Abstract
We report that IL-17 significantly increases the secretion of CXCL1 and CXCL5 from mammary carcinoma cells, which is downregulated by TGF-β through the type II TGF-β receptor (TβRII). Carcinoma cells with conditional knockout of TβRII (Tgfbr2KO) have enhanced sensitivity to IL-17a in the stimulation of chemokine secretion. During polyoma middle T (PyMT) induced tumor progression, levels of Th17 inducing cytokines TGF-β, IL-6, IL-23 were increased in PyMT/Tgfbr2KO tumors, which was associated with an increased number of Th17 cells. IL-17 increased the suppressive function of MDSCs on T cells through the upregulation of Arg, IDO, and COX2. Treatment of PyMT/Tgfbr2KO mice with anti-IL-17 Ab decreased carcinoma growth and metastatic burden. Analysis of human breast cancer transcriptome databases showed a strong association between IL-17 gene expression and poor outcome in lymph node positive, estrogen receptor negative or luminal B subtypes suggesting potential therapeutic approaches.
Introduction
TGF-β plays a major role in the regulation of tumor initiation, progression, and metastasis and requires the type II TGF-β receptor (TβRII) for signaling. It has been shown that decreased expression of TβRII correlated with an increased risk of developing invasive breast cancer (1) and loss of TβRII correlated with high-grade human carcinoma in situ and invasive breast cancer (2). In our laboratory, we have shown that conditional deletion of TβRII in mammary epithelial cells that also express the PyMT oncogene under control of the MMTV promoter resulted in shortened tumor latency and a five-fold increase in lung metastases compared to PyMT tumors with intact TGF-β signaling (3, 4). We identified that TGF-β signaling mediates intrinsic, stromal-epithelial, and host-tumor interactions during breast cancer progression by regulation basal CXCL1, CXCL5 (CXCL1/5), and CCL20 chemokine expression (5).
IL-17 is a cytokine secreted by CD4 and CD8 cells (6–8). The differentiation and regulation of murine Th17 cells has been extensively studied in the past few years and TGF-β, IL-6, and IL-23 have been implicated as critical regulators of the initiation of mouse Th17 cell differentiation (9, 10). Although the function of IL-17 is not fully understood, it is clear that IL-17 amplifies the immune response by inducing the expression of other chemokines, inflammatory cell-surface markers, and inflammatory mediators (11, 12). IL-17-producing cells are detected in cancer patients and tumor-bearing mice (13, 14). Some reports indicate that tumor growth is increased in IL-17−/− mice (15, 16). However, a study by Wang et al indicated that tumor growth is suppressed in IL-17−/− mice (17). Recently, another study has shown that neutralization of IL-17 stunted tumor growth and systemic administration of IL-17 promoted tumor growth. Additional analysis indicated that IL-17 was required for the development and tumor-promoting activity of MDSCs in tumor-bearing mice (18).
In the current study, we examined the indirect role of impaired TGF-β signaling in carcinoma cells on tumor growth. In TβRII knockout tumor cells, we determined basal and IL-17 stimulated secretion of CXCL1/5 and expression of IL-17R. We analyzed the mechanisms that are involved in Th17 differentiation in mice and determined the role of IL-17 in the regulation of suppressive function of MDSCs and macrophages. By using anti-IL-17 Ab in vivo we demonstrated a significant indirect role of impaired TGF-β signaling in carcinoma progression by enhanced Th17 response.
Results
The Expansion of MDSCs During Mammary Tumor Growth in Mice with Deleted TβRII
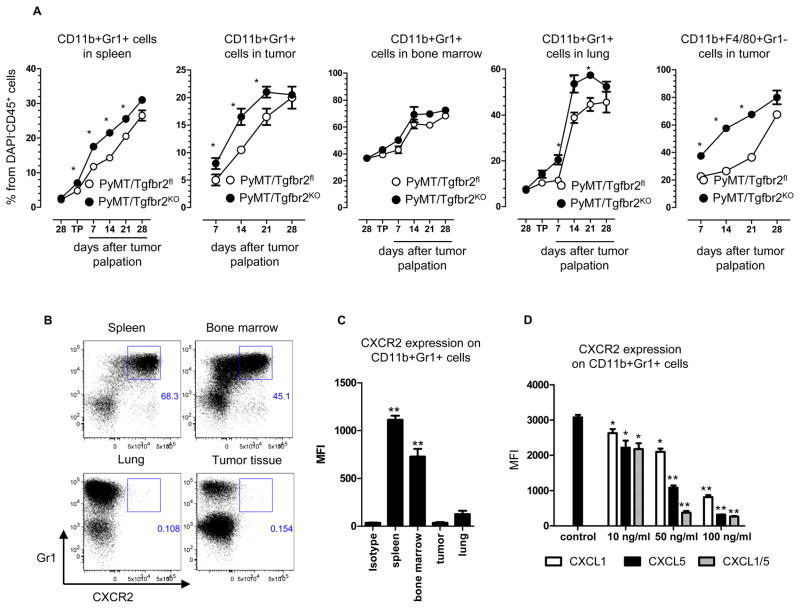
We have previously shown that conditional knockout of TβRII in mammary epithelial cells of MMTV-PyMT mice results in shortened tumor latency and an increased number of metastasis in the lung (3, 4). Additionally, we reported that one week after tumor palpation the number of CD11b+Gr1+ cells (MDSCs) increased in tumor tissue in PyMT/Tgfbr2KO mice. To examine the role of MDSCs in TGF-β mediated tumor progression, tumor tissue, lung, bone marrow, and spleen were collected before tumor progression, on the day of tumor palpation, and 1, 2, 3, and 4 weeks after tumor palpation in PyMT/Tgfbr2fl mice and mice without TβRII in the mammary epithelium. We observed a significant increase of CD11b+Gr1+ cells in spleen and in tumor tissue, at each time point in the PyMT/Tgfbr2KO mice (Figures 1A, Figure S1) except at a late stage of tumor progression – day 28. In the lungs of these tumor-bearing animals we found increased numbers of these cells on days 7, 14, and 21 of tumor progression in PyMT/Tgfbr2KO mice. No differences were found in the bone marrow of these mice. In parallel with the increased number of CD11b+Gr1+ cells, we observed a significant increase of tumor associated macrophages (TAM) in the PyMT/Tgfbr2KO mice (Figures 1A, Figure S1). Localization of CD11b+Gr1+ cells in the tumor tissue was similar to our previously published studies (19) (data not shown). Surprisingly, we found that CD11b+Gr1+ cells are negative for CXCR2 in lung and tumor tissues, but positive in the spleen and bone marrow (Figures 1B, C). We have previously shown that SDF-1/CXCR4 and CXCL5/CXCR2 are involved in the recruitment of MDSCs into tumors of PyMT/Tgfbr2KO mice (19). Therefore, we concentrated our studies on mechanisms driving the migration of MDSCs to tumor tissue, which are recruited by CXCL1/5.
