Abstract
In the title coordination polymer, [Cd(C7H4N3O2)2(C3H7NO)2]n, the CdII atom, lying on an inversion center, is six-coordinated in a distorted N4O2 octahedral geometry. The N atoms of the 4-nitrophenylcyanamide anions form the equatorial plane and the O atoms of the dimethylformamide molecules occupy the axial positions. The anions act as bridging ligands, connecting the Cd atoms into a one-dimensional coordination polymer along [100].
Related literature
For background to phenylcyanamide ligands and their complexes, see: Crutchley (2001 ▶). For polynuclear complexes of phenylcyanamide ligands, see: Ainscough et al. (1991 ▶); Chiniforoshan et al. (2009 ▶, 2010 ▶); Escuer et al. (2004 ▶). For the preparation of 4-nitro-phenylcyanamide used in the synthesis of the title compound, see: Crutchley & Naklicki (1989 ▶).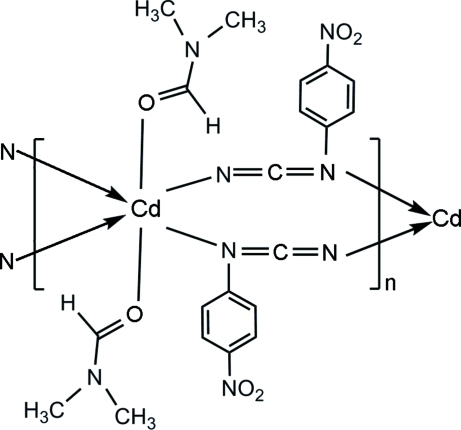
Experimental
Crystal data
[Cd(C7H4N3O2)2(C3H7NO)2]
M r = 582.87
Triclinic,
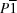
a = 5.6070 (11) Å
b = 9.811 (2) Å
c = 11.679 (2) Å
α = 67.44 (3)°
β = 81.93 (3)°
γ = 84.28 (3)°
V = 586.7 (2) Å3
Z = 1
Mo Kα radiation
μ = 0.98 mm−1
T = 298 K
0.45 × 0.10 × 0.08 mm
Data collection
Stoe IPDS 2T diffractometer
Absorption correction: numerical (X-SHAPE and X-RED32; Stoe & Cie, 2002 ▶) T min = 0.887, T max = 0.923
6589 measured reflections
3150 independent reflections
3038 reflections with I > 2σ(I)
R int = 0.066
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.114
S = 1.11
3150 reflections
162 parameters
H-atom parameters constrained
Δρmax = 0.82 e Å−3
Δρmin = −0.89 e Å−3
Data collection: X-AREA (Stoe & Cie, 2002 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812001924/hy2496sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812001924/hy2496Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Cd1—N1i | 2.287 (3) |
| Cd1—O3 | 2.347 (3) |
| Cd1—N2 | 2.383 (3) |
Symmetry code: (i) 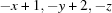 .
.
Acknowledgments
The authors acknowledge financial support from Isfahan University of Technology.
supplementary crystallographic information
Comment
Phenylcyanmide ligands can act as monodentate, bidentate and also as bridging ligands (Crutchley, 2001). In the bridging mode, the cyanamido group (NCN) is coordinated in an end-to-end mode, forming polynuclear complexes (Ainscough et al., 1991; Chiniforoshan et al., 2009, 2010; Escuer et al., 2004).
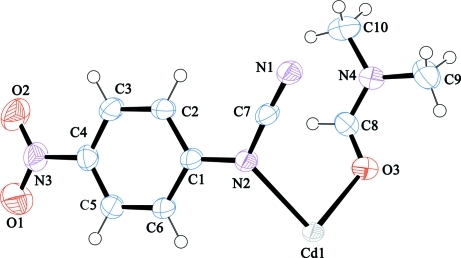
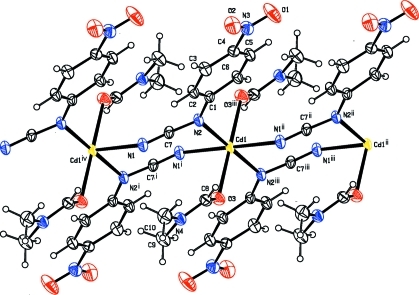
Following our work with this family of ligands, we report here the synthesis and crystal structure of a cadmium(II) coordination polymer of 4-nitro-phenylcyanamide ligand (Crutchley & Naklicki, 1989). The asymmetric unit of the title compound is shown in Fig. 1. In the title compound, the CdII atom lies on an inversion center and has a distorted octahedral geometry (Fig. 2, Table 1). The coordination environment consists of four N atoms from 4-nitro-phenylcyanamide ligands in the equatorial plane and two O atoms from DMF molecules in the axial positions. The one-dimensional structure of the title compound is shown in Fig. 2.
