Abstract
This experiment investigated social referencing as a form of discriminative learning in which maternal facial expressions signaled the consequences of the infant's behavior in an ambiguous context. Eleven 4- and 5-month-old infants and their mothers participated in a discrimination-training procedure using an ABAB design. Different consequences followed infants' reaching toward an unfamiliar object depending on the particular maternal facial expression. During the training phases, a joyful facial expression signaled positive reinforcement for the infant reaching for an ambiguous object, whereas a fearful expression signaled aversive stimulation for the same response. Baseline and extinction conditions were implemented as controls. Mothers' expressions acquired control over infants' approach behavior for all participants. All participants ceased to show discriminated responding during the extinction phase. The results suggest that 4- and 5-month-old infants can learn social referencing via discrimination training.
Keywords: discrimination training, facial expression, infants, joint attention, mother–infant preverbal communication, social referencing
Social referencing can be analyzed as a behavior chain in which the presence of an ambiguous object or event signals the gaze shift of an infant towards another person, typically the mother, whose facial, vocal, and gestural expressions may then serve as discriminative stimuli for a subsequent approach response (Feinman & Lewis, 1983; Gewirtz & Pelaez-Nogueras, 1992; Repacholi, 2009). Some authors have suggested that social referencing is a key component of early communication skills and a prerequisite for later language acquisition (Baldwin, 1995; Pelaez, 2009). Individuals with autism and developmental disabilities present various deficits in communication skills including joint attention, play, imitation, and social referencing (Ozonoff & South, 2001). Longitudinal studies conducted with individuals with autism have shown that deficits in these repertoires are associated with poorer verbal and social achievements later in childhood (Mundy & Gomes, 1998; Stone & Yoder, 2001). Advancements in early diagnosis, coupled with the evidence accrued in favor of early intervention in autism, underline the importance of developing training procedures for infants and young children (Matson & Sipes, 2010). There have been some efforts to teach social referencing to individuals with autism (Brim, Townsend, DeQuinzio, & Poulson, 2009); however, these efforts might benefit from a greater understanding of the development of social referencing in infancy. Identifying procedures that are sufficient for teaching social referencing with infants may contribute to an understanding of both typical and delayed social development.
The most prevalent etiological approach to social referencing is “preformationism.” According to this view, the acquisition of social referencing in infants is “prewired” and is not a result of social learning (Brim et al., 2009; Klinnert, Campos, Sorce, Emde, & Svejda, 1983; Campos & Stenberg, 1981). According to J. J. Campos, “there need be no social learning for an infant to respond appropriately to social signals specifying emotion. Once the perceptual apparatus is attuned to the pattern of visual information going along with positive vs. negative emotions, the infant should be able to respond appropriately” (personal communication, December 8, 2010; see also Campos et al., 2000; Uchiyama et al., 2008). Preformationism does not offer guidance for remediating deficits in social referencing.
By contrast, in a behavior-analytic framework, social referencing can be conceptualized as an operant class that is established through stimulus discrimination and reinforcement (Pelaez, 2009). A typical referencing episode is composed of the following sequence of events: (a) presentation of an ambiguous object or event, (b) infant's gaze shift toward the adult, (c) facial expression posed by the adult, and (d) infant's event-related behavior (e.g., playing, avoidance). Social referencing may be a form of discriminative learning that results from a history of environmental contingencies that operate during early mother–child verbal interactions (Dube, MacDonald, Mansfield, Holcomb, & Ahearn, 2004; Gewirtz & Pelaez-Nogueras, 1992; Holth, 2005; Lewis & Feiring, 1992; Pelaez, Gewirtz, & Wong, 2008).
Figure 1 shows a hypothesized sequence of behavioral and environmental events that may be present in the social referencing process in the natural environment. Maternal facial expressions signal the consequences (reinforcing or aversive) of the infant's reaching toward the ambiguous object. As a result, maternal facial expressions may be established as a conditioned reinforcer for gaze shifting towards the mother's face anytime an ambiguous object or event is presented. There is experimental evidence to suggest that a discriminative stimulus that signals a contingency of reinforcement may be established as a conditioned reinforcer (Dinsmoor, 1950; Lovaas et al., 1966). Moreover, when the value of the chain's final reinforcer is altered, interim reinforcers, which also operate as discriminative stimuli, lose their reinforcing value accordingly (Kuhn, Lerman, Vorndran, & Addison, 2006; Michael, 2000). In summary, the two discriminated responses in the behavior chain are (a) gaze shifting occasioned by the presentation of an unfamiliar object, and (b) event-related behavior (e.g., approaching or avoiding the object) controlled by specific facial expressions (Pelaez, 2009; Pelaez-Nogueras & Gewirtz, 1997). A demonstration of the development of this behavior chain through discrimination training would suggest that social referencing can be acquired via operant learning. Of course, this demonstration does not confirm that social referencing is acquired through an identical process under natural conditions (see Baer, 1973).