Figure 1. Expansion and phenotype of myeloid cells in tumor-bearing mice.
Quantitative data for the presence of TAMs (CD11b+F4/80+Gr1−) and MDSCs (CD11b+Gr1+) and in spleen, lung, bone marrow, tumor (A). “28” – 28 days of age, “TP” – day when tumor was palpated. Five mice per group were analyzed. * -- p<0.05 (B) Flow cytometry analysis of CXCR2 expression on CD11b+Gr1+ cells in Py-MT/Tgfbr2fl mice and quantitative data (C) at 1 week after tumor palpation. Plots are gated as CD45+CD11b+DAPI− cells. (D) Geomean fluorescence intensity (MFI) of CXCR2-APC on CD11b+Gr1+ cells. Gr1+ cells were isolated by Gr1 magnetic microbeads from spleen of PyMT/Tgfbr2KO mice at 28th day of tumor progression. 5×105 cells were incubated 24 hr with different concentration of CXCL chemokines. Five mice per group were analyzed.
TGF-β regulates IL-17-stimulated secretion of CXCL1/5in carcinoma cells
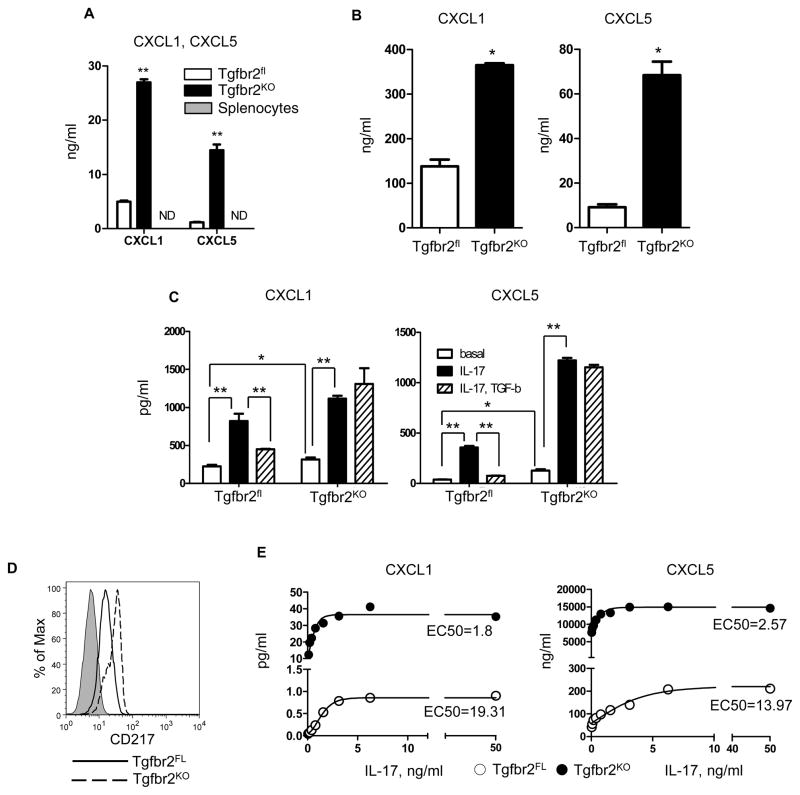
CXCL1/5 are the primary chemokines that signal through CXCR2 to induce myeloid cell chemotaxis. However, it is interesting that CXCR2 is not expressed in CD11b+Gr1+ cells found in the tumor tissue. To address this issue, we isolated CD11b+Gr1+ cells from the spleen of tumor-bearing mice and incubated these cells with varying concentrations of CXCL1/5. We observed a significant decrease in expression of CXCR2 starting at a concentration of 10 ng/ml. Incubation with higher concentrations of chemokines resulted in an almost complete loss of CXCR2 expression (Figure 1D). As seen previously with CXCL5(19), loss of TβRII in PyMT tumor cell lines resulted in an increased secretion of CXCL1 (Figure 2A). Moreover, the significantly high level of CXCL1 secretion indicates a potentially important role in the induction of MDSCs chemotaxis. This data is further supported by the analysis of CXCL1/5 in tumor explants in which we also found an increased level of CXCL1/5in PyMT/Tgfbr2KO mice (Figure 2B).
Figure 2. Regulation of CXCL1 and CXCL5 secretion by IL-17 and TGF-β.
(A) Carcinoma cells from established cell lines (1.5×105/ml) and splenocytes were incubated 24 hr in 3 ml of DMEM, 5% FBS. Level of chemokines was measured by ELISA in conditional medium. (B) Tumor explant was prepared as described in Material and Methods and analyzed for CXCL1and CXCL5by ELISA (R&D System). Data correspond to the mean ± SEM of three individual mice from three experiments. (C) Sorted carcinoma cells from freshly isolated tumor tissue (1.5×105/ml) were incubated 24 hr in 1 ml of DMEM, 5% FBS. Level of CXCL1/5 was measured by ELISA in conditional medium. IL-17 was used at a concentration of 10 ng/ml and TGF-β at a concentration 1 ng/ml. (D) Cultured tumor epithelial cells were stained with IL-17Ra/CD217-PE. Cells were analyzed on FACSCalibur. Representative FACS plot is showing from three experiments. (E) Cultured carcinoma cells (2.5×104 cells/ml) were incubated with IL-17a concentrations from 0 to 50 ng/ml. Data correspond to the mean from three experiments. Conditioned medium was analyzed by ELISA (R&D System), EC50 was analyzed by GraphPad Prizm software. * - p<0.05, ** - p<0.01. PyMT/Tgfbr2fl is Tgfbr2FL, PyMT/Tgfbr2KO is Tgfbr2KO. Data correspond to the mean ± SEM.
We observed a strong effect of IL-17 in promoting increased secretion of CXCL1/5 from carcinoma cells and this effect depended on TβRII. Carcinoma cells (Ep-CAM+CD45−) were sorted from primary tumor tissue of PyMT/Tgfbr2fl and PyMT/Tgfbr2KO mice (Figure S2) and incubated in the presence of IL-17 with or without TGF-β. Reinforcing previous work done using cultured cell lines, these sorted epithelial cells displayed the same profile of CXCL1/5 chemokine expression (Figure 2C). Incubation with IL-17 increased secretion of CXCL1/5 from both types of tumor cells with a more intense increase in CXCL5. Importantly, we observed that TGF-β completely inhibits IL-17 stimulated secretion of CXCL1/5 from PyMT/Tgfbr2fl cells. The data indicate that loss of TGF-β signaling in tumor cells results in a subsequent increase in CXCL1/5 production and acts to exacerbate further induction of these chemokines by other factors by eliminating TGF-β’s regulatory role over their production. Thus, in vivo levels of CXCL1/5 could far exceed those measured ex vivo and lends credence to the increased infiltration of CD11b+Gr1+ cells into tumors with abrogated TGF-β signaling.