Experimental
4-Nitrophenylcynamide (Crutchley & Naklicki, 1989) (0.163 g, 1 mmol) was dissolved in methanol (25 ml) and was added slowly to a solution of cadmium(II) acetate (0.133 g, 0.5 mmol) in methanol (25 ml). The mixture was stirred for 3 hrs. The resulting solid was filtered off. Yellow needles of the title compound were obtained by n-hexane diffusion into a DMF solution of the title compound after 4 weeks.
Refinement
H atoms were positioned geometrically and refined as riding atoms, with C—H = 0.93 (CH) and 0.96 (CH3) Å and with Uiso(H) = 1.2(1.5 for methyl)Ueq(C).
Figures
Fig. 1.
The asymmetric unit of the title compound with displacement ellipsoids drawn at 50% probability level.
Fig. 2.
The one-dimensional polymeric structure of the title compound. [Symmetry codes: (i) 1-x, 2-y, -z; (ii) -1+x, y, z; (iii) -x, 2-y, -z; (iv) 1+x, y, z.]
Crystal data
| [Cd(C7H4N3O2)2(C3H7NO)2] | Z = 1 |
| Mr = 582.87 | F(000) = 294 |
| Triclinic, P1 | Dx = 1.650 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 5.6070 (11) Å | Cell parameters from 3150 reflections |
| b = 9.811 (2) Å | θ = 2.3–29.2° |
| c = 11.679 (2) Å | µ = 0.98 mm−1 |
| α = 67.44 (3)° | T = 298 K |
| β = 81.93 (3)° | Needle, yellow |
| γ = 84.28 (3)° | 0.45 × 0.10 × 0.08 mm |
| V = 586.7 (2) Å3 |
Data collection
| Stoe IPDS 2T diffractometer | 3150 independent reflections |
| Radiation source: fine-focus sealed tube | 3038 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.066 |
| ω scans | θmax = 29.2°, θmin = 2.3° |
| Absorption correction: numerical (X-SHAPE and X-RED32; Stoe & Cie, 2002) | h = −7→7 |
| Tmin = 0.887, Tmax = 0.923 | k = −13→13 |
| 6589 measured reflections | l = −15→16 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.11 | w = 1/[σ2(Fo2) + (0.0672P)2 + 0.308P] where P = (Fo2 + 2Fc2)/3 |
| 3150 reflections | (Δ/σ)max < 0.001 |
| 162 parameters | Δρmax = 0.82 e Å−3 |
| 0 restraints | Δρmin = −0.89 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cd1 | 0.0000 | 1.0000 | 0.0000 | 0.03092 (12) | |
| O1 | −0.1591 (9) | 0.2034 (5) | 0.4718 (4) | 0.0897 (14) | |
| O2 | 0.1354 (7) | 0.1984 (4) | 0.5719 (3) | 0.0705 (10) | |
| O3 | 0.0654 (5) | 1.1593 (3) | 0.0984 (3) | 0.0493 (6) | |
| N1 | 0.6524 (5) | 0.9163 (3) | 0.1262 (3) | 0.0404 (6) | |
| N2 | 0.2627 (5) | 0.8176 (3) | 0.1293 (3) | 0.0362 (5) | |
