Abstract
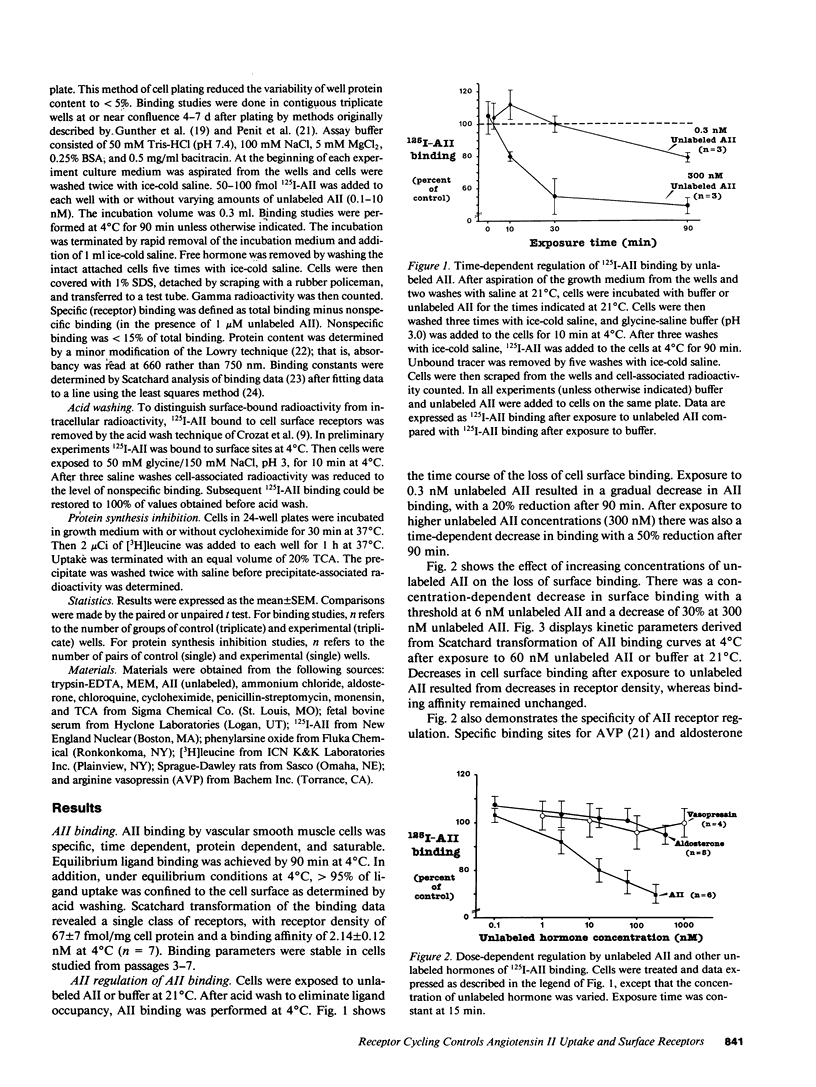
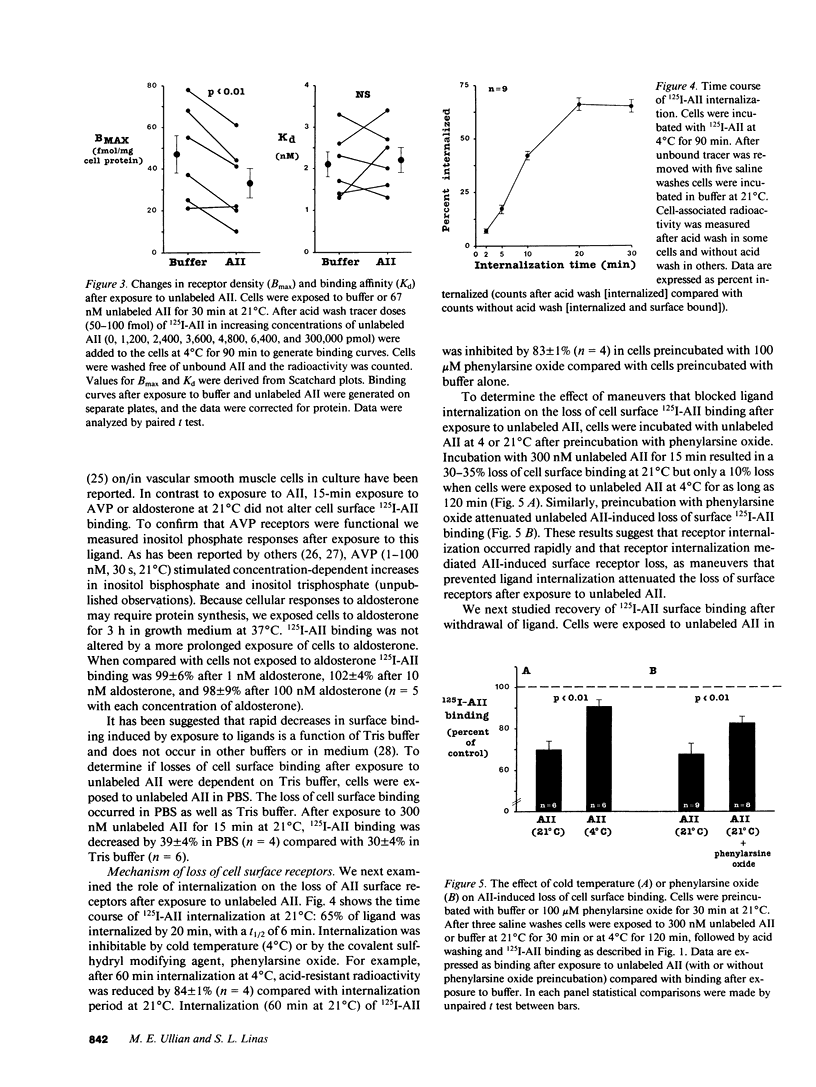
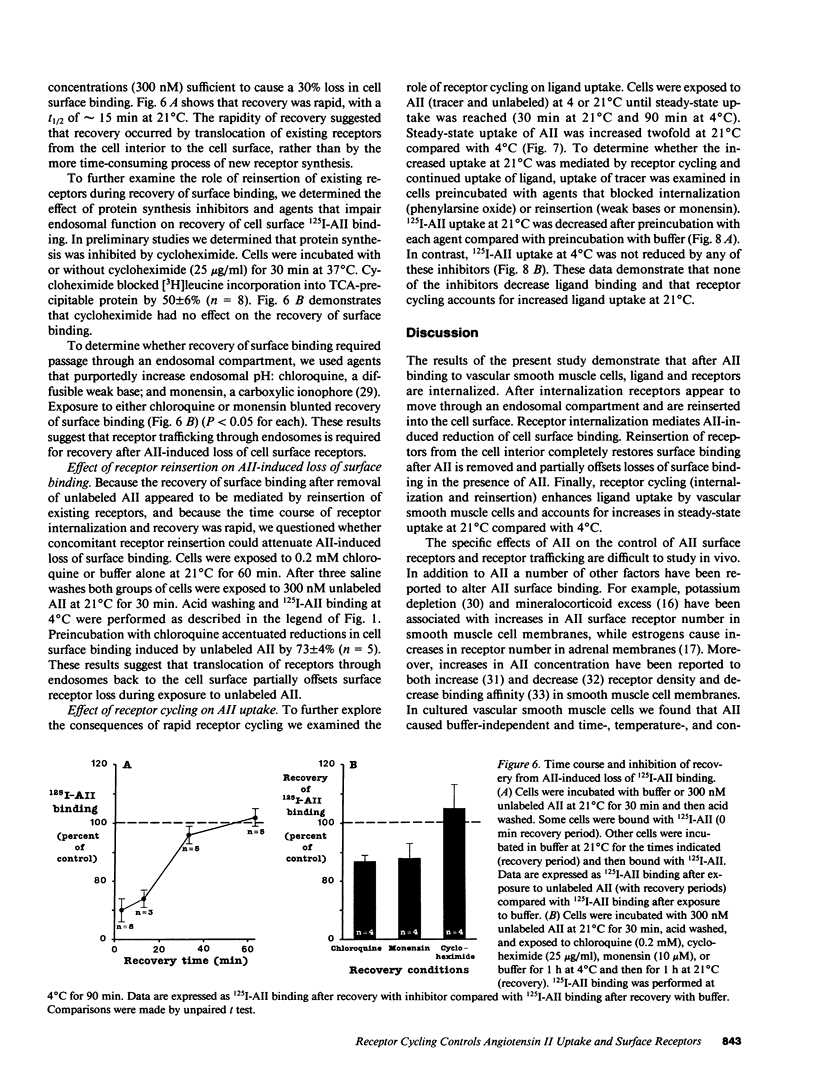
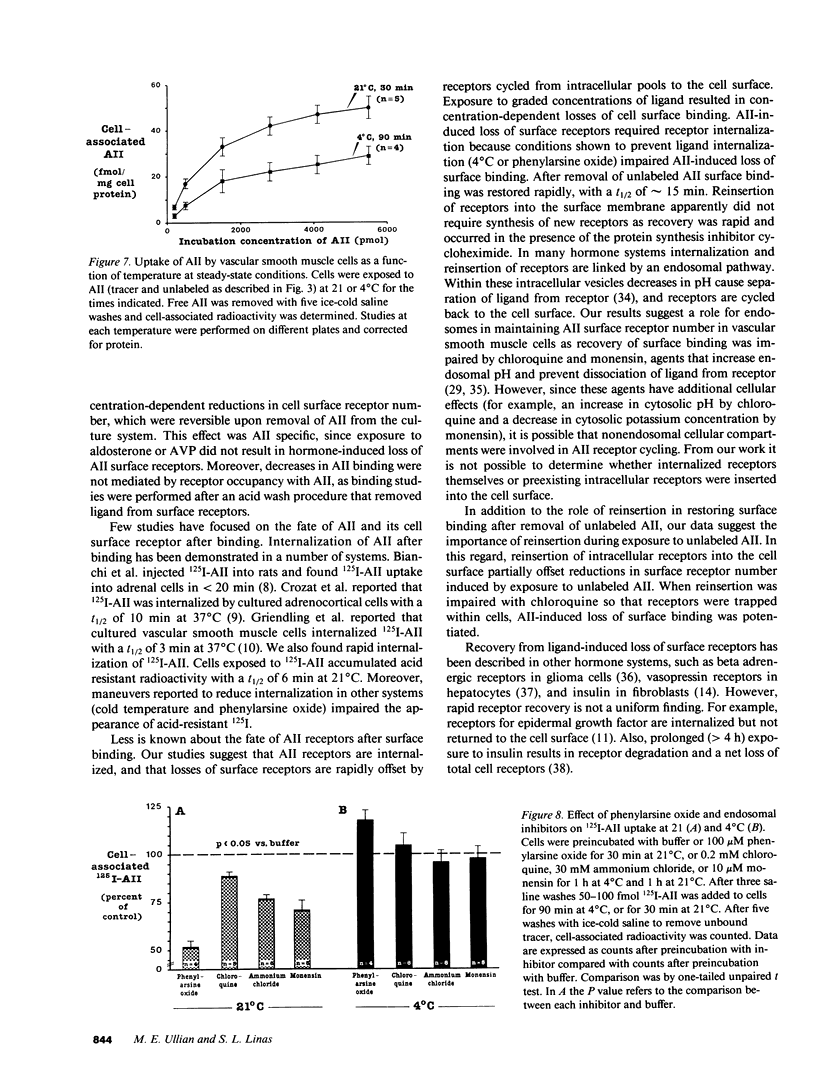
In vivo data on the factors controlling angiotensin II (AII) cell surface binding are conflicting. We studied the specific effects of AII on AII binding in rat mesenteric artery vascular smooth muscle cells in culture. Incubation with unlabeled AII at 21 degrees C resulted in time- and concentration-dependent decreases in AII surface binding at 4 degrees C, with a 30% reduction after exposure to 300 nM AII for 15 min. Reductions in cell surface binding were due to decrements in receptor number rather than changes in binding affinity. Loss of surface receptors was mediated by receptor internalization as maneuvers that blocked ligand internalization (cold temperature and phenylarsine oxide [PAO]) attenuated AII-induced loss of surface receptors. After removal of AII, recovery of surface binding was rapid (t1/2 = 15 min) and was mediated by reinsertion of a preexisting pool of receptors into the surface membrane rather than by new receptor synthesis. To determine the role of receptor cycling on AII-induced surface receptor loss, cells were incubated with the endosomal inhibitor chloroquine during exposure to AII at 21 degrees C. Incubation with AII plus chloroquine resulted in a 70% greater loss of surface binding than after incubation with AII alone. To determine the role of receptor cycling on uptake of ligand, cells were incubated with PAO or endosomal inhibitors during exposure to