IL-17R is the primary receptor for IL-17 signaling (7) and because of the more robust response of PyMT/Tgfbr2KO cells to IL-17 stimulation, alterations in IL-17R expression (CD217) due to loss of TβRII expression were examined. Increased expression of CD217 was observed on PyMT/Tgfbr2KO cells (Figure 2D). After incubating cells with varying concentrations of IL-17, we found that PyMT/Tgfbr2KO carcinoma cells are more sensitive to IL-17, resulting in enhanced secretion of CXCL1/5 (Figure 2E). Additional analysis showed that PyMT/Tgfbr2KO cells are four times more sensitive to low concentrations of IL-17(50 pg/ml)in stimulation of CXCL1/5secretion(data not shown). Deletion of TβRII on carcinoma cells displayed a double effect on CXCL1/5 secretion. First, deletion of TβRII increased expression of CD217 and as a consequence sensitized carcinoma cells to IL-17, and second, deletion of TβRII prevented inhibition by TGF-β of IL-17 stimulated secretion of chemokines.
Deletion of TβRII in carcinoma cells increases the number of Th17 cells
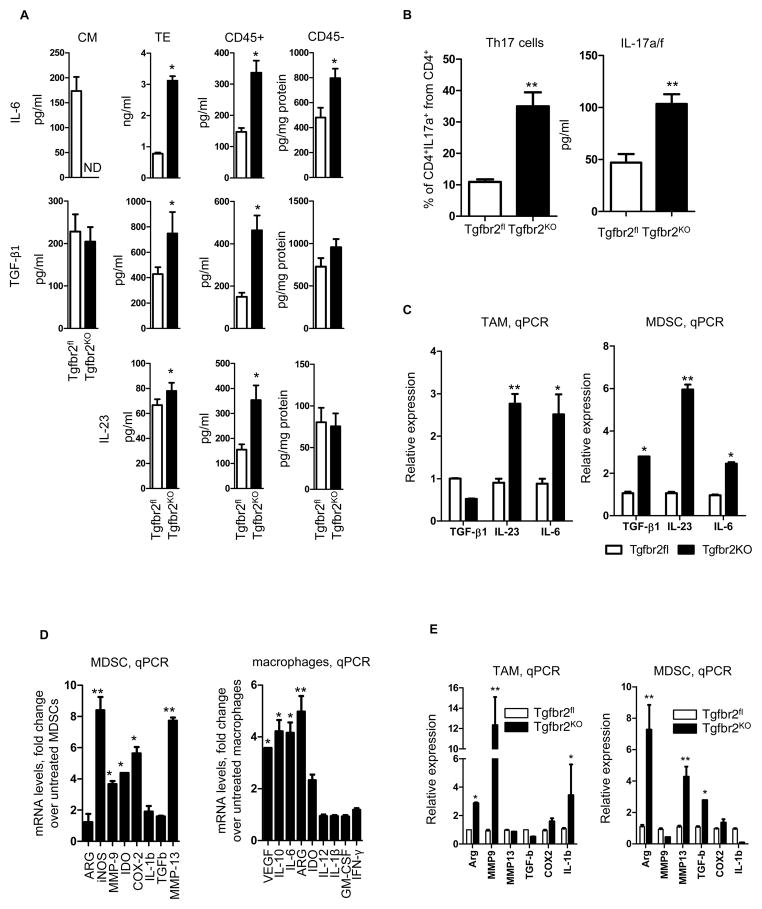
The presence of IL-6, TGF-β, and IL-23 preferentially promotes differentiation of CD4+ T cells into CD4+Th17 cells (9). Tumor tissues from PyMT/Tgfbr2KO mice were characterized by more myeloid cell infiltration, which acts as an additional source of IL-6, TGF-β, and IL-23. Thus, we hypothesized that the PyMT/Tgfbr2KO tumors might be creating a cytokine milieu that promotes differentiation of CD4+T cells into Th17 cells. To identify the primary source of TGF-β, IL-6, and IL-23, the presence of these cytokines in conditioned media of cultured tumor cells, tumor explants, isolated immune cells (CD45+), and non-immune cells (CD45−) from tumor tissue was analyzed by ELISA (Figure 3A). It was observed that PyMT/Tgfbr2KO carcinoma cells do not secrete significant levels of IL-6. However, tumor explants, CD45+, and CD45− cells from tumor tissue have an increased level of IL-6 in PyMT/Tgfbr2KO mice. Basal secretion of TGF-β by cultured carcinoma cells doesn’t depend on TβRII expression, and the level of TGF-β in PyMT/Tgfbr2KO cells was the same as in cells with intact TGF-β signaling. Levels of TGF-β in tumor explants and in conditioned media of CD45+ cells showed the same pattern as IL-6, with increased expression in PyMT/Tgbfr2KO mice, but no differences in secretion by CD45− cells. Neither carcinoma cell type secreted significant levels of IL-23. Also, there were no significant differences in the concentration of IL-23 in conditioned medium from CD45− cells. However, tumor explant and CD45+ cells from tumor tissue have increased levels of IL-23 in the PyMT/Tgfbr2KO mice. Thus, we demonstrate that the primary source of the cytokines necessary for development of Th17 cells is immune cells (CD45+) that have migrated into the tumor tissue. Also, expression of IL-6, TGF-β, and IL-23 (p19) was examined by real-time PCR in PyMT/Tgbr2fl vs PyMT/Tgfbr2KO tumors using freshly resected and PBS perfused tissues (to avoid contamination from circulating cells) and these data correlated with our data shown in Figure 3E (Figure S3A).
Figure 3. Epithelial loss of TβRII results in increased Th17 cell development.