| N3 | 0.0180 (6) | 0.2570 (4) | 0.4842 (3) | 0.0511 (8) | |
| N4 | 0.3441 (5) | 1.1798 (4) | 0.2129 (3) | 0.0417 (6) | |
| C1 | 0.2102 (5) | 0.6769 (3) | 0.2158 (3) | 0.0326 (6) | |
| C2 | 0.3446 (6) | 0.6018 (4) | 0.3172 (3) | 0.0392 (7) | |
| H2 | 0.4765 | 0.6457 | 0.3264 | 0.047* | |
| C3 | 0.2838 (7) | 0.4640 (4) | 0.4033 (3) | 0.0437 (7) | |
| H3 | 0.3748 | 0.4149 | 0.4694 | 0.052* | |
| C4 | 0.0864 (6) | 0.4002 (4) | 0.3899 (3) | 0.0387 (7) | |
| C5 | −0.0458 (6) | 0.4682 (4) | 0.2903 (4) | 0.0446 (8) | |
| H5 | −0.1761 | 0.4225 | 0.2818 | 0.053* | |
| C6 | 0.0172 (6) | 0.6057 (4) | 0.2026 (4) | 0.0413 (7) | |
| H6 | −0.0697 | 0.6511 | 0.1344 | 0.050* | |
| C7 | 0.4701 (5) | 0.8656 (4) | 0.1296 (3) | 0.0338 (6) | |
| C8 | 0.1668 (6) | 1.1147 (4) | 0.1947 (4) | 0.0412 (7) | |
| H8 | 0.1137 | 1.0288 | 0.2590 | 0.049* | |
| C9 | 0.4414 (9) | 1.3125 (5) | 0.1153 (5) | 0.0584 (10) | |
| H9A | 0.6050 | 1.2913 | 0.0873 | 0.088* | |
| H9B | 0.4371 | 1.3892 | 0.1476 | 0.088* | |
| H9C | 0.3461 | 1.3444 | 0.0466 | 0.088* | |
| C10 | 0.4716 (9) | 1.1095 (6) | 0.3235 (5) | 0.0598 (11) | |
| H10A | 0.3882 | 1.0246 | 0.3806 | 0.090* | |
| H10B | 0.4778 | 1.1784 | 0.3631 | 0.090* | |
| H10C | 0.6327 | 1.0793 | 0.2994 | 0.090* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cd1 | 0.01969 (14) | 0.03075 (16) | 0.03821 (18) | −0.00441 (9) | −0.00930 (10) | −0.00556 (12) |
| O1 | 0.092 (3) | 0.066 (2) | 0.089 (3) | −0.048 (2) | −0.027 (2) | 0.011 (2) |
| O2 | 0.073 (2) | 0.0528 (18) | 0.0584 (19) | −0.0116 (16) | −0.0145 (16) | 0.0133 (15) |
| O3 | 0.0510 (14) | 0.0441 (14) | 0.0569 (16) | −0.0031 (11) | −0.0226 (12) | −0.0175 (12) |
| N1 | 0.0274 (11) | 0.0406 (14) | 0.0439 (15) | −0.0065 (10) | −0.0072 (10) | −0.0035 (12) |
| N2 | 0.0253 (11) | 0.0335 (13) | 0.0425 (14) | −0.0060 (9) | −0.0082 (10) | −0.0037 (11) |
| N3 | 0.0499 (17) | 0.0391 (16) | 0.0529 (18) | −0.0092 (13) | −0.0037 (14) | −0.0038 (14) |
| N4 | 0.0381 (13) | 0.0435 (15) | 0.0451 (15) | −0.0046 (11) | −0.0100 (11) | −0.0159 (13) |
| C1 | 0.0256 (12) | 0.0321 (14) | 0.0362 (14) | −0.0014 (10) | −0.0073 (10) | −0.0072 (11) |
| C2 | 0.0347 (14) | 0.0401 (16) | 0.0398 (16) | −0.0078 (12) | −0.0126 (12) | −0.0075 (13) |
| C3 | 0.0431 (17) | 0.0400 (17) | 0.0389 (17) | −0.0069 (13) | −0.0139 (13) | −0.0003 (13) |
| C4 | 0.0380 (15) | 0.0302 (14) | 0.0436 (17) | −0.0045 (11) | −0.0024 (13) | −0.0092 (13) |
