Abstract
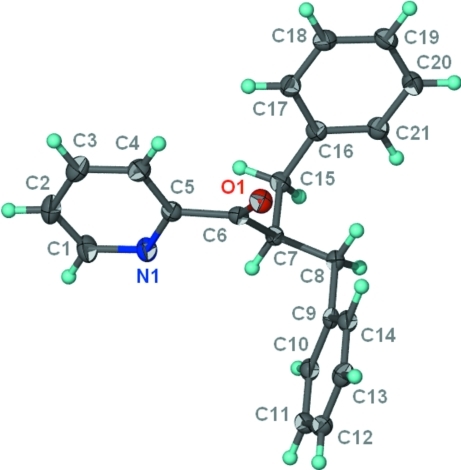
Molecules of the title compound, C21H19NO, assume an approximate propellar shape, with the three aromatic rings being nearly perpendicularly aligned with respect to the plane formed by the C atoms that are connected to the methine C atom [dihedral angles: pyridyl 79.82 (4)°, phenyl 80.12 (3)° and phenyl 86.93 (3)°].
Related literature
For background to fast aldol reactions, see: Nugent et al. (2010 ▶).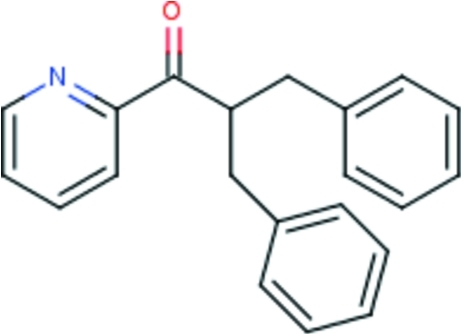
Experimental
Crystal data
C21H19NO
M r = 301.37
Monoclinic,
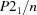
a = 15.1569 (3) Å
b = 5.6333 (1) Å
c = 19.5468 (4) Å
β = 109.295 (2)°
V = 1575.22 (5) Å3
Z = 4
Cu Kα radiation
μ = 0.60 mm−1
T = 100 K
0.30 × 0.20 × 0.10 mm
Data collection
Agilent SuperNova Dual diffractometer with Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011 ▶) T min = 0.840, T max = 0.942
25210 measured reflections
3299 independent reflections
3133 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.098
S = 1.04
3299 reflections
208 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.21 e Å−3
Data collection: CrysAlis PRO (Agilent, 2011 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812003686/bt5803sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812003686/bt5803Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812003686/bt5803Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the Higher Education Commission of Pakistan and the Ministry of Higher Education of Malaysia (grant No. UM.C/HIR/MOHE/SC/12) for supporting this study.
supplementary crystallographic information
Comment
2-Benzyl-3-phenyl-1-(pyridin-2-yl)propan-1-one (Scheme I), in the optically active form, was synthesized for use in fast aldol condensations (Nugent et al., 2010). The molecule assumes an approximate propellar shape (Fig. 1), with the three aromatic rings being nearly perpendicularly aligned at with respect to the plane formed by the C atoms that are connected to the methine C atom [dihedral angles: pyridyl 79.82 (4), phenyl 80.12 (3), phenyl 86.93 (3)°].
Experimental
In a 250 ml flask was added sodium borohydride (4 equiv, 1.2 mg, 48 mmol) in anhydrous toluene (40 ml) followed by the addition of 18-crown-6 (0.1 equiv, 0.32 mg, 1.2 mmol), and acetyl pyridine (1 equiv, 1.35 ml, 12 mmol). Benzyl bromide (2.5 equiv, 3.6 ml, 30 mmol) was added. The reaction mixture was stirred at 323 K for 5 h under an inert atmosphere. The reaction was monitored by TLC and GC. The reaction was quenched by adding saturated ammonium chloride. The organic compound was extracted with ethyl acetate. The organic layer was dried over sodium sulfate and the solvent removed to give a yellow oil. This was submitted to flash chromatography and eluted with 5% ethyl acetate/hexane to give the desired ketone product (70% yield).
Refinement
H-atoms were placed in calculated positions [C—H 0.95 to 0.99 Å, Uiso(H) 1.2Ueq(C)] and were included in the refinement in the riding model approximation.
Figures
Fig. 1.