(A) Data from ELISA analysis of IL-6, IL-23 and TGF-β1. Cultured carcinoma cells (1×105/ml) were incubated for 24hr in DMEM + 5% FBS. Tumor explants were prepared as described in Materials and Methods. CD45+ and CD45− cell were isolated by CD45 magnetic microbeads and incubated 24hr in 1×106/ml in RPMI + 10% FBS. CM – conditional medium of carcinoma cells, TE – tumor explant. (B) Quantitative data of CD4+ T cells isolated from tumor tissue of PyMT/Tgfbr2fl and PyMT/Tgfbr2KO mice on day 9 of tumor progression. Before flow cytometry analysis cells were incubated 6 hr with CD3/CD28 beads (Left). Isolated CD4+ cells from tumor tissue of PyMT/Tgfbr2fl and PyMT/Tgbfr2KO mice were incubated for 24 hr with CD3/CD28 microbeads and then the levels of IL-17a/f in conditional medium was measured by ELISA following the manufacturer’s protocol (right). Data correspond to the mean ± SEM of three individual mice from three experiments. (C) qPCR of TGF-β1, IL-23 (p19) and IL-6 from sorted TAMs (left) and MDSCs (right) from tumor tissue of PyMT/Tgfbr2fl and PyMT/Tgfbr2KO mice on day 9 of tumor progression. Cells were sorted by FACSAria. (D) MDSCs (CD11b+Gr1+) were isolated from spleen of MMTV-PyMT FVB tumor-bearing mice by magnetic microbeads (Gr1) and then incubated for 24hr with IL-17 (10ng/ml), then total RNA was isolated and expression of genes was analyzed by qRT-PCR. The fold change in expression by treated vs. untreated cells is shown (left). Monocytes were differentiated from bone marrow of naive FVB mice in presence of M-CSF (10 ng/ml). Suspension cells were removed and attached cells were harvested by trypsin and analyzed. One portion of cells was analyzed by flow cytometry using the CD11b marker. The remaining cells were incubated 24hr with IL-17 (10ng/ml) and then total RNA was isolated and expression of genes was analyzed by qRT-PCR. The fold change in expression by treatment over untreated cells is shown (right). Data correspond to the mean ± SEM of three individual mice from two experiments. (E) CD11b+F4/80+Gr1− cells (TAMs) and CD11b+Gr1+ cells (MDSCs) were sorted by FACSAria from tumor tissue of PyMT/Tgfbr2fl and PyMT/Tgfbr2KO mice on day 9th of tumor progression. Total RNA was isolated immediately after sorting and expression of genes was analyzed by qRT-PCR. Data correspond to the mean ± SEM of two to three individual mice from three experiments. PyMT/Tgfbr2fl is Tgfbr2FL, PyMT/Tgfbr2KO is Tgfbr2KO, ND - not detected. * - p<0.05, ** -p<0.01.
Additionally, we sorted MDSCs and TAMs from tumor tissue (Figure S3B) and analyzed the expression of the IL-6, IL-23 (p19), and TGF-β genes by RT-PCR. Previously, it has been shown that MDSCs are a major source of TGF-β in PyMT/Tgfbr2KO tumors (19). Analysis of MDSCs from tumor tissue recapitulated these findings (Figure 3B), but it was also observed that MDSCs have increased expression of IL-23(p19) and IL-6 in PyMT/Tgfbr2KO. TAMs from PyMT/Tgbr2KO tumors have similar patterns of gene expression except for TGF-β. As a result, it was determined that two sources for increased expression of TGF-β, IL-6, and IL-23exist. First, secretion of these cytokines is increased in PyMT/Tgfbr2KO tumors mediated by increased immune cell infiltration. Migration of immune cells to tumor tissue with deleted TβRII is increased as a consequence of enhanced basal secretion of CXCL1/5. Second, the PyMT/Tgfbr2KO tumor microenvironment up-regulates gene expression of TGF-β, IL-6, and IL-23 in myeloid cells.
Quantification of Th17 cells in the tumor microenvironment was performed using both flow cytometry for cellular identification and ELISA analysis of Th17 specific cytokine production. A significant increase in the number of CD4+IL-17a+ cells in PyMT/Tgfbr2KO mice was observed (Figure S3C). Similar results were obtained using another tumor model, 4T1 mammary carcinoma cells, which produce more CXCL1/5 than PyMT/Tgfbr2KO carcinoma cells (Figure S4). Quantitative analysis of the proportion of CD4+IL17a+ cells to the total number of CD4+ cells showed that PyMT/Tgfbr2KO tumors contain on average 3.5 times more Th17 cells and also CD4+ T cells isolated from knockout tumors produced more IL-17s (Figure 3C).
IL-17 increases the pro-tumorigenic properties of MDSCs and monocytes
Because we found that the number of Th17 cells in tumor tissue is increased in PyMT/Tgfbr2KO mice, we hypothesized that myeloid cells from tumor tissue of these mice have increased expression of pro-tumorigenic genes due to the increased presence of IL-17. First, we checked the possible role of IL-17 in functions of myeloid cells. We incubated MDSCs with IL-17 and found an up-regulation in the expression of genes that correlated with the suppressive function of MDSCs such as Arg, MMP-9, IDO, COX-2, and MMP-13 (Figure 3D). An increased suppressive function of MDSCs on T cell proliferation was observed after incubation with IL-17 as well (Figure S3D). In monocytes we found that IL-17 up-regulates gene expression corresponding to the M2 type of TAMs, such as VEGF, IL-10, IL-6, Arg, and IDO (Figure 3D). Second, we sorted MDSCs and TAMs from tumor tissue and analyzed gene expression. We found that MDSCs from PyMT/Tgfbr2KO mice have increased Arg, MMP-13, and TGF-β expression while TAMs from these mice have increased Arg, MMP-9, and IL-1β expression compared to PyMT/Tgfbr2fl mice (Figure 3E). The data suggest that increased infiltration of myeloid cells mediated by CXCL1/5 to the tumor tissue of PyMT/Tgfbr2KO mice switches anti-tumorigenic properties of these cells to pro-tumorigenic in the presence of IL-17.