| C5 | 0.0341 (15) | 0.0359 (16) | 0.059 (2) | −0.0078 (12) | −0.0133 (14) | −0.0081 (15) |
| C6 | 0.0332 (14) | 0.0350 (15) | 0.0507 (19) | −0.0046 (11) | −0.0181 (13) | −0.0052 (14) |
| C7 | 0.0256 (12) | 0.0349 (14) | 0.0340 (14) | 0.0007 (10) | −0.0073 (10) | −0.0045 (11) |
| C8 | 0.0410 (16) | 0.0386 (16) | 0.0467 (18) | −0.0055 (13) | −0.0073 (13) | −0.0172 (14) |
| C9 | 0.059 (2) | 0.054 (2) | 0.060 (2) | −0.0214 (19) | −0.0045 (19) | −0.015 (2) |
| C10 | 0.056 (2) | 0.072 (3) | 0.056 (2) | 0.001 (2) | −0.0242 (19) | −0.024 (2) |
Geometric parameters (Å, º)
| Cd1—N1i | 2.287 (3) | C2—C3 | 1.381 (5) |
| Cd1—O3 | 2.347 (3) | C2—H2 | 0.9300 |
| Cd1—N2 | 2.383 (3) | C3—C4 | 1.380 (5) |
| O1—N3 | 1.219 (5) | C3—H3 | 0.9300 |
| O2—N3 | 1.214 (5) | C4—C5 | 1.377 (5) |
| O3—C8 | 1.238 (5) | C5—C6 | 1.388 (5) |
| N1—C7 | 1.170 (4) | C5—H5 | 0.9300 |
| N1—Cd1ii | 2.287 (3) | C6—H6 | 0.9300 |
| N2—C7 | 1.299 (4) | C8—H8 | 0.9300 |
| N2—C1 | 1.392 (4) | C9—H9A | 0.9600 |
| N3—C4 | 1.461 (4) | C9—H9B | 0.9600 |
| N4—C8 | 1.316 (4) | C9—H9C | 0.9600 |
| N4—C9 | 1.456 (5) | C10—H10A | 0.9600 |
| N4—C10 | 1.460 (5) | C10—H10B | 0.9600 |
| C1—C6 | 1.401 (4) | C10—H10C | 0.9600 |
| C1—C2 | 1.409 (4) | ||
| N1i—Cd1—N1iii | 180.000 (1) | C3—C2—H2 | 119.5 |
| N1i—Cd1—O3 | 86.19 (12) | C1—C2—H2 | 119.5 |
| N1iii—Cd1—O3 | 93.81 (12) | C4—C3—C2 | 119.2 (3) |
| N1i—Cd1—O3iv | 93.81 (12) | C4—C3—H3 | 120.4 |
| N1iii—Cd1—O3iv | 86.19 (12) | C2—C3—H3 | 120.4 |
| O3—Cd1—O3iv | 180.00 (10) | C5—C4—C3 | 121.5 (3) |
| N1i—Cd1—N2iv | 95.72 (10) | C5—C4—N3 | 119.8 (3) |
| N1iii—Cd1—N2iv | 84.28 (10) | C3—C4—N3 | 118.7 (3) |
| O3—Cd1—N2iv | 90.74 (10) | C4—C5—C6 | 119.4 (3) |
| O3iv—Cd1—N2iv | 89.26 (10) | C4—C5—H5 | 120.3 |
| N1i—Cd1—N2 | 84.28 (10) | C6—C5—H5 | 120.3 |
| N1iii—Cd1—N2 | 95.72 (10) | C5—C6—C1 | 120.8 (3) |
| O3—Cd1—N2 | 89.26 (10) | C5—C6—H6 | 119.6 |
| O3iv—Cd1—N2 | 90.74 (10) | C1—C6—H6 | 119.6 |
| N2iv—Cd1—N2 | 180.0 | N1—C7—N2 | 176.4 (3) |
| C8—O3—Cd1 | 121.5 (2) | O3—C8—N4 | 124.9 (4) |
| C7—N1—Cd1ii | 142.5 (3) | O3—C8—H8 | 117.6 |
| C7—N2—C1 | 116.9 (3) | N4—C8—H8 | 117.6 |
| C7—N2—Cd1 | 113.9 (2) | N4—C9—H9A | 109.5 |
| C1—N2—Cd1 | 128.50 (19) | N4—C9—H9B | 109.5 |
| O2—N3—O1 | 123.1 (4) | H9A—C9—H9B | 109.5 |
| O2—N3—C4 | 119.1 (3) | N4—C9—H9C | 109.5 |