Anisotropic displacement ellipsoid plot (Barbour, 2001) of C21H19NO at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C21H19NO | F(000) = 640 |
| Mr = 301.37 | Dx = 1.271 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: -P 2yn | Cell parameters from 14175 reflections |
| a = 15.1569 (3) Å | θ = 3.1–76.1° |
| b = 5.6333 (1) Å | µ = 0.60 mm−1 |
| c = 19.5468 (4) Å | T = 100 K |
| β = 109.295 (2)° | Block, colourless |
| V = 1575.22 (5) Å3 | 0.30 × 0.20 × 0.10 mm |
| Z = 4 |
Data collection
| Agilent SuperNova Dual diffractometer with Atlas detector | 3299 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 3133 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.034 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 76.3°, θmin = 3.2° |
| ω scans | h = −19→19 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011) | k = −7→5 |
| Tmin = 0.840, Tmax = 0.942 | l = −24→24 |
| 25210 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.098 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0537P)2 + 0.5533P] where P = (Fo2 + 2Fc2)/3 |
| 3299 reflections | (Δ/σ)max = 0.001 |
| 208 parameters | Δρmax = 0.26 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.52050 (5) | 0.37525 (13) | 0.78840 (4) | 0.01980 (17) | |
| N1 | 0.46273 (6) | 0.74653 (15) | 0.63746 (5) | 0.0198 (2) | |
| C1 | 0.42604 (8) | 0.7372 (2) | 0.56524 (6) | 0.0238 (2) | |
| H1 | 0.4365 | 0.8677 | 0.5381 | 0.029* | |
| C2 | 0.37344 (8) | 0.5477 (2) | 0.52749 (6) | 0.0238 (2) | |
| H2 | 0.3482 | 0.5497 | 0.4761 | 0.029* | |
| C3 | 0.35863 (8) | 0.3560 (2) | 0.56657 (6) | 0.0234 (2) | |
| H3 | 0.3218 | 0.2252 | 0.5425 | 0.028* | |
| C4 | 0.39850 (7) | 0.35810 (19) | 0.64159 (6) | 0.0199 (2) | |
| H4 | 0.3908 | 0.2274 | 0.6698 | 0.024* | |
| C5 | 0.45003 (7) | 0.55637 (17) | 0.67445 (5) | 0.0156 (2) | |
| C6 | 0.49749 (6) | 0.56118 (17) | 0.75561 (5) | 0.0149 (2) | |
| C7 | 0.51069 (7) | 0.80051 (17) | 0.79253 (5) | 0.0146 (2) | |
| H7 | 0.5282 | 0.9173 | 0.7607 | 0.018* | |
| C8 | 0.58912 (7) | 0.79632 (18) | 0.86598 (5) | 0.0162 (2) | |
| H8A | 0.5892 | 0.9489 | 0.8911 | 0.019* | |
| H8B | 0.5763 | 0.6684 | 0.8961 | 0.019* | |
| C9 | 0.68460 (7) | 0.75692 (17) | 0.85936 (5) | 0.0152 (2) | |
| C10 | 0.72381 (7) | 0.92961 (18) | 0.82695 (5) | 0.0173 (2) | |
| H10 | 0.6902 | 1.0716 | 0.8093 | 0.021* | |
| C11 | 0.81153 (7) | 0.89629 (19) | 0.82020 (5) | 0.0198 (2) | |
| H11 | 0.8372 | 1.0150 | 0.7978 | 0.024* | |
| C12 | 0.86173 (7) | 0.68974 (19) | 0.84614 (5) | 0.0204 (2) | |
