analyses necessarily trade-off collating data from a sufficient sample of species, with data quality–with considerable noise deriving from factors that influence the dispersion of pheromones (plume structure and environmental turbidities) and population abundance (see earlier). Additionally, as our understanding of moth phylogenetic relationships becomes clearer, the interpretation of how often, and why, elaborate antennae have evolved is likely to be modified. Nevertheless, we can derive two main conclusions about the evolution of antennae in moths. First, the elaborate male antennae borne by some moth species are associated with larger body size, suggesting a cost to these structures. Accordingly, there must be equally strong benefits, that we would hypothesize most likely derive from sexual selection for this type of antennal morphology. Second, small pheromone titres, low male abundance, and diffusion properties of the pheromone do not directly account for why males of some species have simple antennae and others elaborate antennae. However, the latter two factors may influence the size of those structures in species that have elaborate antennae. More generally, it is evident that other factors, perhaps associated with reproductive ecology and mating system, may select for the evolution of these remarkable lepidopteran characteristics.
Acknowledgments
We thank G. Holwell and two anonymous reviewers for their constructive comments on the earlier version of this manuscript. This research was funded by a Discovery Project grant from the Australian Research Council (DP0987360).
References
- Afonin AN, Greene SL, Dzyubenko NI, Frolov AN, editors. 2008. Interactive agricultural ecological atlas of Russia and neighboring countries. Available at < http://www.agroatlas.ru>.
- Alford DV. Pests of fruit crops: a colour handbook. London, U.K: Manson Publishing; 2007. [Google Scholar]
- Anonymous. 2011. Carposina niponensis. EPPO datasheet on quarantine pests. Available at < http://www.eppo.org/QUARANTINE/insects/Carposina_sasakii/CARSSA_ds.pdf>.
- Arnett RH. American insects: a handbook of the insects of America north of Mexico. Boca Raton: CRC Press; 2000. [Google Scholar]
- Association Lepiforum. 2011. Lepiforum: Bestimmung von Schmetterlingen (Lepidoptera) und ihren Präimaginalstadien. Available at < http://www.lepiforum.de>.
- Australian Moths Online. 1994. 2011. CSIRO Ecosystem Sciences. Available at < http://www1.ala.org.au/gallery2/main.php>.
- Bae YS, Park KT. Systematics study of the genus Grapholita Treitschke (Lepidoptera, Tortricidae) from Korea. Korean J. Biol. Sci. 1997;1:539–547. [Google Scholar]
- Berlov E, Berlov O. 1999. 2011. 1000 Siberian butterflies and moths. Available at < http://catocala.narod.ru>.
- BioLib. 1999. 2011. Biological library. Available at < http://www.biolib.cz>.
- Birch MC, Haynes KF. Insect pheromones. London, U.K: Edward Arnold; 1982. [Google Scholar]
- Blackburn TM, Brown VK, Doube BM, Greenwood JJD, Lawton JH, Stork NE. The relationship between abundance and body size in natural animal assemblages. J. Anim. Ecol. 1993;62:519–528. [Google Scholar]
- Bonduriansky R. Sexual selection and allometry: a reappraisal of the evidence and ideas. Evolution. 2007;61:838–849. doi: 10.1111/j.1558-5646.2007.00081.x. [DOI] [PubMed] [Google Scholar]
- Bossert WH, Wilson EO. The analysis of olfactory communication among animals. J. Theoret. Biol. 1963;5:443–469. doi: 10.1016/0022-5193(63)90089-4. [DOI] [PubMed] [Google Scholar]
- Brier H. 2010. Etiella behrii (Lucerne seed web moth). IPM Insect Pests. Queensland government. Primary industry and fisheries. Available at < http://www.dpi.qld.gov.au/26_7796.htm>.
- Brown JW, Razowski J. Description of Ptychocroca, a new genus from Chile and Argentina, with comments on the Bonagota Razowski group of genera (Lepidoptera: Tortricidae: Euliiini) Zootaxa. 2003;303:1–31. [Google Scholar]
- BugGuide.Net. 2011. Iowa State University Entomology. Available at < http://bugguide.net>.
- Burnham KP, Anderson DR. Model selection and multimodel inference. 2nd ed. New York, NY: Springer; 2002. [Google Scholar]
- Butler LI, McDonough LM. Insect sex pheromones: evaporation rates of alcohols and acetates from natural rubber septa. J. Chem. Ecol. 1981;7:627–633. doi: 10.1007/BF00987710. [DOI] [PubMed] [Google Scholar]
- Butterflies and Moths of North America. 2011. Available at < http://www.butterfliesandmoths.org>.
- Byers JA. Active space of pheromone plume and its relationship to effective attraction radius in applied models. J. Chem. Ecol. 2008;34:1134–1145. doi: 10.1007/s10886-008-9509-0. [DOI] [PubMed] [Google Scholar]
- Canadian Biodiversity Information Facility. 2011. Available at < http://www.cbif.gc.ca>.