Figure 1.
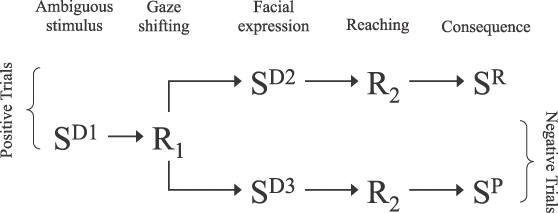
Two-component behavior chains describing the behavioral events hypothesized to be present in social referencing and programmed in the positive and negative trials in this study. SD1: ambiguous object; SD2: joyful facial expression; SD3: fearful facial expression; R1: gaze shifting; R2: reaching; SP: punishing stimuli; SR: reinforcing stimuli.
In an earlier study, Gewirtz and Pelaez-Nogueras (1992) explored whether arbitrary maternal expressions acquired stimulus control over infant responses. The originally meaningless expressions consisted of hand-to-face movements made by the mother and were trained as cues for the behavior of 9-month-old infants toward an ambiguous object (covered puppet). One maternal hand-to-face gesture (i.e., hands opened on either side of head, palms facing the infant) signaled the availability of reinforcement (i.e., soft lights and music) for approaching the ambiguous object, whereas a second hand-to-face sign (i.e., clenched right fist to the nose) was correlated with aversive stimulation for approaching responses (i.e., harsh door buzzer). As a result of this procedure, the infants showed differential responding to the two signs. That is, the infants differentially reached for the ambiguous objects after the positive sign and avoided objects following the presentation of signs paired with aversive consequences. However, because meaningless cues (rather than socially relevant maternal emotional expressions) were used, the ecological validity of the study was not optimal.
The current study expanded on the findings of Gewirtz and Pelaez-Nogueras (1991, 1992) by presenting a laboratory demonstration of the development of social referencing using socially relevant maternal emotional expressions that consisted of smiling and a fearful expression.
METHOD
Participants and Setting
Infants and mothers were recruited through the Dade County birth records in the greater Miami metropolitan area. Mothers initially were contacted via mail and subsequently were reached by phone. Mother–infant dyads were selected from mid-socioeconomic strata. Mothers were asked to participate voluntarily in a study on communication development in babies. Mothers signed an informed consent form developed according to the Helsinki declaration guidelines. No monetary compensation was offered for participation. Mothers and their children received a certificate for their contribution to research and science. Only those participants who did not show the target discrimination before the first differential reinforcement phase (DR1) could be included in the study. None of the infants were excluded by this criterion, however. There were eight drop outs: five infants were unable to complete the experiment due to excessive crying or difficulties in scheduling a second visit to finish data collection, two mothers did not follow instructions, and one mother decided to stop participating. Eleven typically developing infants (six boys and five girls, eight of whom were 4 months old and three of whom were 5 months old; six were Caucasian, and five were Hispanic) and their mothers participated in this experiment. Participants were not receiving any specialized developmental or medical services at the time of the study. Young infants were selected to ensure that the fewest possible components of social referencing existed in their behavioral repertoires. The literature suggests that social referencing is not present reliably in infants younger than 12 months of age (Hornik, Risenhoover, & Gunnar, 1987; Stenberg, 2009).
Sessions were conducted during weekdays. No more than one session was conducted daily; session duration averaged 30 min (range, 20 to 40 min). Sessions were conducted in an experimental room with an adjacent control room.
Equipment in the control room included a TV monitor connected to a special-effects generator to deliver stimuli, a microphone, a time–date generator, and a videocassette recorder. Two camcorders equipped with wide-angle lenses recorded all activities in the experimental room. One camcorder was placed in the right corner (1.25 m away from the baby, behind the mother) to capture the infant responses. The second camera was located in the left corner (1.25 m away from the mother, behind the child) to capture both the mother's face and the puppet theater. Both cameras were activated simultaneously, and their timers were synchronized to allow retrospective coding.