| H12 | 0.9218 | 0.6672 | 0.8418 | 0.024* | |
| C13 | 0.82336 (7) | 0.51680 (18) | 0.87838 (5) | 0.0197 (2) | |
| H13 | 0.8573 | 0.3755 | 0.8963 | 0.024* | |
| C14 | 0.73529 (7) | 0.54951 (18) | 0.88463 (5) | 0.0173 (2) | |
| H14 | 0.7094 | 0.4293 | 0.9063 | 0.021* | |
| C15 | 0.41552 (7) | 0.87940 (17) | 0.79944 (5) | 0.0163 (2) | |
| H15A | 0.4214 | 1.0450 | 0.8173 | 0.020* | |
| H15B | 0.3674 | 0.8773 | 0.7507 | 0.020* | |
| C16 | 0.38254 (7) | 0.72582 (17) | 0.84959 (5) | 0.0159 (2) | |
| C17 | 0.32976 (7) | 0.52092 (18) | 0.82459 (5) | 0.0180 (2) | |
| H17 | 0.3112 | 0.4813 | 0.7746 | 0.022* | |
| C18 | 0.30400 (7) | 0.37407 (18) | 0.87213 (6) | 0.0197 (2) | |
| H18 | 0.2680 | 0.2356 | 0.8544 | 0.024* | |
| C19 | 0.33078 (7) | 0.42930 (19) | 0.94531 (6) | 0.0211 (2) | |
| H19 | 0.3140 | 0.3278 | 0.9778 | 0.025* | |
| C20 | 0.38230 (8) | 0.6343 (2) | 0.97072 (6) | 0.0220 (2) | |
| H20 | 0.4005 | 0.6737 | 1.0207 | 0.026* | |
| C21 | 0.40717 (7) | 0.78150 (18) | 0.92306 (6) | 0.0192 (2) | |
| H21 | 0.4415 | 0.9224 | 0.9408 | 0.023* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0225 (4) | 0.0148 (4) | 0.0208 (4) | 0.0004 (3) | 0.0055 (3) | 0.0034 (3) |
| N1 | 0.0237 (4) | 0.0177 (4) | 0.0170 (4) | −0.0013 (3) | 0.0055 (3) | 0.0008 (3) |
| C1 | 0.0300 (6) | 0.0227 (5) | 0.0173 (5) | 0.0001 (4) | 0.0060 (4) | 0.0025 (4) |
| C2 | 0.0255 (5) | 0.0263 (6) | 0.0161 (5) | 0.0040 (4) | 0.0021 (4) | −0.0016 (4) |
| C3 | 0.0218 (5) | 0.0211 (5) | 0.0235 (5) | −0.0015 (4) | 0.0024 (4) | −0.0054 (4) |
| C4 | 0.0196 (5) | 0.0169 (5) | 0.0225 (5) | −0.0012 (4) | 0.0061 (4) | −0.0005 (4) |
| C5 | 0.0146 (4) | 0.0152 (5) | 0.0172 (5) | 0.0017 (3) | 0.0054 (4) | −0.0002 (4) |
| C6 | 0.0127 (4) | 0.0153 (5) | 0.0177 (5) | −0.0008 (3) | 0.0062 (4) | 0.0008 (4) |
| C7 | 0.0156 (4) | 0.0140 (4) | 0.0138 (4) | −0.0001 (3) | 0.0043 (4) | 0.0013 (3) |
| C8 | 0.0165 (5) | 0.0179 (5) | 0.0138 (4) | −0.0009 (4) | 0.0043 (4) | −0.0007 (3) |
| C9 | 0.0163 (5) | 0.0173 (5) | 0.0110 (4) | −0.0024 (4) | 0.0029 (3) | −0.0026 (3) |
| C10 | 0.0196 (5) | 0.0168 (5) | 0.0141 (4) | −0.0011 (4) | 0.0037 (4) | −0.0001 (4) |
| C11 | 0.0217 (5) | 0.0223 (5) | 0.0166 (5) | −0.0053 (4) | 0.0077 (4) | −0.0015 (4) |
| C12 | 0.0172 (5) | 0.0250 (5) | 0.0196 (5) | −0.0020 (4) | 0.0071 (4) | −0.0057 (4) |
| C13 | 0.0200 (5) | 0.0181 (5) | 0.0191 (5) | 0.0013 (4) | 0.0040 (4) | −0.0027 (4) |
| C14 | 0.0197 (5) | 0.0169 (5) | 0.0147 (4) | −0.0024 (4) | 0.0048 (4) | −0.0007 (4) |
| C15 | 0.0166 (5) | 0.0151 (5) | 0.0171 (5) | 0.0019 (3) | 0.0052 (4) | 0.0018 (3) |