| O1—N3—C4 | 117.7 (4) | H9A—C9—H9C | 109.5 |
| C8—N4—C9 | 120.7 (3) | H9B—C9—H9C | 109.5 |
| C8—N4—C10 | 120.8 (4) | N4—C10—H10A | 109.5 |
| C9—N4—C10 | 117.9 (4) | N4—C10—H10B | 109.5 |
| N2—C1—C6 | 119.5 (3) | H10A—C10—H10B | 109.5 |
| N2—C1—C2 | 122.5 (3) | N4—C10—H10C | 109.5 |
| C6—C1—C2 | 118.0 (3) | H10A—C10—H10C | 109.5 |
| C3—C2—C1 | 121.1 (3) | H10B—C10—H10C | 109.5 |
| N1i—Cd1—O3—C8 | −96.1 (3) | C6—C1—C2—C3 | −1.9 (5) |
| N1iii—Cd1—O3—C8 | 83.9 (3) | C1—C2—C3—C4 | −0.7 (6) |
| N2iv—Cd1—O3—C8 | 168.2 (3) | C2—C3—C4—C5 | 2.5 (6) |
| N2—Cd1—O3—C8 | −11.8 (3) | C2—C3—C4—N3 | −177.6 (4) |
| N1i—Cd1—N2—C7 | 35.1 (3) | O2—N3—C4—C5 | −179.7 (4) |
| N1iii—Cd1—N2—C7 | −144.9 (3) | O1—N3—C4—C5 | −1.2 (6) |
| O3—Cd1—N2—C7 | −51.2 (3) | O2—N3—C4—C3 | 0.4 (6) |
| O3iv—Cd1—N2—C7 | 128.8 (3) | O1—N3—C4—C3 | 178.9 (5) |
| N1i—Cd1—N2—C1 | −155.1 (3) | C3—C4—C5—C6 | −1.5 (6) |
| N1iii—Cd1—N2—C1 | 24.9 (3) | N3—C4—C5—C6 | 178.6 (4) |
| O3—Cd1—N2—C1 | 118.6 (3) | C4—C5—C6—C1 | −1.2 (6) |
| O3iv—Cd1—N2—C1 | −61.4 (3) | N2—C1—C6—C5 | −177.2 (3) |
| C7—N2—C1—C6 | −165.8 (3) | C2—C1—C6—C5 | 2.9 (5) |
| Cd1—N2—C1—C6 | 24.6 (5) | Cd1—O3—C8—N4 | 131.9 (3) |
| C7—N2—C1—C2 | 14.0 (5) | C9—N4—C8—O3 | −1.7 (6) |
| Cd1—N2—C1—C2 | −155.5 (3) | C10—N4—C8—O3 | −172.6 (4) |
| N2—C1—C2—C3 | 178.2 (3) |
Symmetry codes: (i) −x+1, −y+2, −z; (ii) x+1, y, z; (iii) x−1, y, z; (iv) −x, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HY2496).
References
- Ainscough, E. W., Baker, E. N., Brader, M. L. & Brodie, A. M. (1991). J. Chem. Soc. Dalton Trans. pp. 1243–1249.
- Chiniforoshan, H., Jalilpour, S., Shirinfar, B. & Khavasi, H. R. (2009). Acta Cryst. E65, m386. [DOI] [PMC free article] [PubMed]
- Chiniforoshan, H., Shirinfar, B., Jalilpour, S. & Khavasi, H. R. (2010). Acta Cryst. E66, m331. [DOI] [PMC free article] [PubMed]
- Crutchley, R. J. (2001). Coord. Chem. Rev. 219, 125–155.
- Crutchley, R. J. & Naklicki, M. L. (1989). Inorg. Chem. 28, 1955–1958.
- Escuer, A., Mautner, F. A., Sanz, N. & Vicente, R. (2004). Polyhedron, 23, 1409–1417.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe & Cie (2002). X-AREA, X-RED32 and X-SHAPE Stoe & Cie, Darmstadt, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812001924/hy2496sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812001924/hy2496Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report