- Cardé RT, Haynes KF. Structure of the pheromone communication channel in moths. In: Cardé RT, Millar JG, editors. Advances in chemical ecology. Cambridge, U.K: Cambridge Univ. Press; 2004. pp. 283–332. [Google Scholar]
- Cardé RT, Willis MA. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008;34:854–866. doi: 10.1007/s10886-008-9484-5. [DOI] [PubMed] [Google Scholar]
- Cardé RT, Baker TC. Sexual communication with pheromones. In: Bell WJ, Cardé RT, editors. Chemical ecology of insects. London, U.K: Chapman and Hall; 1984. pp. 355–383. [Google Scholar]
- Cardé RT. Some taxonomic notes on the Nearctic Holomelina (Arctiidae) with a partial key to the species. J. Lepidopt. Soc. 1965;19:69–76. [Google Scholar]
- Carter DJ. Pest Lepidoptera of Europe. Dordrecht: Dr. W. Junk Publishers; 1984. [Google Scholar]
- Chapman RF. Chemoreception: the significance of receptor numbers. Adv. Insect Physiol. 1982;16:247–356. [Google Scholar]
- Cho S, Mitchell A, Mitter C, Regier J, Matthews M, Robertson R. Molecular phylogenetics of heliothine moths (Lepidoptera: Noctuidae: Heliothinae), with comments on the evolution of host range and pest status. Syst. Entomol. 2008;33:581–594. [Google Scholar]
- Conner WE, editor. Tiger moths and woolly bears. Behavior, ecology and evolution of the Arctiidae. New York: Oxford Univ. Press; 2008. [Google Scholar]
- Cotgreave P. The relationship between body size and population abundance in animals. Trends Ecol. Evol. 1993;8:244–248. doi: 10.1016/0169-5347(93)90199-Y. [DOI] [PubMed] [Google Scholar]
- Cox PD, Pinniger DB, Mueller D. Monitoring populations of the webbing clothes moth Tineola bisselliella, using pheromone lures. In: Wildey KB, editor. Proceedings of the Second International Conference on Urban Pests. U.K: Exeter Press; 1996. pp. 541–545. [Google Scholar]
- Crozier L. 1996. The Blueberry leaftier. Lowbush Blueberry Fact Sheet. Wild Blueberry Network Information Centre. Available at < http://nsac.ca/wildblue/facts/insects/leaftier.asp>.
- Currie DJ. What shape is the relationship between body size and population-density? Oikos. 1993;66:353–358. [Google Scholar]
- Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. [Google Scholar]
- Delisle J, Vincent C. Modified pheromone communication associated with insecticidal resistance in the obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Tortricidae) Chemoecology. 2002;12:47–51. [Google Scholar]
- Delisle J, West RJ, Bowers WW. The relative performance of pheromone and light traps in monitoring the seasonal activity of both sexes of the eastern hemlock looper, Lambdina fiscellaria fiscellaria. Entomol. Exp. Appl. 1998;89:87–98. [Google Scholar]
- Dickens JC, Smith JW, Light DM. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep.: Noctuidae) Chemoecology. 1993;4:175–177. [Google Scholar]
- Doud CW, Phillips TW. Activity of Plodia interpunctella (Lepidoptera: Pyralidae) in and around flour mills. J. Econ. Entomol. 2000;93:1842–1847. doi: 10.1603/0022-0493-93.6.1842. [DOI] [PubMed] [Google Scholar]
- Duckworth WD, Eichlin TD. A classification of the Sesiidae of America North of Mexico (Lepidoptera: Sesioidea) Sacramento, CA: State of California Department of Food and Agriculture; 1977. Occasional Papers in Entomology 26. [Google Scholar]
- Dugdale J, Kristensen NP, Robinson GS, Scoble MJ. The Yponomeutoidea. In: Kristensen NP, editor. Handbook of Zoology: Lepidoptera, moths and butterflies. Berlin: Walter de Gruyter; 1999. pp. 119–130. [Google Scholar]
- Dugdale JS. Entomology of the Aucklands and other islands south of New Zealand: Lepidoptera, excluding non-crambine Pyralidae. Pacific Insects Monogr. 1971;27:55–172. [Google Scholar]
- E. H. Strickland Entomological Museum. Entomology collection. 2001. 2011. University of Alberta. Available at < http://www.entomology.ualberta.ca/index.html>.
- El-Sayed AM. 2011. The Pherobase: database of insect pheromones and semiochemicals. Available at http://www.pherobase.com.
- Elkinton JS, Cardé RT. Odor dispersion. In: Bell WJ, Cardé RT, editors. Chemical ecology of insects. London, U.K: Chapman and Hall; 1984. pp. 73–91. [Google Scholar]
- Ellington CP. Aerodynamics and the origin of insect flight. Adv. Insect Physiol. 1991;23:171–210. [Google Scholar]
- Elliott HJ, Bashford R. The life history of Mnesampela privata (Guen.) (Lepidoptera: Geometridae) a defoliator of young eucalypts. J. Aust. Entomol. Soci. 1978;17:201–204. [Google Scholar]
- Emlen DJ, Allen CE. Genotype to phenotype: physiological control of trait size and scaling in insects. Integr. Comp. Biol. 2004;43:617–634. doi: 10.1093/icb/43.5.617. [DOI] [PubMed] [Google Scholar]
- Evenden M, Gries R. Plasticity of male response to sex pheromone depends on physiological state in a long-lived moth. Anim. Behav. 2008;75:663–672. [Google Scholar]
- Featured Creatures. 1996. 2011. University of Florida Department of Entomology. Available at < http://entnemdept.ufl.edu/creatures/>.