Neutralization of IL-17 decrease tumor growth and number of metastasis
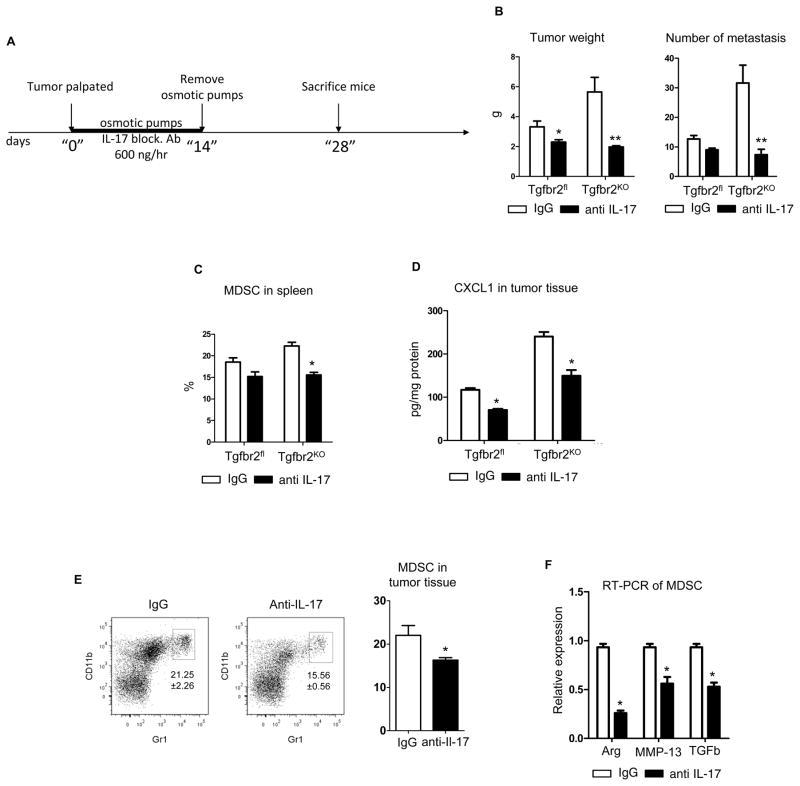
Osmotic pumps were implanted on the day of tumor palpation with anti-IL-17 Ab or with Rat IgG for the control group. After two weeks, the osmotic pumps were removed and the tumors were allowed to develop for an additional two weeks. On the 28th day after tumor palpation, analysis of tumor size, immune cell infiltration, and metastasis was performed (Figure 4). It was found that treatment with anti-IL-17 Ab decreased tumor growth (Figure 4B). The treatment effect on PyMT/Tgfbr2KO mice was more pronounced. Analysis of lung metastases showed that the number of foci in the lungs isolated from PyMT/Tgfbr2KO mice treated with IL-17 blocking Ab decreased dramatically. Flow cytometry analysis of immune cells showed a decreased number of MDSCs in the spleens of PyMT/Tgfbr2KO mice (Figure 4C). One possible explanation for the significant decrease in the MDSC population could be decreased secretion of CXL1/5 from carcinoma cells which can be stimulated by IL-17. To evaluate this hypothesis, the level of CXCL1 was measured in tumor tissue lysates and a significant decrease in CXCL1 expression in PyMT/Tgbr2KO mice after treatment with anti-IL-17 Ab was observed (Figure 4D). Flow cytometry analysis showed a decreased percentage of CD11b+Gr1+ cells in tumor tissues of PyMT/Tgfbr2KO mice (Figure 4E). In addition we sorted MDSCs from tumor tissues of PyMT/Tgfbr2KO mice and found decreased expression of the Arg, MMP-13, and TGF-β genes, which are the primary mediators of the suppressive function of MDSCs (Figure 4F). Taken together these data indicate that IL-17 plays a major role in tumor progression, through the modulation of MDSC migration and function, and the effect of IL-17 is increased in mice with deleted TβRII in carcinoma cells.
Figure 4. In vivo inhibition of IL-17 abrogates tumor promoting effect of myeloid cells.
(A) Scheme of treatment with IL-17 blocking Ab, (B) Weight of total tumor tissue isolated from all 10 mammary glands of mice (left) and number of metastasis in lung counted by whole mounted staining (right), (C) Quantitative data for the presence of CD11b+Gr1+ (MDSC) cells in spleen of PyMT/Tgfbr2fl and PyMT/Tgfbr2KO mice with IgG and Anti-IL-17 Ab treatment, (D) CXCL1 in tumor tissue lysates was measured by ELISA (R&D Systems) and then recalculated on 1 mg of protein,, (E) Representative FACS plots and quantitative data of percentage CD11b+Gr1+ cells in tumor tissue on PyMT/Tgfbr2KO mice with IgG and anti-IL-17 treatment (F) MDSCs were sorted from tumor tissue on day 28 from PyMT/Tgfbr2KO mice with treatment of IgG and anti-IL-17 Ab. After sorting total RNA was isolated and used for qRT-PCR. * - p<0.05, ** - p<0.01. Data correspond to the mean ± SEM of five individual mice from two experiments.
Importance of IL-17 in human breast cancers
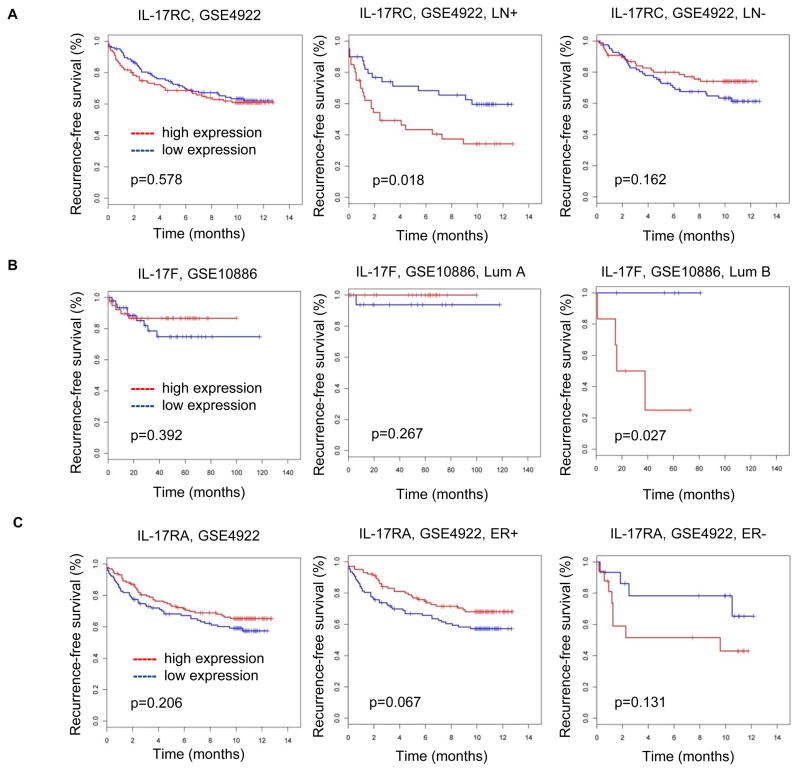
To determine the relevance of these findings to human breast cancer, we analyzed microarray profiles of human breast cancer tissues with well-documented clinical data related to lymph node (LN) involvement, ER status, and time of relapse detection over 10-year period. In a mouse model, we found that deletion of TβRII in epithelial cells correlated with increased Th17 response. Therefore we first conducted a correlation analysis between expression of genes from TGF-β signaling and genes that associated with Th17 response. We found that only two Th17 genes had a significant correlation with TGF-β superfamily genes: IL-17A and IL-17F (data not shown). A large number of genes associated with TGF-β signaling had a negative correlation with IL-17A/F (data not shown). Secondly, we analyzed the correlation between IL-17 and the CXCL1/5 genes and found a very strong positive correlation between IL-17A and CXCL1 and IL-17A and CXCL5, r=0.25, p=0.0001 and r=0.22, p=0.0007 respectively[also, there is a marginal effect between IL-17RA/CXCL1 (r=0.12, p=0.07) and IL-17RC/CXCL5 (r=0.12, p=0.07)]. These findings indicate that in human breast cancer, as well as in mouse models, decreased TGF-β signaling is correlated with increased CXCL1/5 genes and Th17 response. Next, we examined the association of Th17 genes with tumor subtype and relapse-free survival (RFS) by testing whether LN status and ER status would modify the association (Figure 5). Three genes from Th17 family had a significant interaction with RFS: IL-17RC, IL17RA, and IL-17F. IL17-RC was associated with reduced RFS in LN+ breast cancer patients. IL17F had a significant association with reduced RFS in patients with luminal B subtype breast cancer. IL17RA had marginal association with increased RFS in ER+ patients, but this association was not significant in ER− patients. RFS in ER− patients with IL17RA is opposite to ER+ cancer patients, suggesting a potential role of IL17RA to decrease RFS in ER− patients.