AII at 4 and 21 degrees C. Compared with buffer these agents did not alter AII uptake at 4 degrees C, but decreased uptake by 12-50% at 21 degrees C. These results indicate that after binding AII receptors cycle and that receptor cycling attenuates AII-induced losses of surface receptors and enhances ligand uptake by providing a continuous source of receptors to the cell surface.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti R. G., Linas S. L. Mechanism of decreased vascular response to angiotensin II in renal vascular hypertension. Kidney Int. 1987 Apr;31(4):906–912. doi: 10.1038/ki.1987.84. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C., Gutkowska J., De Léan A., Ballak M., Anand-Srivastava M. B., Genest J., Cantin M. Fate of [125I]angiotensin II in adrenal zona glomerulosa cells. Endocrinology. 1986 Jun;118(6):2605–2607. doi: 10.1210/endo-118-6-2605. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Bruns C., Marmé D. Pertussis toxin inhibits the angiotensin II and serotonin-induced rise of free cytoplasmic calcium in cultured smooth muscle cells from rat aorta. FEBS Lett. 1987 Feb 9;212(1):40–44. doi: 10.1016/0014-5793(87)81552-1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat A., Penhoat A., Saez J. M. Processing of angiotensin II (A-II) and (Sar1,Ala8)A-II by cultured bovine adrenocortical cells. Endocrinology. 1986 Jun;118(6):2312–2318. doi: 10.1210/endo-118-6-2312. [DOI] [PubMed] [Google Scholar]