- Ferrara FAA, Vilela EF, Jham GN, Eiras AE, Picanco MC, Attygalle AB, Svatos A, Frighetto RTS, Meinwald J. Evaluation of the synthetic major component of the sex pheromone of Tuta absoluta (Meyrick) (Lepidoptera: Gelechidae) J. Chem. Ecol. 2001;27:907–917. doi: 10.1023/a:1010378818318. [DOI] [PubMed] [Google Scholar]
- Forbes WPM. Pectinate antennae in the Geometridae. Psyche. 1925;32:106–112. [Google Scholar]
- Foster SP, Howard AJ, Atera RH. Age-related changes in reproductive characters of four species of tortricid moths. New Zeal. J. Zool. 1995;22:271–280. [Google Scholar]
- Fraval A. 2011. HYPPZ. Encyclopédie des ravageurs européens. Institut National de la Researche Agronomiques. Available at < http://www.inra.fr/hyppz/pa.htm>.
- Freckleton RP. The seven deadly sins of comparative analysis. J. Evol Biol. 2009;22:1367–1375. doi: 10.1111/j.1420-9101.2009.01757.x. [DOI] [PubMed] [Google Scholar]
- Gage MJG. Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc R Soc Lond B. 1994;258:247–254. [Google Scholar]
- Gorbunov OG, Arita Y. Review of the genus Paradoxecia Hampson, 1919 (Lepidoptera, Sesiidae, Tithiinae) Bonner Zool. Beitrage. 1997;47:59–68. [Google Scholar]
- Greenfield MD. Moth sex pheromones: an evolutionary perspective. Fla. Entomol. 1981;64:4–17. [Google Scholar]
- Gustaffson B. 2003. Svenska fjärilar (Swedish fauna): Lepidoptera. Swedish Museum of Natural History. Available at < http://www2.nrm.se/en/svenska_fjarilar/>.
- Hambäck PA, Summerville KS, Steffan-Dewenter I, Krauss J, Englund G, Crist TO. Habitat specialization, body size, and family identity explain lepidopteran density-area relationships in a cross-continental comparison. Proc. Nat. Acad. Sci. USA. 2007;104:8368–8373. doi: 10.1073/pnas.0611462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hants Moths Group. 2011. Hantsmoths. Available at < http://www.hantsmoths.org.uk/index.htm>.
- Harari AR, Zahavi T, Thiéry D. Fitness cost of pheromone production in signalling female moths. Evolution. 2011;65:1572–1582. doi: 10.1111/j.1558-5646.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford, U.K: Oxford Univ. Press; 1991. [Google Scholar]
- Heppner JB, Dekle GW. Mimosa webworm2Homodaula anisocentraMeyrick (Lepidoptera: Plutellidae) Florida Department of Agriculture and Consumer Services Division of Plant Industry, Gainesville FLA; 1975. [Google Scholar]
- Herbison-Evans D, Crossley S. 2011. Pictures of moths in Australia. Available at < http://lepidoptera.butterfly-house.com.au/moths-imago.html>.
- Hill DS. Pests of crops in warmer climates and their control. Berlin: Springer; 2008. [Google Scholar]
- Hoare RJB, Rhode BE, Emmerson AW. 2011. Larger moths of New Zealand: image gallery and online guide. Available at < http://largemoths.landcareresearch.co.nz/>.
- Hogmire HW, editor. Mid-Atlantic orchard monitoring guide. Ithaca, NY: Northeast Regional Agricultural Engineering Service Publication 75; 1995. [Google Scholar]
- Holloway JD. The moths of Borneo. Part 14. The family Noctuidae. Subfamiles: Eutellinae, Stictopterinae, Plusiinae, Pantheinae. Malayan Nat. J. 1985;38:157–317. Available at < http://www.mothsofborneo.com>. [Google Scholar]
- Holwell GI, Barry KL, Herberstein ME. Mate location, antennal morphology, and ecology in two praying mantids (Insecta: Mantodea) Biol. J. Linn. Soc. 2007;91:307–313. [Google Scholar]
- Hoover GA, Haydt TR. Fall CankerwormAlsophila pometariaEntomological Notes TS-39. Pennsylvania State University; 2001. Available at < http://ento.psu.edu/extension/factsheets/pdf/fallcankerworm.pdf>. [Google Scholar]
- Hulcr J, Miller SE, Setliff GP, Darrow K, Mueller ND, Hebert PDN, Weiblen GD. DNA barcoding confirms polyphagy in a generalist moth Homona mermerodes (Lepidoptera: Tortricidae) Mol. Ecol. Notes. 2007;7:549–557. [Google Scholar]
- Hunter AF. The ecology and evolution of reduced wings in forest macrolepidoptera. Evol. Ecol. 1995;9:275–287. [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- Insects of Japan. 2011. Euproctis pulverea. Available at < http://www.insects.jp/kon-gagomafuridoku.htm>.
