Abstract
This study assessed the effects of a contingency management (CM) intervention for alcohol consumption in 10 alcohol-dependent participants. An ABCA design was used. Vouchers were provided contingent on results of ethyl glucuronide (EtG) urine tests (an alcohol biomarker with a 2-day detection period) and alcohol breath tests during the C phase. The percentage of negative urines was 35% during the first baseline phase, 69% during the C phase, and 20% during the return-to-baseline phase. Results suggest that EtG urine tests may be a feasible method to deliver CM to promote alcohol abstinence.
Keywords: addiction, alcohol, contingency management, biomarker, ethyl glucuronide
Contingency management (CM) has been supported extensively as a strategy to promote abstinence from illicit drugs (Prendergast, Podus, Finney, Greenwell, & Roll, 2006). Widespread investigation of CM as a treatment for drug use has been based, in part, on the availability of low-cost immunoassay urine drug tests, which immediately detect drug use for at least 2 days prior to testing and provide immediate results. These tests can be administered two to three times a week to verify abstinence.
Despite initial findings that support CM as a treatment for alcohol use (Petry, Martin, Cooney, & Kranzler, 2000), continued investigations have been limited primarily because breath tests, the most commonly used objective measure of alcohol use, detect use only up to 12 hr (Warner & Sharma, 2009). In contrast, ethyl glucuronide (EtG), a metabolite of alcohol, has a detection period in urine of up to 3 days (see Litten, Bradley, & Moss, 2010, for a review), depending on the amount of alcohol consumed and individual factors (e.g., an individual's weight). EtG testing is increasingly being used in forensic, employment screening, and treatment settings (Litten et al., 2010).
EtG can be assessed in urine using liquid chromatography mass spectrometry (LC/MS) or a relatively low-cost immunoassay (EtG-I). EtG-I analyses are available through commercial drug-testing laboratories or by using an onsite nonlaboratory-based analyzer. EtG-I is an alcohol biomarker that may be superior to breath tests and is suitable for use in a CM intervention.
This study investigated the effects of a voucher-based CM intervention in 15 alcohol-dependent adults using an ABCA design. The EtG-I biomarker and alcohol breath tests served as the basis for reinforcement of abstinence.
METHOD
Participants
Participants were 15 adults (21 to 65 years-old, M = 29; SD = 8.8) who met criteria for current alcohol dependence. Ten participants had a self-reported history of methamphetamine use. The majority of participants were male (87%, n = 13), Caucasian (80%, n = 12), and unemployed (67%, n = 10). Participants were included in the study if they were 21 to 65 years of age and reported at least four drinking episodes each week (three or more drinks per episode) during 30 days prior to study entry. Individuals were excluded if they had a history of alcohol withdrawal, were using methamphetamine during the 30 days prior to study entry, or were unsafe to participate. Participants were not enrolled in alcohol treatment.
Measures
Urine and breath samples were collected at an initial intake assessment and on every Monday and Thursday during the 9 weeks of participation. Urine samples were sent to American Drug Testing, Spokane, Washington, where EtG-I urine tests were conducted using a Microgenics MGC-240 analyzer and the DRI ethyl glucuronide enzyme immunoassay (Thermo-Fisher Scientific). A cutoff level of 500 ng/ml was used (Bottcher, Beck, & Helander, 2008). Twice weekly, monitoring of alcohol use was based on monitoring schedules used in CM studies of stimulants (which are detectable in urine for 2 to 3 days; Petry et al., 2005). Alcohol breath tests (Alcosensor Breathalyzer) were considered positive if blood alcohol concentration was greater than 0.001%. The alcohol Timeline Follow Back (TLFB; Sobell & Sobell, 2000) was completed at all study sessions (after the delivery of reinforcers) to assess drinking since the last study visit.
Procedure
Approval for this study was provided by appropriate institutional review boards. Participants were recruited using online advertisements that requested individuals who had a history of problematic alcohol and methamphetamine use. Individuals with a history of comorbid methamphetamine use were recruited because of the research team's interest in improving treatment options for this population. Sixty-nine persons responded to the advertisement. Forty-five were screened for initial inclusion criteria over the telephone. Twenty-nine individuals were deemed eligible to participate. Fourteen of these individuals were no longer interested or did not attend an initial study appointment. Fifteen individuals provided written informed consent and participated in study procedures, which were conducted at Washington State University.
Design
An ABCA design was used. During the A and B phases, participants received $5 for each study visit if they provided both urine and breath samples. The baseline and return-to-baseline phases lasted 2 weeks. During a 1-week B phase, payment continued for urine and breath tests, and participants were warned that alcohol use during this week could result in positive EtG-I tests during the 1st week of the CM phase and failure to earn vouchers. This phase was included due to concerns about the oversensitivity and possible detection window of EtG-I.