- Douglas J. G., Brown G. P. Effect of prolonged low dose infusion of angiotensin II and aldosterone on rat smooth muscle and adrenal angiotensin II receptors. Endocrinology. 1982 Sep;111(3):988–992. doi: 10.1210/endo-111-3-988. [DOI] [PubMed] [Google Scholar]
- Douglas J. G. Estrogen effects on angiotensin receptors are modulated by pituitary in female rats. Am J Physiol. 1987 Jan;252(1 Pt 1):E57–E62. doi: 10.1152/ajpendo.1987.252.1.E57. [DOI] [PubMed] [Google Scholar]
- Douglas J. G. Mechanism of adrenal angiotensin II receptor changes after nephrectomy in rats. J Clin Invest. 1981 Apr;67(4):1171–1176. doi: 10.1172/JCI110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J. B., Dickey B. F., Bucher N. L., Fine R. E. Internalization, recycling, and redistribution of vasopressin receptors in rat hepatocytes. J Biol Chem. 1985 Oct 15;260(23):12641–12646. [PubMed] [Google Scholar]
- Griendling K. K., Delafontaine P., Rittenhouse S. E., Gimbrone M. A., Jr, Alexander R. W. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987 Oct 25;262(30):14555–14562. [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S., Gimbrone M. A., Jr, Alexander R. W. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature. 1980 Sep 18;287(5779):230–232. doi: 10.1038/287230a0. [DOI] [PubMed] [Google Scholar]
- Harford J., Wolkoff A. W., Ashwell G., Klausner R. D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J Cell Biol. 1983 Jun;96(6):1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel C., Staehelin M. Reappearance of beta-adrenergic receptors after isoproterenol treatment in intact C6-cells. J Cell Biol. 1983 Nov;97(5 Pt 1):1538–1543. doi: 10.1083/jcb.97.5.