- IPM North Carolina. 1997a. Cranberry fruitworm. Available at < http://ipm.ncsu.edu/small_fruit/cranworm.html>.
- IPM North Carolina. 1997b. Southern armyworm. Available at < http://ipm.ncsu.edu/ag295/html/southern_armyworm.htm>.
- Ishikawa Y, Takanashi T, Kim C, Hoshizaki S, Tatsuki S, Huang Y. Ostrinia spp. in Japan: their host plants and sex pheromones. Entomol. Exp. Appl. 1999;91:237–244. [Google Scholar]
- Israeli Ministry of Agriculture and Rural Development. 2011. Plant protection and inspection services. Available at < http://www.ppis.moag.gov.il>.
- Jaffe K, Mirás B, Cabrera A. Mate selection in the moth Neoleucinodes elegantalis: evidence for a supernormal chemical stimulus in sexual attraction. Anim. Behav. 2007;73:727–734. [Google Scholar]
- Jiménez-Pérez A, Wang Q. Sexual selection in Cnephasia jactatana (Lepidoptera: Tortricidae) in relation to age, virginity, and body size. Ann. Entomol. Soc. Am. 2004;97:819–824. [Google Scholar]
- Johansson BG, Jones TM. The role of chemical communication in mate choice. Biol. Rev. 2007;82:265–289. doi: 10.1111/j.1469-185X.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- Jonko C. 2011. European butterflies and moths. Available at < http://www.lepidoptera.pl>.
- Jpmoth.org. 2011. An identification guide of Japanese moths. Available at < http://www.jpmoth.org>.
- Kaila L. Phylogeny of the superfamily Gelechioidea (Lepidoptera: Ditrysia): an exemplar approach. Cladistics. 2004;20:303–340. doi: 10.1111/j.1096-0031.2004.00027.x. [DOI] [PubMed] [Google Scholar]
- Kawano K. Sexual dimorphism and the making of oversized male characters in beetles (Coleoptera) Ann. Entomol. Soc. Am. 2006;99:327–341. [Google Scholar]
- Kehat M, Greenberg S, Tamaki Y. Field evaluation of the synthetic sex pheromone, as an attractant for males of the cotton leafworm, Spodoptera littoralis (Boisd.), in Israel. Appl. Entomol. Zool. 1976;11:45–52. [Google Scholar]
- King ABS, Saunders JL. The invertebrate pests of annual food crops in Central America. London: Overseas Development Administration; 1984. [Google Scholar]
- Komai F, Nasu Y. Four species of Olethreutinae (Lepidoptera: Tortricidae) associated with viviparous seedlings of the mangrove Rhizophoraceae in the Ryukyu Island, Japan. Invertebr. Syst. 2003;17:75–87. [Google Scholar]
- Komai F. A new species of the genus Grapholita Treitschke from Japan allied to the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae) Appl. Entomol. Zool. 1979;14:133–136. [Google Scholar]
- Komai F. A taxonomic review of the genus Grapholita and allied genera (Lepidoptera: Tortricidae) in the Palearctic region. Entomol. Scand. 1999;55:1–226. [Google Scholar]
- Korean Natural History Research Information System. 2011. . Available at < http://www.naris.go.kr>.
- Korycinska A, Eyre D. Plant Pest Factsheet. The Food and Environment Research Agency (FERA); 2011. Box tree caterpillar Cydalima perspectalis. Available at < http://www.fera.defra.gov.uk/plants/publications/documents/factsheets/box0TreeCaterpillar2011.pdf>. [Google Scholar]
- Kristensen NP, Scoble MJ, Karsholt O. Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa. 2007;1668:699–747. [Google Scholar]
- Kurz MA, Kurz ME. 2000. 2011. Naturkundliches Informationssystem. Available at < http://www.nkis.info/index.html>.
- Lafontaine JD, Fibiger M. Revised higher classification of the Noctuoidea (Lepidoptera). Can. Entomol. 2006;138:610–635. [Google Scholar]
- Landry J-F. Taxonomic review of the leek moth genus Acrolepiosis (Lepidoptera: Acrolepiidae) in North America. Can. Entomol. 2007;139:319–353. [Google Scholar]
- Lee S, Hodges RW, Brown RL. Checklist of Gelechiidae (Lepidoptera) in America north of Mexico. Zootaxa. 2009;2231:1–39. [Google Scholar]
- Lee SY, Park H, Boo KS, Park K-T, Cho S. Molecular identification of Adoxophyes honmai (Yasuda) (Lepidoptera: Tortricidae) based on mitochondrial COI gene sequences. Mol. Cells. 2005;19:391–397. [PubMed] [Google Scholar]
- Lim H, Park KC, Baker TC, Greenfield MD. Perception of conspecific female pheromone stimulates female calling in an arctiid moth, Utetheisa ornatrix. J. Chem. Ecol. 2007;33:1257–1271. doi: 10.1007/s10886-007-9291-4. [DOI] [PubMed] [Google Scholar]
- Lindström J, Kaila L, Niemalä P. Polyphagy and adult body size in geometrid moths. Oecologia. 1994;98:130–132. doi: 10.1007/BF00341463. [DOI] [PubMed] [Google Scholar]
- Line L. 2007. Moths of Maryland. Available at < http://www.marylandmoths.com>.