Target Behaviors and Stimuli
The occurrence of the following responses was coded during each trial: use of prompts for gaze shifting, infant's gaze shifts towards the mother's face, mother's facial expressions (joyful or fearful), use of prompts for reaching, infant's reaching for the object, and presentation of consequences (positive or aversive). Three independent observers were trained, but only one or two collected data for each session. Observers coded all trials from videotaped recordings. The dependent variable was the percentage of trials per eight-trial block with reaching responses. A trial consisted of the following sequence of events: (a) ambiguous object presentation, (b) gaze shift, (c) facial expression, (d) reaching or not reaching response, and (e) reinforcing or punishing stimuli. The sequence was not considered a trial if any of these events was absent. Operational definitions are presented below.
Gaze shifting was defined as looking back at the mother's face within 5 s of the presentation of the target object. Looking back at the mother was defined as the infant orienting his or her head toward the mother (∼90° turn) while staring at the mother's face for a continuous period of 2 s or more.
Reaching was defined as the infant emitting gross motor movements (of the upper body plus the extension of the arm) toward the target object such that he or she could touch the object or get less than 5 cm from the object. This definition also applied to prompted instances of reaching.
Joyful and fearful facial expressions were modeled and practiced by the mothers before the session started (training took a few minutes) based on the descriptions and photographs for the expressions of happiness and fear by Ekman (1975). A joyful expression was defined as (a) corners of the lips drawn back and up, (b) mouth parted with teeth exposed, (c) wrinkle (naso-labial fold) running down from the nose to the outer edge beyond the lip corners, (d) raised cheeks, and (e) wrinkles below the lower eyelid (Ekman, Figure 50A, p. 112). A fearful expression was defined as (a) eyebrows raised and drawn together, (b) eyes open and lower lid tensed, and (c) both lips stretched back (Ekman, Figure 22B, p. 50). We requested that mothers maintain a neutral facial expression any time that a joyful or fearful expression was not required. Neutral expression was defined as eyes open without tension in the orbital area and mouth closed with lips and cheek muscles relaxed and still.
Punishing stimuli
A 2-s obnoxious sound (food blender, door buzzer, or whistle) coupled with a blue light were used as aversive consequences contingent on reaching after a fearful expression. To preclude habituation to the obnoxious sounds, the sound volume was adjusted between 75 dB (equivalent to a hair dryer) to 85 dB (equivalent to busy city traffic), depending on each infant's reaction to sound. Specifically, stimulus intensity was adjusted in those participants who did not show a noticeable startle response in one or more of the trials in the preceding block. The startle response was defined as a diffuse musculoskeletal response immediately following the presentation of the aversive stimulus. Aversive stimuli sometimes triggered emotional behaviors (infant crying, gazing away), although these were not considered necessary to determine that the aversive stimulus was effective. None of the mothers complained that a stimulus was inappropriate or too harsh. Sounds never exceeded 85 dB. Noise levels and durations were well below the permissible exposure times and levels recommended by the National Institute for Occupational Safety and Health (1998). Although some participants dropped out of the study due to excessive crying, crying seemed to be a function of exposure to the experimental setting rather than to any particular stimulus in our protocol. However, we did not record the occurrence of emotional behavior elicited by aversive stimuli. On the rare occasions that an infant cried after hearing the aversive stimulus (e.g., blender noise), we were able to resume the trials after a break of a few seconds or minutes.
Reinforcing stimuli
A portable tape recorder that played 3 s of baby melodies and brightly colored lights (connected to a switch) were used as reinforcers for reaching after joyful maternal expressions.
General Procedure
Positive and negative trials
A positive trial was composed of the following sequence of events: (a) object presentation, (b) gaze shifting towards the mother, (c) mother's joyful facial expression, (d) child response (reaching response or no response), and (e) reinforcing stimuli in the form of lights and music contingent on reaching (during differential reinforcement only). A negative trial was composed of the following sequence: (a) object presentation, (b) gaze shifting towards the mother, (c) mother presents a fearful facial expression contingent on gaze shifting, (d) child response (reaching response or no response), and (e) punishing stimulus in the form of an obnoxious noise contingent on reaching (during differential reinforcement only) or no consequence if the infant did not reach for the object. Either of these sequences of events composed a complete referencing episode if reaching ended a positive trial or if a negative trial ended with reaching omission. We used the term referencing to refer to these two behavior chains. On the other hand, we used the term reaching to refer narrowly to the infant response that ended the chain and served as the dependent variable.