During the 4-week CM (C) phase of the study, voucher values were based on an escalating schedule of reinforcement (Roll & Higgins, 2000). Participants received $5 for the first contingent phase visit in which alcohol-free breath and urine tests were provided. The value of subsequent vouchers increased by $2 for each consecutive visit in which negative alcohol samples were provided. Positive alcohol samples or nonattendance resulted in no voucher for that visit and a reset of the voucher value to $5, which escalated thereafter in the manner described above. Continuously abstinent participants could earn up to $96. Voucher values were selected to be consistent with previous research that demonstrated increased abstinence using similar voucher values (Higgins et al., 1994). Because an offsite laboratory analyzed the urine samples, EtG-I results were available prior to the next study visit. Thus, vouchers were contingent on the EtG-I result from the previous visit and the breath test from the current visit. The additional contingency of alcohol-negative breath tests was selected to ensure that reinforcers were not provided to individuals who recently used alcohol or were intoxicated.
During the final study visit, participants received a brief intervention that was comprised of motivational interviewing and referrals to alcohol treatment providers. Those who completed all study visits received a $30 gift card.
Statistical Analysis
To supplement visual analysis, effects of CM on alcohol abstinence were assessed using generalized estimating equations (GEE), in which the main effect of time was entered and compared outcomes during Phases B and C to the baseline phase. GEE is a method of analyzing data that is similar to repeated measures ANOVA; however, GEE allows continuous and binomial outcomes (Hardin & Hilbe, 2003). Missing EtG-I samples were coded as positive. Because no alcohol breath tests were positive, these data were not analyzed statistically. TLFB data also were analyzed using GEE. Alpha was set at .05. Odds ratios were calculated using GEE analyses. In addition, measures of sensitivity (true positives divided by true positives plus false negatives) and specificity (true negatives divided by true negatives plus false positives) were calculated to assess the performance of the EtG-I test. Sensitivity indicates the ability of the test to detect a positive result, and specificity indicates the ability of the test to detect a negative result. The “standard” in this analysis was self-reported drinking assessed via the TLFB. Self-reported drinking was used as the standard because vouchers were not contingent on self-report data, the TLFB has been validated extensively in previous research (Sobell & Sobell, 2000), and other validity outcomes, such as objective observation of use or quantitative EtG testing (e.g., LC/MS), were not feasible as part of this small study.
RESULTS AND DISCUSSION
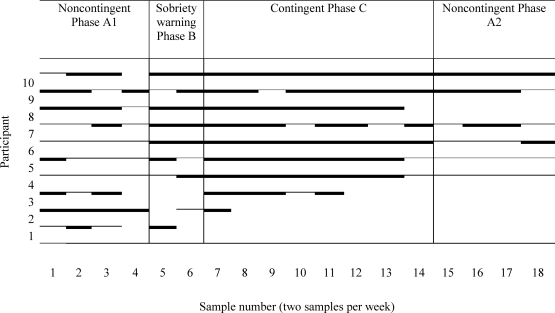
Nine of 15 participants submitted an alcohol-negative EtG-I sample at study intake. Two participants did not complete the baseline phase of the study, and three participants were continuously abstinent throughout the baseline and sobriety warning phases. Therefore, it was not possible to investigate changes in abstinence associated with CM for these five participants. Figure 1 shows the alcohol urine-test results for the remaining 10 participants. The percentage of alcohol-negative EtG-I tests across the study was 35% (first baseline phase), 60% (B phase), 69% (C phase), and 20% (return-to-baseline phase). Statistical analyses of these 10 participants indicated that they were significantly more likely to submit alcohol-negative urines during the CM treatment phase of the study than in the baseline phase (odds ratio = 4.71, χ2 = (1) 8.15, p < .05). Although alcohol-negative samples were more likely to be submitted during the sobriety warning phase than in the baseline phase, this difference was not statistically significant. The self-reports of alcohol use (TLFB) revealed a similar pattern relative to the urine tests. Relative to the first baseline phase (37% days abstinent), participants reported significantly higher levels of self-reported days of abstinence during the sobriety warning phase (65% days abstinent; χ2 = (1) 16.67, p < .05), and the CM phase (63% days abstinent; χ2 = (1) 35.31, p < .05). Participants reported abstinence for 43% of days during the return-to-baseline phase. Participants earned an average of $77 (SD = $27) during the CM phase.
Figure 1.
Alcohol urinalysis results for individual participants. The solid thick lines indicate a negative result. The solid thinner lines indicate a positive result. A break in the line indicates that the participant did not provide a urine sample.