- Lloyd JE. Sexual selection in luminescent beetles. In: Blum MS, Blum NA, editors. Sexual selection and reproductive competition in insects. New York: Academic Press; 1979. pp. 293–342. [Google Scholar]
- Loudon C, Koehl MAR. Sniffing by a silkworm moth: wing fanning enhances air penetration through and pheromone interception by antennae. J. Exp. Biol. 2000;203:2977–2990. doi: 10.1242/jeb.203.19.2977. [DOI] [PubMed] [Google Scholar]
- Löfstedt C, Herrebout WM. Sex pheromones of three small ermine moths found on the European spindle tree. Entomol. Exp. Appl. 1988;46:29–38. [Google Scholar]
- Löfstedt C, Herrebout WM, Menken SBJ. Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae) Chemoecology. 1991;2:20–28. [Google Scholar]
- Maddison WP, Maddison DR. 2010. Mesquite: a modular system for evolutionary analysis. Ver. 2.73. Available at http://mesquiteproject.org.
- Mankin RW, Mayer MS. The insect antenna is not a molecular sieve. Experientia. 1984;40:1251–1252. [Google Scholar]
- Martins EP, Hansen TF. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. [Google Scholar]
- Martins EP. Phylogenies, spatial autoregression, and the comparative method: a computer simulation test. Evolution. 1996;50:1750–1765. doi: 10.1111/j.1558-5646.1996.tb03562.x. [DOI] [PubMed] [Google Scholar]
- Martins EP. COMPARE, version 4.6b. Computer programs for the statistical analysis of comparative data. Bloomington. Available at: Department of Biology, Indiana University; 2004. http://compare.bio.indiana.edu/ [Google Scholar]
- McNeil JN. Behavioral ecology of pheromone-mediated communication in moths and its importance in the use of pheromone traps. Ann. Rev. Entomol. 1991;36:407–430. [Google Scholar]
- Medvedev GS, editor. Keys to the insects of the European part of the USSR. Vol. 4. Leiden: E. J. Brill; 1990. [Google Scholar]
- Meijerman L, Ulenberg SA. 2000. Arthropods of economic importance: Eurasian Tortricidae. Available at < http://nlbif.eti.uva.nl/bis/tortricidae.php>.
- Melifronides ID, Zyngas JP, Markoullis G. Control of the spiny bollworm (Earias insulana [Boisd.]) in Cyprus. EPPO Bull. 1978;8:37–41. [Google Scholar]
- Menzel CM, Waite GK. Litchi and longan: botany, production and uses. Wallingford, U.K: CABI Press; 2005. [Google Scholar]
- Miller JC, Hammond PC. Macromoths of northwest forests and woodlands. United States Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia. FHTET-98-18. Jamestown, ND. Available at <: Northern Prairte Wildlife Research Center Online; 2000. http://www.npwrc.usgs.gov/resource/insects/macronw/index.htm>. [Google Scholar]
- Miller WE. Wing measure as a size index in Lepidoptera: the family Olethreutidae. Ann. Entomol. Soc. Am. 1977;70:253–256. [Google Scholar]
- Miller WE. Body weight as related to wing measure in hawkmoths (Sphingidae) J. Lepid. Soc. 1997;51:91–92. [Google Scholar]
- Minet J. Tentative reconstruction of the ditrysian phylogeny (Lepidoptera: Glossata). Entomol. Scand. 1991;22:69–95. [Google Scholar]
- Mitchell A, Mitter C, Regier JC. Systematics and evolution of the cutworm moths (Lepidoptera: Noctuidae): evidence from two protein-coding nuclear genes. Syst. Entomol. 2006;31:21–46. [Google Scholar]
- Mohn DL. 1993. 2005. The Critters Page. Moths great and small. Available at < http://ccs-hk.org/DM/butterfly/moths.html>.
- Mulder PG, Grantham RA. The pecan nut casebearer. Oklahoma: Oklahoma State University; 2003. Oklahoma Cooperative Extensions Service Publication 7189. [Google Scholar]
- Nagata K, Tamaki Y, Noguchi H, Yushima T. Changes in sex pheromone activity in adult females of the smaller tea tortrix moth Adoxophyes fasciata. J. Insect Physiol. 1972;18:339–346. [Google Scholar]
- Naka H, Kobayashi N, Tsuchida K, Sakurai H. A method for rearing the persimmon fruit moth, Stathmopoda masinissa (Lepidoptera: Stathmopodidae) using cultured tip tissue of the Japanese persimmon, Diospyros kaki. Jap. J. Appl. Entomol. Zool. 1998;42:221–226. [Google Scholar]
- National Museums Northern Ireland. 2009. 2011. Habitas. Available at < http://www.habitas.org.uk>.