Positive and negative trials were implemented in all experimental conditions with the only variation that no consequences were presented contingent on reaching during baseline and extinction. If gaze shifts did not occur spontaneously, prompting was used in both positive and negative trials. Therefore, gaze shifts were considered an invariable component of each trial. Prompts for gaze shifting were recorded for treatment fidelity purposes. For a trial to be recorded, the sequence of object presentation, gaze shift, and facial expression needed to be in place. Therefore, a trial would not be counted if a mother failed to present the appropriate facial expression at the time of the infant gaze shift. In addition to this sequence, a complete referencing episode required a reaching response (positive trials) or a response omission (negative trials). No trials were omitted based on whether the child reached or not. Positive and negative trials were interspersed within 16-trial sequences that contained eight positive and eight negative trials. The following constraints were enforced: (a) Each daily series began with a positive trial, and (b) there were never more than two positive or negative trials in succession. For graphing purposes, data were grouped in eight-trial blocks.
Session description
A 5-min period at the start of each daily visit enabled the mother and infant to become comfortable in the laboratory. During this period, the mother received training in the facial expressions to be posed during that session. An experimenter modeled the joyful and fearful expressions as described by Ekman (1975) and asked the mother to imitate him. The mother was required to respond within 2 s from the model presentation of the appropriate facial expression and to be 100% accurate in emitting a series of no fewer than 16 interspersed fearful and joyful facial expressions before the experiment commenced. During the actual experimental sessions, mothers followed the instructions of a second experimenter (via earphone), who was located in the control room. For each trial, the mother was instructed to pose a joyful, fearful, or neutral facial expression. Accuracy of each mother's facial expression was monitored throughout the experiment by one of the research assistants. Any time the mother's expressions departed from the operational definition, the trial was canceled and additional instructions or training was provided as needed. We resumed trials as soon as possible, continuing in the same trial number in which the session was discontinued. We did not record the number of times that sessions were interrupted. However, this was a concern primarily with the two mothers who had difficulty following instructions and who eventually dropped out of the study.
Each daily session consisted of an interspersed set of eight positive and eight negative trials, each lasting 40 to 60 s (50 s mean duration) throughout all phases of the study. The exact length of each trial depended on the latency to gaze shifting, the latency to reaching, and the duration of the prompting protocol if in place for that particular trial (prompting for gaze shifting, reaching, or both). Intertrial intervals lasted 8 s, with the exception of the few occasions when the aversive stimulus elicited emotional responses (e.g., crying). In those cases intertrial intervals lasted up to 5 min.
The experimenter in the control room instructed the experimenter behind the theater as to when to uncover an object and present the positive or negative consequences, as well as when to initiate and terminate each trial. Twenty-four different toys (e.g., small train) and puppets were alternated randomly and used as ambiguous objects. Any of these objects could be used in any trial type or study phase. However, they were uncovered only at the point of delivering the reinforcer during positive trials. Objects could be presented more than once for a particular subject over the course of the study.
At the beginning of every trial, the object was placed out of the infant's reach and was covered by a white cloth. To enhance ambiguity, the covered object was shaken while an unfamiliar sound was played (i.e., whoopee whistle regardless of the study phase or trial type). At this point, the mother prompted the infant to look at the object (still covered under a cloth) by pointing to the object (index finger tip oriented toward the object; finger 5 cm from the object). Prompting for looking at the object continued for 10 to 15 s. If the infant did not look at the object after 15 s, we terminated the session and started over after a short break. Subsequently, the infant was expected to turn gaze direction from the object towards the mother. If an infant did not shift gaze direction to look at the mother's face after 5 s, the mother prompted gaze shifting by using a rattle sound or a bell. This prompt was available throughout all trials regardless of phase or trial type. As soon as the infant looked at the mother, she posed one of the two facial expressions. After the infant emitted the first gaze shift, the experimenter moved the ambiguous object forward within the infant's reach (about 25 cm from the infant's trunk). If a reaching response occurred within 10 s after the point at which the facial expression was presented, the experimenter immediately delivered the appropriate consequence. In positive trials we presented lights, music, and the toy under the cloth as consequent events, whereas in negative trials we played the obnoxious sound (e.g., blender noise) and removed the covered object (still under the cloth) from the stage.