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Schultz G. S., Galardy R. E., Jamieson J. D. Preparation of functional smooth muscle cells from the rabbit aorta. J Exp Med. 1978 Nov 1;148(5):1400–1413. doi: 10.1084/jem.148.5.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson V. P., Ronnett G. V., Lane M. D. Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J Biol Chem. 1983 Oct 25;258(20):12139–12142. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linas S. L., Marzec-Calvert R., Ullian M. E., O'Brien R. F. Mechanism of the antihypertensive effect of K depletion in the spontaneously hypertensive rat. Kidney Int. 1988 Jul;34(1):18–25. doi: 10.1038/ki.1988.140. [DOI] [PubMed] [Google Scholar]
- Marshall S., Garvey W. T., Geller M. Primary culture of isolated adipocytes. A new model to study insulin receptor regulation and insulin action. J Biol Chem. 1984 May 25;259(10):6376–6384. [PubMed] [Google Scholar]
- Marshall S., Green A., Olefsky J. M. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981 Nov 25;256(22):11464–11470. [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Tris (hydroxmethyl) aminomethane permits the expression of insulin-induced receptor loss in isolated rat adipocytes. Biochem Biophys Res Commun. 1981 Sep 30;102(2):646–653. doi: 10.1016/s0006-291x(81)80181-7. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Meyer-Lehnert H., Caramelo C., Tsai P., Schrier R. W. Interaction of atriopeptin III and vasopressin on calcium kinetics and contraction of aortic smooth muscle cells. J Clin Invest. 