Figure 5. Increased expression of IL17 genes correlated with worse outcome in ER−, LN+, Lum B human breast cancers.
(A) In human LN+ breast cancer patients, the expression of IL-17RC correlated with reduced relapse free survival (RFS) in the GSE4922 data set. No significant differences in RFS was observed in correlation with the IL-17RC in LN− patients, (B) In human luminal B subtype breast cancer patients, the IL17f correlated with reduced RFS in GSE108869 data set, (C) In ER+ breast cancer patients, the IL-17RA has marginal correlation with increased RFS, but not with correlations in ER− patients.
Our data suggest that some particular genes in human breast cancer may correlate with Th17 response and associate with reduced RFS in subsets of breast cancer patients. The non-significant correlations or marginal effects may be due to the lack of study power or the heterogeneity of the human data and we conclude that further testing in larger human data sets may be necessary to confirm that our findings have a direct impact on the patient population. Collectively, our findings indicate a strategy for targeting tumor growth and tumor metastasis by disrupting Th17 cell development in selected breast cancer cases.
Discussion
An important role of MDSCs in tumor-associated immune response has been established in a large number of studies. Here, we have shown the important role of TGF-β signaling in carcinoma cells in the stimulation of MDSC migration to the tumor site and enhanced pro-tumorigenic properties of these cells through increased numbers of Th17 cells. We found significantly increased numbers of MDSCs in spleen, bone marrow, lung, and tumor tissue during the course of tumor progression. The role of MDSCs in metastasis initiation and formation has been well established (19–22). We suggest that the presence of increased MDSCs in both the tumor and lung of PyMT/Tgfbr2KO mice is a basic mechanism of acceleration of tumor growth and increased lung metastasis. Previously, we found that MDSCs in tumor tissue can express high levels of CXCR4 in PyMT/Tgfbr2KO mice (19). In this study we found that CD11b+Gr1+ cells from tumor and lung did not express CXCR2. In vitro analysis of MDSCs indicates that CXCR2 is internalized as cells migrate to areas with a high concentration of CXCL1 and/or CXCL5. We suggest that the high level of CXCL1/5secretion is the primary cause for CXCR2 internalization by tumor infiltrating MDSCs and this suggests that CXCL1/5-CXCR2 axis plays a major role in the recruitment of MDSCs into tumor tissue. Secretion of the CXCL1/5 chemokines can be induced by IL-1, LPS, TNFα (23), PGE2 (24), adenosine (25), IL-17 (12), and other factors. In parallel with increasing production of CXCL chemokines, IL-17 increases the local production of chemokines such as IL-8 (26) andMCP-1 (27), thereby promoting the recruitment of myeloid cells (28). In this study, we demonstrated that IL-17 stimulates secretion of CXCL1/5 from carcinoma cells, but TGF-β inhibits IL-17 stimulated secretion from carcinoma cells with an intact TGF-β signaling pathway. This indicates an important role of TβRII in regulation of IL-17 stimulated secretion of chemokines. Moreover we suggest that signaling through TβRII regulates expression of CD217 and sensitivity to IL-17 on tumor cells.
AlthoughIL-17 production is increased in inflammatory reactions and is considered to be an inflammatory cytokine-promoting factor in tumor development (29, 30), it was not known whether IL-17 has an effect on MDSCs in the tumor-bearing hosts. To address this question, Xu’s group showed that IL-17 is required for the development of MDSCs in tumor-bearing mice (18). Using IL-17R−/− mice and Ad-IL-17 mice, they showed that IL-17 can regulate expression of Arg-I, A100/A8/A9, and MMP9 molecules (18), which are known to be mediators of MDSC-mediated immunosuppression and tumor promotion (31, 32). However, this effect could be due to the systemic effects of loss or gain of IL-17 function. In this study, by using a different approach, we showed that IL-17 increases expression of Arg, MMP-9, IDO, COX-2, and MMP-13 (Figure 4). Furthermore, after incubating MDSCs with IL-17, these cells have a significantly increased immunosuppressive effect on T cell proliferation. Also we found that IL-17 up-regulates gene expression corresponding to M2 type TAMs. Recent work by the Coussens group (33) in the same mouse model as we used in this work, but with intact TGF-β signaling, showed an important role for T cells in tumor progression. IL-4 secreted by Th2 cells can switch the phenotype of TAMs from M1 to M2. Moreover, they showed that activation of TAMs by IL-4, in combination with factors derived from mammary epithelial cells (MEC), such as SDF1, regulates expression of EGF, which in turn stimulates EGFR-induced MEC invasive behavior in vitro and MEC entry into peripheral blood and pulmonary metastasis in vivo. We find additional mechanisms in mammary tumor progression in mice with loss of TGF-β signaling in carcinoma cells. The early stages of carcinoma progression are dependent on Th17 cells which secrete IL-17 to switch the phenotype of TAMs to M2 and increase the number and suppressive activity of MDSCs. Late stages of tumor progression can be dependent on Th2 cells, which in parallel with increased numbers of MDSCs, increase pro-tumorigenic and metastasis properties of M2 TAMs.
These data demonstrate that IL-17 signaling is required not only for increased migration of MDSCs by up-regulating CXCL1/5 but also for the tumor-promoting and immunosuppressive activity of myeloid cells. The administration of IL-17 Ab in vivo during tumor growth was used to support our hypothesis that deletion of TβRII in PyMT carcinoma cells increases tumor growth and metastasis by increasing the host’s Th17 response. We demonstrated that administration of anti-IL-17 Ab significantly decreased tumor growth in both groups of mice with an even more significant reduction in PyMT/Tgfbr2KO mice. The decreased number of metastases was more pronounced and significant in PyMT/Tgfbr2KO mice (31.67±6.01 with IgG vs 7.33±1.86 with anti-IL-17), which demonstrated an increased Th17 response in mice with abrogated TGF-β signaling.
It was recently shown that a gene expression signature associated with complete abrogation of TGF-β signaling correlated with reduced RFS in breast cancer patients; moreover, the strongest association was observed in patients with ER+, specifically within the luminal A subtype (5). It has also been reported that IL-17 associated with macrophages is increased in LN+ Grade III human breast cancer (34). Lonning’s group found increased IL-17 mRNA in stage II and stage III human breast cancer samples compared with normal breast (14). Oft’s group showed significant up-regulation of IL-23(p19) mRNA in the overwhelming majority of carcinoma samples from various organ types when compared with their adjacent normal tissue. The expression of IL-17 was also found to be significantly elevated in human tumors, consistent with activation of IL-23-induced processes (35). Based on these findings, we hypothesized that human breast cancer associated with decreased TGF-β signaling would also correlate with increased Th17 response. We observed that increased Th17 response in human breast cancer patients correlated with reduced RFS in patients with ER-tumors, LN+, specifically within the luminal B subtype and decreased TGF-β signaling in human breast cancer also correlated with enhanced Th17 response similar to the mouse studies.