- National Taiwan University Insect Museum Digital Archives Project. 2011. Milionia basalis pryeri. Available at < http://www.imdap.entomol.ntu.edu.tw/SelectedDA/sda-8.html>.
- Natural Resources Canada. 2009. Biology of the white-marked tussock moth (Orgyia leucostigma). Canadian Forest Service Insect Production Services. Available at < http://insect.glfc.cfs.nrcan.gc.ca/files/562.pdf>.
- Norfolk Moths. 2011. The macro and micro moths of Norfolk. Available at < http://www.norfolkmoths.co.uk/>.
- North American Moth Photographers Group. 2007. Digital guide. Available at < http://mothphotographersgroup.msstate.edu>.
- Oehlke W. 2011. Manduca sexta sexta. Sphingidae of the Americas. Available at < http://www.silkmoths.bizland.com/msextsex.htm>.
- Ohno S, Hoshizaki S, Ishikawa Y, Tatsuki S, Akimoto S. Allometry of the male genitalia in a lepidopteran species, Ostrinia latipennis (Lepidoptera: Crambidae) Appl. Entomol. Zool. 2003;38:313–319. [Google Scholar]
- O’Connell RJ, Grant AJ, Mayer MS, Mankin RW. Morphological correlates of differences in pheromone sensitivity in insect sensilla. Science. 1983;220:1408–1410. doi: 10.1126/science.220.4604.1408. [DOI] [PubMed] [Google Scholar]
- Papillons de Poitou-Charentes. 2011. . Available at < http://www.papillon-poitou-charentes.org>.
- Pashley DP. Biosystematic study in Tortricidae (Lepidoptera), with a note on evolutionary rates of allozymes. Ann. Entomol. Soc. Am. 1983;76:139–148. [Google Scholar]
- Pest and Diseases Image Library. 2011. . Available at < http://www.padil.gov.au>.
- Phelan PL. Evolution of sex pheromones and the role of asymmetric tracking. In: Roitberg RD, Isman MB, editors. Insect chemical ecology. New York, NY: Chapman and Hall; 1992. pp. 265–314. [Google Scholar]
- Phillips C. 1992. Gum-leaf skeletonizer—Uraba lugens. Government of South Australia, primary industries and resources SA. Available at < http://www.pir.sa.gov.au/_data/assets/pdf_file/0019/32923/Number_16_Gum-leaf_Skeletonizer.pdf>.
- Pittaway AR, Kitching IJ. 2000. 2011. Sphingidae of the Eastern Palaearctic. Available at < http://tpittaway.tripod.com/china/china.htm>.
- Quero C, Bau J, Guerrero A, Breuer M, De Loof A, Kontzog HG, Camps F. Sex pheromone of the oak processionary moth Thaumetopoea processionea. Identification and biological activity. J. Agric. Food Chem. 2003;51:2987–2991. doi: 10.1021/jf021035g. [DOI] [PubMed] [Google Scholar]
- Raina AK, Klun JA, Stadelbacher EA. Diel periodicity and effect of age and mating on female sex pheromone titer in Heliothis zea (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am. 1986;79:128–131. [Google Scholar]
- Ramel A. 2011. Les insectes. Available at < http://aramel.free.fr/>.
- Rauf A. Overview of the cocoa pod borer Conopomorpha cramerella (Snellen) (Lepidoptera: Gracillariidae) Southeast Asia Regional IPM Network. 2008 [Google Scholar]
- Rauscher S, Arn H, Guerin P. Effects of dodecyl acetate and Z-10-tridecenyl acetate on attraction of Eupoecilia ambiguella males to the main sex pheromone component, Z-9-dodecenyl acetate. J. Chem. Ecol. 1984;10:253–264. doi: 10.1007/BF00987853. [DOI] [PubMed] [Google Scholar]
- Regier JC, Cook CP, Mitter C, Hussey A. A phylogenetic study of the ‘bombycoid complex’ (Lepidoptera) using five protein-coding nuclear genes, with comments on the problem of macrolepidopteran phylogeny. Syst. Entomol. 2008;33:175–189. [Google Scholar]
- Regnier FE, Law JH. Insect pheromones. J. Lipid Res. 1968;9:541–551. [PubMed] [Google Scholar]
- Renou M, Descoins C, Priesner E, Gallois M, Lettere M. A study of the sex pheromone of the leek moth, Acrolepiopsis assectella (Lepidoptera: Acrolepiidae) Entomol. Exp. Appl. 1981;29:198–208. [Google Scholar]
- Roelofs WL, Brown RL. Pheromones and evolutionary relationships of Tortricidae. Annu. Rev. Ecol. Syst. 1982;13:395–422. [Google Scholar]
- Russell IPM. 2011. Tuta absoluta. Available at < http://www.russellipm-agriculture.com/insect.php?insect_id=125&lang=en>.