1988 Oct;82(4):1407–1414. doi: 10.1172/JCI113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Ochi S., Fujiwara Y., Orita Y., Tanaka Y., Shin S., Takama T., Wada A., Ueda N., Kamada T. Phosphoinositide turnover enhanced by angiotensin II in isolated rat glomeruli. Biochim Biophys Acta. 1987 Jan 19;927(1):100–105. doi: 10.1016/0167-4889(87)90071-1. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Douglas J. G., Linas S. L. Mechanism of decreased vascular reactivity to angiotensin II in conscious, potassium-deficient rats. J Clin Invest. 1984 Jan;73(1):79–86. doi: 10.1172/JCI111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Bauer C. Pertussis toxin abolishes angiotensin II-induced phosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Biochem J. 1986 May 15;236(1):289–294. doi: 10.1042/bj2360289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. A., Lawrence B., Nguyen H. H., Meyer W. J., 3rd Aldosterone and dexamethasone binding in human arterial smooth muscle cells. J Hypertens. 1987 Dec;5(6):739–744. doi: 10.1097/00004872-198712000-00018. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Brown E. R., Barnes D., Sabir M. A., Davis J. S., Farese R. V. Angiotensin II rapidly increases phosphatidate-phosphoinositide synthesis and phosphoinositide hydrolysis and mobilizes intracellular calcium in cultured arterial muscle cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7812–7816. doi: 10.1073/pnas.81.24.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell. 1981 Aug;25(2):433–440. doi: 10.1016/0092-8674(81)90061-1. [DOI] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]