In summary, our results demonstrated that deletion of TβRII in carcinoma cells promotes mammary tumor growth by increasing the number of infiltrating Th17 cells. The mechanism behind this is increased tumor secretion of CXCL1/5. The result of increased cytokine secretion is an increase in the number of infiltrating MDSCs and TAMs in the tumor tissue. These infiltrated myeloid cells may enhance differentiation of Th17 cells through increased secretion of IL-6, TGF-β, and IL-23. Th17 cells in turn act to induce CXCL1/5 in mammary carcinoma cells through IL-17 signaling, which is regulated by TβRII. IL-17 secreted by Th17 cells has a tumor-promoting effect by up-regulating CXCL1/5 secretion by epithelial cells and increasing the pro-tumorigenic properties of myeloid cells (Figure 6). Our studies provide insights into a novel mechanism by which epithelial TGF-β signaling modulates the tumor microenvironment and is involved in tumor progression. The importance of TGF-β signaling in tumor progression is irrefutable and has been implicated in a variety of cancers and at numerous stages of tumor progression. As the effects of pharmacological targeting of the TGF-β pathway in vivo on tumor progression remains controversial due to the dual roles of TGF-β on tumor progression, the targeting of TGF-β effects is a viable option. As IL-17 has pro-tumorigenic effects on breast cancer, the targeting of this cytokine could be considered when it is associated with impaired TGF-β signaling in carcinoma cells.
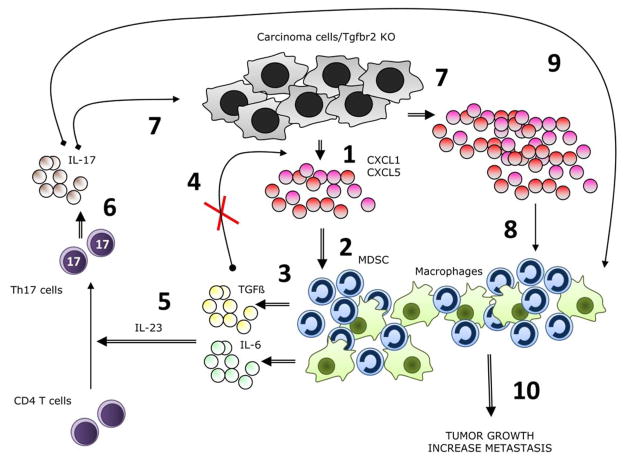
Figure 6. Schematic summarizing data and working hypotheses.
The following numbers refer to the numbers in the schematic. (1) PyMT/Tgfbr2KO carcinomas secrete abundant CXCL1/5 due to lack of TGF-β suppression of chemokine expression. (2) The chemokines recruit to the tumor microenvironment TAMs and MDSCs. (3) TAMs and MDSCs secrete TGF-β and IL-6. (4) TGF-β would suppress expression of CXCL1/5 in TGF-β responsive cells, but cannot in this model because of the lack of TβRII. (5) Together with TGF-β and IL-6, IL-23 stimulates naive CD4 T cells to differentiate into Th17 cells. (6) Th17 cells secrete IL-17. (7) IL-17 stimulates increased secretion of CXCL1/5 by the carcinoma cells. (8) The increase in chemokine expression results in the recruitment of even more TAMs and MDSCs. (9) IL-17 causes MDSCs to be even more immunosuppressive. (10) The net result is enhanced tumor growth and metastases.
Materials and Methods
Cell lines and mice
4T1 breast cancer cell line (CRL-2539) was obtained from ATCC (Manassas, VA) and maintained following the manufacturer’s protocols. Tgfbr2fl and Tgfbr2KO carcinoma cell lines were derived from primary tumors of MMTV-PyMT/Tgfbr2flox/flox and MMTV-PyMT/Tgfbr2KO mice respectively, established and cultured in DMEM/F12 with 5% adult bovine serum as previously described (5, 19). All studies were performed on MMTV-PyMT/Tgfbr2flox/flox (PyMT/Tgfbr2fl) and MMTV-PyMT/Tgfbr2KO (PyMT/Tgfbr2KO) mice which were established and maintained as described (3). MMTV-Cre mice were used to delete Tgfbr2 in the mammary epithelium. These mice have pure FVB background and have spontaneous tumor formation of mammary gland on 28–34th day of age. For additional experiments 8–10 week-old female C57bl/6 and FVB mice were purchased from Harlan Inc. (Indianapolis, IN). To examine the effect of neutralizing IL-17 on tumor growth, mice were implanted with osmotic pumps (DURECT Corporation, Cupertino, CA) with normal rat IgG or rat anti-mouse IL-17 mAb (600 ng/hr for 2 weeks) (R&D Systems, Inc., Minneapolis, MN). Pumps were implanted on day of tumor palpation and removed after 2 weeks. The studies were approved by IACUC at Vanderbilt University Medical Center.
Flow Cytometry Analysis
Single-cell suspensions were made from spleens, BM and lungs of normal and tumor-bearing mice (25, 36), and tumor tissues (37). These cells were labeled with fluorescence-conjugated Abs (Biolegend, eBiosciense, BD) and isotype-matched IgG controls. The cells were analyzed on LSRII flow cytometry (Becton Dickinson) and the data were analyzed with FlowJo software. For intracellular staining, cells were fixed and permeabilized by using BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit following the manufacturer’s protocol. T cells were stimulated with Dynabeads Mouse T-Activator CD3 CD28 (Invitrogen) or with Leukocyte Activation Cocktail, with BD GolgiPlug (BD, San Diego, CA) following the manufacturer’s protocols.
Magnetic Cell Separation
Tumor-infiltrating host immune cells and T cells were magnetically separated from tumor tissue of mammary gland using CD45 or CD4 magnetic microbeads following application protocols of the manufacturer (Miltenyi Biotec Inc., Auburn, CA).
Single-Cell Sorting
Splenocytes from tumor-bearing mice and single cell suspensions of tumor tissue were stained with fluorescence-labeled Abs and sorted with a FACSAria flow cytometer (Becton Dickinson). EpCAM+CD45−, CD11b+Gr1+, CD11b+Gr1−F4/80+ and CD4+CD45+CD11b−DAPI− cells were collected for gene expression and cytokine secretion analysis.
Whole-Lung Mounting
Mice were sacrificed by anesthetic overdose. Lungs were processed as described (38). The tumor nodules in lung were then counted.