- Ruther J, Matschke M, Garbe L-A, Steiner S. Quantity matters: male sex pheromone signals mate quality in the parasitic wasp Nasonia vitripennis. Proc. R. Soc. Lond. B. 2009;276:3303–3310. doi: 10.1098/rspb.2009.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutowski RL. Mate choice and lepidopteran mating behaviour. Fla. Entomol. 1984;65:72–82. [Google Scholar]
- Ryan MF. Insect chemoreception: fundamental and applied. New York, NY: Kluwer; 1992. [Google Scholar]
- Safonkin AF, Triseleva TA. Pheromone composition, the mtDNA COI locus variation, and phylogenetic relationships in leafrollers of the tribe Archipini (Lepidoptera: Tortricidae) Russ. J. Genet. 2008;44:413–417. [PubMed] [Google Scholar]
- Salas-Reyes VA. 1985. Studies of the sex pheromone of a blueberry leafroller (Cheimophila salicella (Hubner)) and on the synthesis of chiral pheromones from carbohydrates. PhD diss, Simon Fraser University, Burnaby, Canada.
- Sanders CJ, Lucuik GS. Factors affecting calling by female eastern spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae) Can. Entomol. 1972;104:1751–1762. [Google Scholar]
- Sane SP, Dieudonné A, Willis MA, Daniel TL. Antennal mechanosensors mediate flight control in moths. Science. 2007;315:863–866. doi: 10.1126/science.1133598. [DOI] [PubMed] [Google Scholar]
- Savela M. 2011. Finnish Lepidoptera. Available at < http://www.funet.fi/pub/sci/bio/life/intro.html>.
- Schal C, Charlton RE, Cardé RT. Temporal patterns of sex pheromone titers and release rates in Holomelina lamae (Lepidoptera: Arctiidae) J. Chem. Ecol. 1987;13:1115–1129. doi: 10.1007/BF01020542. [DOI] [PubMed] [Google Scholar]
- Schneider RWS, Lanzen J, Moore PA. Boundary-layer effect on chemical signal movement near the antennae of the sphinx moth, Manduca sexta: temporal filters for olfaction. J. Comp. Physiol. A. 1997;182:287–298. [Google Scholar]
- Scott DL. 2001. 2011. Lynn Scott's Lepidoptera Images. Available at < http://www.acleris.com/dls/tn-mothindex.html>.
- Shirai Y. Factors influencing flight ability of male adults of the diamondback moth, Plutella xylostella, with special reference to temperature conditions during the larval stage. Appl. Entomol. Zool. 1993;28:291–301. [Google Scholar]
- Simmons RB, Pogue MG. Redescription of two often-confused noctuid pests, Copitarsia decolora and Copitarsia incommode (Lepidoptera: Noctuidae: Cuculliinae) An. Entomol. Soc. Am. 2004;97:1159–1164. [Google Scholar]
- Simonsen TJ. Phylogeny of the cactus-feeding phycitines and their relatives (Lepidoptera, Pyralidae) based on adult morphology: evaluation of adult character-systems in phycitine systematics and evidence for a single origin of Cactaceae-feeding larvae. Insect Syst. Evol. 2008;39:303–325. [Google Scholar]
- Skou P. The Geometroid moths of North Europe (Lepidoptera, Drepanidae and Geometridae). Entomonograph. Vol. 6. Copenhagen: E.J. Brill/Scandinavian Science Press; 1986. [Google Scholar]
- Solis MA. Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera) Rev. Colomb. Entomol. 2007;33:1–9. [Google Scholar]
- Spaethe J, Brockmann A, Halbig C, Tautz J. Size determines antennal sensitivity and behavioural threshold to odors in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Steinbrecht RA. Structure and function of insect olfactory sensilla. In: Bock GR, Cardew G, editors. Olfaction in mosquito-host interactions. CIBA Foundation Symposium 2000. London, U.K: Wiley; 1996. pp. 158–177. [DOI] [PubMed] [Google Scholar]
- Stichting TINEA. 2011. Kleine vinders. Available at < http://www.kleinevlinders.nl>.
- Strauss RE. Patterns of quantitative variation in lepidopteran wing morphology: the convergent groups Heliconiinae and Ithomiinae (Papilionoidea: Nymphalidae) Evolution. 1990;44:86–103. doi: 10.1111/j.1558-5646.1990.tb04281.x. [DOI] [PubMed] [Google Scholar]
- Summerville KS, Wilson TD, Veech JA, Crist TO. Do body size and diet breadth affect partitioning of species diversity? A test with forest Lepidoptera. Diversity Distrib. 2006;12:91–99. [Google Scholar]
- Svensson M. Sexual selection in moths: the role of chemical communication. Biol. Rev. 1996;71:113–135. [Google Scholar]
- Tamaki Y. Sex pheromones. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Vol. 9. New York, NY: Pergamon Press; 1985. pp. 145–191. [Google Scholar]
- The Virginia Fruit Page. 2011. . Available at < http://www.virginiafruit.ento.vt.edu/>.