| C16 | 0.0138 (4) | 0.0157 (5) | 0.0185 (5) | 0.0030 (3) | 0.0056 (4) | 0.0016 (4) |
| C17 | 0.0164 (4) | 0.0196 (5) | 0.0169 (4) | 0.0003 (4) | 0.0040 (4) | −0.0003 (4) |
| C18 | 0.0169 (5) | 0.0183 (5) | 0.0230 (5) | −0.0018 (4) | 0.0054 (4) | 0.0007 (4) |
| C19 | 0.0203 (5) | 0.0226 (5) | 0.0219 (5) | 0.0003 (4) | 0.0092 (4) | 0.0048 (4) |
| C20 | 0.0237 (5) | 0.0262 (5) | 0.0174 (5) | −0.0005 (4) | 0.0084 (4) | −0.0007 (4) |
| C21 | 0.0197 (5) | 0.0177 (5) | 0.0204 (5) | −0.0012 (4) | 0.0071 (4) | −0.0023 (4) |
Geometric parameters (Å, º)
| O1—C6 | 1.2172 (12) | C10—H10 | 0.9500 |
| N1—C1 | 1.3365 (14) | C11—C12 | 1.3910 (15) |
| N1—C5 | 1.3414 (13) | C11—H11 | 0.9500 |
| C1—C2 | 1.3897 (16) | C12—C13 | 1.3881 (15) |
| C1—H1 | 0.9500 | C12—H12 | 0.9500 |
| C2—C3 | 1.3829 (16) | C13—C14 | 1.3922 (14) |
| C2—H2 | 0.9500 | C13—H13 | 0.9500 |
| C3—C4 | 1.3893 (15) | C14—H14 | 0.9500 |
| C3—H3 | 0.9500 | C15—C16 | 1.5111 (13) |
| C4—C5 | 1.3922 (14) | C15—H15A | 0.9900 |
| C4—H4 | 0.9500 | C15—H15B | 0.9900 |
| C5—C6 | 1.5103 (13) | C16—C21 | 1.3950 (14) |
| C6—C7 | 1.5110 (13) | C16—C17 | 1.3974 (14) |
| C7—C8 | 1.5320 (13) | C17—C18 | 1.3928 (14) |
| C7—C15 | 1.5573 (13) | C17—H17 | 0.9500 |
| C7—H7 | 1.0000 | C18—C19 | 1.3869 (15) |
| C8—C9 | 1.5113 (13) | C18—H18 | 0.9500 |
| C8—H8A | 0.9900 | C19—C20 | 1.3905 (15) |
| C8—H8B | 0.9900 | C19—H19 | 0.9500 |
| C9—C10 | 1.3963 (14) | C20—C21 | 1.3892 (15) |
| C9—C14 | 1.3960 (14) | C20—H20 | 0.9500 |
| C10—C11 | 1.3916 (14) | C21—H21 | 0.9500 |
| C1—N1—C5 | 117.01 (9) | C12—C11—C10 | 120.20 (9) |
| N1—C1—C2 | 123.81 (10) | C12—C11—H11 | 119.9 |
| N1—C1—H1 | 118.1 | C10—C11—H11 | 119.9 |
| C2—C1—H1 | 118.1 | C13—C12—C11 | 119.46 (9) |
| C3—C2—C1 | 118.42 (10) | C13—C12—H12 | 120.3 |
| C3—C2—H2 | 120.8 | C11—C12—H12 | 120.3 |
| C1—C2—H2 | 120.8 | C12—C13—C14 | 120.31 (10) |
| C2—C3—C4 | 118.89 (10) | C12—C13—H13 | 119.8 |
| C2—C3—H3 | 120.6 | C14—C13—H13 | 119.8 |
| C4—C3—H3 | 120.6 | C13—C14—C9 | 120.75 (9) |
| C3—C4—C5 | 118.41 (10) | C13—C14—H14 | 119.6 |
| C3—C4—H4 | 120.8 | C9—C14—H14 | 119.6 |
| C5—C4—H4 | 120.8 | C16—C15—C7 | 114.03 (8) |
| N1—C5—C4 | 123.40 (9) | C16—C15—H15A | 108.7 |
| N1—C5—C6 | 116.65 (8) | C7—C15—H15A | 108.7 |
| C4—C5—C6 | 119.92 (9) | C16—C15—H15B | 108.7 |
| O1—C6—C5 | 119.46 (9) | C7—C15—H15B | 108.7 |
| O1—C6—C7 | 123.14 (8) | H15A—C15—H15B | 107.6 |
| C5—C6—C7 | 117.36 (8) | C21—C16—C17 | 118.25 (9) |
| C6—C7—C8 | 111.91 (8) | C21—C16—C15 | 120.38 (9) |