Agreement between EtG-I and self-report was 74% (142 of 193 samples) when EtG-I results were compared to self-reported drinking of at least two standard drinks per day over the 2 days prior to collection of each urine sample (a detection period that has been supported in previous research; McDonell et al., 2011). Using self-reported drinking as a validity outcome, the specificity of EtG-I was high (97%). The high specificity of EtG-I observed in this study does not support previous concerns regarding inadvertent positive tests due to ingestion of mouthwash or other products that contain small amounts of ethanol (Litten et al., 2010).
The sensitivity of EtG-I using a cutoff of 500 ng/ml was superior to breath tests (sensitivity = 0). EtG-I was able to accurately detect self-reported drinking 75% and 50% of the time when participants reported drinking 1 or 2 days prior to EtG-I testing, respectively. EtG-I was more likely to detect self-reported drinking (sensitivity = 75%, specificity = 90%) when drinking occurred almost daily (i.e., two or more standard drinks were consumed during at least 3 of the 4 days prior to EtG-I testing). Therefore, using a 500 ng/ml cutoff, EtG-I was able to detect low levels of drinking the day prior to testing, as well as drinking that occurred nearly daily. Detecting infrequent drinking, particularly when it occurs 2 or more days prior to testing, may require use of a lower EtG-I cutoff level.
Several limitations of this study should be noted. First, although none of the breath tests were positive for alcohol use, it is possible that administration of breath tests, and not EtG-I tests alone, influenced participants' behavior during the CM phase. Future studies using an on-site analyzer would allow immediate EtG-I results and delivery of reinforcers without using breath tests. Although purchase of an analyzer involves a high initial cost (approximately $30,000 to $35,000), each EtG-I test can be conducted at a cost (as low as $1 to $2 per test) that is substantially below the fee charged for EtG-I testing by commercial drug-testing companies ($10 to $12 per test). Purchasing an on-site analyzer might be a cost-effective option for treatment agencies that implement a high-volume CM intervention. Second, the study design does not allow us to isolate the variables that were responsible for the changes in abstinence during the sobriety warning (B) phase. It is possible that participants' behavior might have been influenced by rule-governed behavior (e.g., the verbal warning about the upcoming contingency). Finally, this study was not designed to be a formal evaluation of EtG-I accuracy. In particular, the small sample size and use of self-reported drinking as a validity outcome limit conclusions about EtG-I accuracy. Evaluating EtG-I accuracy should involve a larger sample, include observation and verification of drinking behavior, and administer quantitative EtG analyses with established accuracy (e.g., LC/MS).
Despite these limitations, the results suggest that a CM intervention based on EtG-I has the potential to provide clinically significant reductions in alcohol use. Future studies are needed to determine if the intervention (using an on-site analyzer with lower cutoffs for a positive test) with a longer duration is both feasible and effective in promoting alcohol abstinence.
Acknowledgments
This study was supported by Grant CKWX0382 from the U.S. Department of Justice. We thank Rob Packer, Geetha Gujjarlapudi, and Arlana Byers for their assistance.
REFERENCES
- Bottcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol and Alcoholism. 2008;43:46–48. doi: 10.1093/alcalc/agm153. [DOI] [PubMed] [Google Scholar]
- Hardin J.W, Hilbe J.M. Generalized estimating equations. Boca Raton, FL: Chapman and Hall/CRC; 2003. [Google Scholar]
- Higgins S.T, Budney A.J, Bickel W.K, Foerg F.E, Donham R, Badger G.J. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Litten R.Z, Bradley A.M, Moss H.B. Alcohol biomarkers in applied settings: Recent advances and future research opportunities. Alcoholism: Clinical and Experimental Research. 2010;34:955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- McDonell M.G, Howell D, Srebnik D, Angelo F, Sugar A, Rainey C, et al. Monitoring alcohol abstinence using ethyl glucuronide urinalysis in alcohol dependent outpatients with comorbid drug dependence and mental illness. The American Journal on Addictions. 2011;20:482–484. doi: 10.1111/j.1521-0391.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N.M, Martin B, Cooney J.L, Kranzler H.R. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psycholology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry N.M, Peirce J.M, Stitzer M.L, Blaine J, Roll J.M, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Sobell L.C, Sobell M.B. Handbook of psychiatric measures. Washington DC: American Psychiatric Association; 2000. Alcohol timeline followback (TLFB) [Google Scholar]
- Warner E.A, Sharma N. Principles of addiction medicine (4th ed., pp. 295–304) Philadelphia: Lippincot Williams & Wilkens; 2009. Laboratory diagnosis. In R. K. Ries, S. C. Miller, D. A. Fiellen, & R. Saitz (Eds.), [Google Scholar]