- Trematerra P, Brown JW. Argentine Argyrotaenia (Lepidoptera: Tortricidae): Synopsis and descriptions of two new species. Zootaxa. 2004;574:1–12. [Google Scholar]
- Tumlison R, Benjamin K. Henderson State University Department of Biology; 2011. Moths of Arkansas. Available at < http://www.hsu.edu/interior2.aspx?id=13240>. [Google Scholar]
- Turgeon JJ, McNeil JN, Roelofs WL. Responsiveness of Pseudaletia unipuncta males to female sex pheromone. Physiol. Entomol. 1983;8:339–344. [Google Scholar]
- Tuskes PM, Tuttle JP, Collins MM. The wild silk moths of North America: a natural history of the Saturniidae of the United States and Canada. Ithaca, NY: Cornell Univ Press; 1996. [Google Scholar]
- Tóth M, Szöcs G, Majoros B, Bellas TE, Novak L. Experiments with a two-component sex attractant of the silver Y moth (Autographa gamma L), and some evidence for the presence of both components in natural female sex pheromone. J. Chem. Ecol. 1983;9:1317–1325. doi: 10.1007/BF00994800. [DOI] [PubMed] [Google Scholar]
- Moths UK. 2011. Your guide to the moths of Great Britain and Ireland. Available at < http://ukmoths.org.uk/>.
- United States Department of Agriculture. Riverdale, Maryland. Available at <: Animal Plant Health Inspection Service, Plant Protection and Quarantine, Emergency and Domestic Programs; 2010. New pest response guidelines: false codling moth Thaumatotibia leucotreta. http://www.aphis.usda.gov/import_export/plants/manuals/online_manuals.shtml>. [Google Scholar]
- United States Department of Agriculture. 2011. Pecan nut casebearer (Acrobasis nuxvorella Neunzig). Pecan Breeding Program, Aggie Horticulture. Available at < http://aggie-horticulture.tamu.edu/CARYA/Manual/PNCproc.html>.
- Venette RC, Davis EE, Heisler H, Larson M. Mini risk assessment: Silver Y mothAutographa gramma(L.) (Lepidoptera: Noctuidae) United States Department of Agriculture; 2003. . Available at < http://www.aphis.usda.gov/plant_health/plant_pest_info/pest_detection/downloads/pra/agammapra.pdf>. [Google Scholar]
- Vickers NJ. Winging it: moth flight behaviour and responses of olfactory neurons are shaped by pheromone plume dynamics. Chem. Senses. 2006;31:155–166. doi: 10.1093/chemse/bjj011. [DOI] [PubMed] [Google Scholar]
- Waller JM, Bigger M, Hillocks RJ. Coffee pests, diseases and their management. Wallingford, U.K: CABI Publishing; 2007. [Google Scholar]
- Watson L, Dallwitz MJ. 2003. 2011. British insects: the families of Lepidoptera. Available at < http://delta-intkey.com>.
- Wcislo WT. Sensilla numbers and antennal morphology of parasitic and non-parasitic bees (Hymenoptera: Apoidea) Int. J. Insect Morphol. Embryol. 1995;24:63–81. [Google Scholar]
- Western Australian Department of Agriculture. 2011. Entomology. Available at < http://agspsrv34.agric.wa.gov.au/ento/>.
- White EP, Ernest SKM, Kerkhoff AJ, Enquist BJ. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007;22:323–330. doi: 10.1016/j.tree.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Whittle CP, Bellas TE, Horak M, Pinese B. The sex pheromone and taxonomic status of Homona spargotis Meyrick sp. rev., an Australian pest species of the coffearia group (Lepidoptera: Tortricidiae: Tortricinae) J. Aust. Entomol. Soc. 1987;26:169–179. [Google Scholar]
- Williams RN, Fickle DS, Welty C, Ellis M. Insects and mite pests of grapes in Ohio and the Midwest. Grape IPM Team. Ohio State University; 2011. . Available at < http://www.oardc.ohio-state.edu/grapeipm/index.htm>. [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour. Cambridge, U.K: Cambridge Univ. Press; 2003. [Google Scholar]
- Yamamoto S, Sota T. Phylogeny of the Geometridae and the evolution of winter moths inferred from a simultaneous analysis of mitochondrial and nuclear genes. Mol. Phyl. Evol. 2007;44:711–723. doi: 10.1016/j.ympev.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Young CJ. Characterisation of the Australian Nacophorini using adult morphology, and phylogeny of the Geometridae based on morphological characters. Zootaxa. 2008;1736:1–141. [Google Scholar]
- Zhang A, Polavarapu S. Sex pheromone of the blueberry leafminer, Caloptilia porphyretica. J. Chem. Ecol. 2004;30:1513–1527. doi: 10.1023/b:joec.0000042066.77560.59. [DOI] [PubMed] [Google Scholar]