Functional assays
T cell proliferation was measured using a mixed leukocyte reaction. Dendritic cells (DC)from C57bl/6 mice were differentiated from BM cells for 6 days with GM-CSF (10ng/ml) and IL-4 (10ng/ml) in RPMI 1640 medium. T cells from naïve FVB mice were isolated using R&D Systems Mouse T cell Enrichment columns, according to the manufacturer’s protocol. T cells were plated at 105 T cells per well in 96 wells plate. Each well contained 100,000 T cells, 25,000 MDSCs and different number of DC. Cells were incubated for 72 hours. 3[H]-thymidine was then added at 1μCi per 200uL of cells per well for an additional 18 hours followed by cell harvesting and radioactivity count using a liquid scintillation counter.
Preparation of tumor conditioned medium (explant)
Tumor explants were prepared from freshly isolated tumors. Spontaneous tumors from Tgbr2fl and Tgfbr2KO mice, without ulceration, approximately 1.5 cm in diameter were removed under sterile conditions after euthanizing the mouse. Tumors were minced into pieces <3mm in diameter and digested in 1 mg/mL Collagenase Type I/Dispase Type II at 37°C for two hours. The digested tissue pieces were then pressed through a 70μm mesh screen to create a single cell suspension. Cells were washed with PBS and resuspended in DMEM supplemented with 200 U/mL penicillin plus 50μg/mL streptomycin, and 5% FBS. Cells were cultured overnight at 107cells/mL and the cell free supernatant collected.
Quantitative RT-PCR
Total RNA was extracted from sorted CD11b+Gr1+, CD11b+Gr1− F4/80+ and CD4+ cells as described using an RNeasy Mini Kit (QIAGEN Inc., Valencia, CA). cDNA was synthesized using Invitrogen Superscripttm First-strand synthesis system for RT-PCR (Invitrogen Inc., Carlsbad, CA). Primers specific for Arg, MMP-9, IDO, COX-2, IL-1β, TGF-β, MMP-13, IL-23, IL-6, IL-17a, IL-17f, IL-21, CCL20, IL-10, VEGF, GM-CSF, IFN-γ were used and relative gene expression was determined using ABI PRISM 7900HT Sequence Detection System (PE Applied Biosystems, Foster City, CA). The comparative threshold cycle method was used to calculate gene expression normalized to β-actin as a gene reference. Primer sequences are available upon request.
ELISA
Cytokine levels in CD4+, CD45+, CD45− and Ep-CAM+CD45− cell supernatants or tissue lysates were measured using the Mouse TGF-β1, IL-6, IL-23, IL-17a/f, CXCL1/5 ELISA kits (R&D Systems, Minneapolis, MN) following the manufacturer’s protocol.
Statistical Analysis
Data were presented as mean ± SEM. Multiple comparisons between treatment groups and control untreated group were performed using one-way ANOVA followed by Dunnett’s procedure for multiplicity adjustment. Two group comparison was performed using two-sample t tests. Correlation analyses for the gene expression between the signatures of interest were performed using data representing 1,319 patients from 4 independent, previously reported studies (Gene Expression Omnibus ID: GSE10886, GSE4922, GSE6532, and GSE2845) (5). The gene expression was appropriately normalized across all arrays. Gene symbols were assigned using the manufacturer-provided annotation, but the analysis was performed at probe level. The association between RFS and IL17 family was analyzed using the univariate Cox proportional hazard regression model. The modification effect of ER status and node status was examined by testing the interaction between IL17 gene and ER or node status using the multivariate Cox proportional hazard model. When a significant interaction was identified, a Kaplan-Meier survival curve was created for the “high versus low” of the IL-17 gene expression by dichotoming all the samples among the subtype (Figure 5). All tests were two-tailed. All statistical analyses used a p value cutoff of 0.05 to determine significance. All data analysis for human breast cancer correlation was performed in R (39).
Supplementary Material
Significance.
TGF-β signaling is a major tumor suppressor pathway and is therefore difficult to target therapeutically. Understanding the downstream effects of abrogation of TGF-β signaling in tumor cells may identify processes that can be targeted therapeutically. We present data indicating that targeting IL-17 signaling, a pathway that is greatly enhanced by loss of TGF-β signaling, could provide therapeutic benefit. Analysis of human databases indicated a specific group of patients where treatment could be more efficient.
Acknowledgments
This work is supported by NIH grants CA085492, A102162, and CA126505 and the T.J. Martell Foundation to HLM and CA068485for core laboratory support.
References
- 1.Gobbi H, Dupont WD, Simpson JF, Plummer WD, Jr, Schuyler PA, Olson SJ, et al. Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J Natl Cancer Inst. 1999;91:2096–101. doi: 10.1093/jnci/91.24.2096. [DOI] [PubMed] [Google Scholar]
- 2.Gobbi H, Arteaga CL, Jensen RA, Simpson JF, Dupont WD, Olson SJ, et al. Loss of expression of transforming growth factor beta type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology. 2000;36:168–77. doi: 10.1046/j.1365-2559.2000.00841.x. [DOI] [PubMed] [Google Scholar]
- 3.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 4.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, et al. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–19. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 5.Bierie B, Chung CH, Parker JS, Stover DG, Cheng N, Chytil A, et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–82. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–8. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 11.Spriggs MK. Interleukin-17 and its receptor. J Clin Immunol. 1997;17:366–9. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 13.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–10. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–9. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010 doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–8. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Zhang YK, Liu Y, Ding CZ, Li Q, Zhou Y, et al. Synthesis and evaluation of novel alpha-amino cyclic boronates as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett. 2010;20:3550–6. doi: 10.1016/j.bmcl.2010.04.129. [DOI] [PubMed] [Google Scholar]
- 22.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GRO alpha gene requires both NF-kappa B and novel constitutive factors. J Biol Chem. 1995;270:30619–26. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–51. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–31. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–6. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woltman AM, de Haij S, Boonstra JG, Gobin SJ, Daha MR, van Kooten C. Interleukin-17 and CD40-ligand synergistically enhance cytokine and chemokine production by renal epithelial cells. J Am Soc Nephrol. 2000;11:2044–55. doi: 10.1681/ASN.V11112044. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Reboul P, He Y, Jolicoeur FC, et al. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J Rheumatol. 2001;28:712–8. [PubMed] [Google Scholar]
- 29.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–31. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Ljung BM, Mayall B, Lottich C, Boyer C, Sylvester SS, Leight GS, et al. Cell dissociation techniques in human breast cancer--variations in tumor cell viability and DNA ploidy. Breast Cancer Res Treat. 1989;13:153–9. doi: 10.1007/BF01806527. [DOI] [PubMed] [Google Scholar]
- 38.Jessen KA, Liu SY, Tepper CG, Karrim J, McGoldrick ET, Rosner A, et al. Molecular analysis of metastasis in a polyomavirus middle T mousemodel: the role of osteopontin. Breast Cancer Res. 2004;6:R157–69. doi: 10.1186/bcr768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.www.r-project.org [Internet]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.