| C6—C7—C15 | 108.41 (8) | C17—C16—C15 | 121.33 (9) |
| C8—C7—C15 | 112.17 (8) | C18—C17—C16 | 120.77 (9) |
| C6—C7—H7 | 108.1 | C18—C17—H17 | 119.6 |
| C8—C7—H7 | 108.1 | C16—C17—H17 | 119.6 |
| C15—C7—H7 | 108.1 | C19—C18—C17 | 120.25 (10) |
| C9—C8—C7 | 112.99 (8) | C19—C18—H18 | 119.9 |
| C9—C8—H8A | 109.0 | C17—C18—H18 | 119.9 |
| C7—C8—H8A | 109.0 | C18—C19—C20 | 119.55 (10) |
| C9—C8—H8B | 109.0 | C18—C19—H19 | 120.2 |
| C7—C8—H8B | 109.0 | C20—C19—H19 | 120.2 |
| H8A—C8—H8B | 107.8 | C21—C20—C19 | 120.06 (10) |
| C10—C9—C14 | 118.47 (9) | C21—C20—H20 | 120.0 |
| C10—C9—C8 | 119.98 (9) | C19—C20—H20 | 120.0 |
| C14—C9—C8 | 121.55 (9) | C20—C21—C16 | 121.10 (10) |
| C11—C10—C9 | 120.82 (9) | C20—C21—H21 | 119.5 |
| C11—C10—H10 | 119.6 | C16—C21—H21 | 119.5 |
| C9—C10—H10 | 119.6 | ||
| C5—N1—C1—C2 | 2.48 (16) | C14—C9—C10—C11 | −0.23 (14) |
| N1—C1—C2—C3 | −0.61 (17) | C8—C9—C10—C11 | −179.84 (9) |
| C1—C2—C3—C4 | −1.43 (16) | C9—C10—C11—C12 | −0.31 (15) |
| C2—C3—C4—C5 | 1.50 (16) | C10—C11—C12—C13 | 0.38 (15) |
| C1—N1—C5—C4 | −2.38 (15) | C11—C12—C13—C14 | 0.09 (15) |
| C1—N1—C5—C6 | 175.79 (9) | C12—C13—C14—C9 | −0.64 (15) |
| C3—C4—C5—N1 | 0.44 (15) | C10—C9—C14—C13 | 0.70 (14) |
| C3—C4—C5—C6 | −177.67 (9) | C8—C9—C14—C13 | −179.69 (9) |
| N1—C5—C6—O1 | −151.38 (9) | C6—C7—C15—C16 | 66.21 (10) |
| C4—C5—C6—O1 | 26.85 (13) | C8—C7—C15—C16 | −57.86 (11) |
| N1—C5—C6—C7 | 30.88 (12) | C7—C15—C16—C21 | 90.50 (11) |
| C4—C5—C6—C7 | −150.88 (9) | C7—C15—C16—C17 | −87.20 (11) |
| O1—C6—C7—C8 | 24.09 (13) | C21—C16—C17—C18 | −1.18 (15) |
| C5—C6—C7—C8 | −158.27 (8) | C15—C16—C17—C18 | 176.56 (9) |
| O1—C6—C7—C15 | −100.14 (10) | C16—C17—C18—C19 | −0.18 (15) |
| C5—C6—C7—C15 | 77.51 (10) | C17—C18—C19—C20 | 0.99 (16) |
| C6—C7—C8—C9 | 66.67 (10) | C18—C19—C20—C21 | −0.42 (16) |
| C15—C7—C8—C9 | −171.23 (8) | C19—C20—C21—C16 | −0.98 (16) |
| C7—C8—C9—C10 | 66.63 (11) | C17—C16—C21—C20 | 1.76 (15) |
| C7—C8—C9—C14 | −112.97 (10) | C15—C16—C21—C20 | −176.00 (9) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5803).
References
- Agilent (2011). CrysAlis PRO Agilent Technologies, Yarnton, Oxfordshire, England.
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Nugent, T. C., Umar, M. N. & Bibi, A. (2010). Org. Biomol. Chem. 8, 4085–4089. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812003686/bt5803sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812003686/bt5803Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812003686/bt5803Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report