Design and Conditions
An ABAB design was used to evaluate to what extent social referencing responses could be acquired through discrimination training. The 11 data sets were arranged in a four-tier nonconcurrent multiple baseline design across participants.
Baseline probe sessions
One block of positive trials and one block of negative trials were conducted across 2 consecutive days to assess the initial level of reaching. Positive and negative trials were presented as described above. After the presentation of the ambiguous object, the mother presented joyful or fearful expressions to the infant contingent on gaze shifting towards the mother, but no consequences followed the infant's subsequent behavior. If the difference in percentage of positive versus negative trials with reaching was greater than 25% for a particular subject, we assumed that the target discrimination was already in place and the participant would be dropped from the study. However, no infant was excluded for this reason.
Differential reinforcement (DR1)
Each infant was exposed to eight positive and eight negative trials interspersed within the same session. During positive trials, 3 s of music and lights and four slow metronome-like movements of the ambiguous object followed each reaching response. In addition, the infant was allowed to touch the object for 5 s. Immediately afterward, the object was removed from the stage. During negative trials, infant reaching responses were followed by 2 s of an obnoxious sound (e.g., blender noise) accompanied by a blue light and four rapid metronome-like movements of the covered object. The covered object was removed from the stage immediately after the presentation of the consequent stimuli. The infant was not permitted to touch the object. If the infant did not reach for the object within 10 s after the presentation of the fearful expression, the consequence was not delivered and we ended the trial. Sessions continued until the percentage of reaching responses across positive and negative trial blocks was clearly distinct.
During the first session, the infant was preexposed briefly to the relevant consequence. The consequence was presented for 0.5 s simultaneously with the facial expression before each trial. Preexposed stimuli were identical to the consequence to be presented for that trial in the event of a reaching response (music plus multicolored lights or obnoxious noise plus blue light). Preexposure was intended to facilitate the infant's discriminative learning. Unpaired preexposure to two or more stimuli typically enhances subsequent discriminative learning (see a review by Hall, 1991). Multicolored lights and the obnoxious noise were used solely as consequent stimuli after the preexposure session. Therefore, no form of antecedent stimulus control is to be attributed to these stimuli.
A shaping and prompting protocol for reaching was conducted when an infant did not exhibit a full reaching response toward the ambiguous object during some of the positive trials. A prompting protocol to facilitate response omission was also available for a fraction of the negative trials. Prompts (positive and negative trials) and shaping (positive trials only) for reaching were available during 50% of the trials of the first two sessions of DR1 and for 25% of trials of the third DR1 session. No prompts for reaching or not reaching were presented after the third DR1 session (notice that only S6 through S11 had additional DR1 sessions after this point). During positive trials with prompting for reaching, we requested mothers to reach and touch the object and to produce vocal cues (see operational definitions) as extrastimulus prompts to facilitate responding. If the child did not reach for the object in a positive trial within 10 s of the presentation of the facial expression, the mother reached and touched the object and vocalized while she continued to present the facial expression. Mothers were instructed to use high-pitched low-intensity nasal vocalizations with /m/ and /n/ phonemes for a period of 3 to 5 s for positive trials, which were signaled by joyful expressions (e.g., “mnnn”). In addition, the shaping protocol consisted of the delivery of musical sounds used as reinforcers for successive approximations to reaching: (a) extending arm and (b) extending arm in the direction of the object or touching the object. On the other hand, during negative trials with prompting for reaching omission, we used extrastimulus prompting that consisted of high-pitched high-intensity sounds using /α:/ phoneme and lasting 3 to 5 s (e.g., “aaaah!”) for trials signaled by a fearful expression. We delivered extrastimulus prompts if the child reached within 10 s of the presentation of the fearful expression. No shaping protocol was in place during negative trials.
Extinction
Extinction trials were conducted exactly as described for baseline. We presented the ambiguous stimulus until the infant made a gaze shift, followed by a facial expression by the mother. No consequence followed the infant's subsequent behavior. These sessions continued until reaching showed a substantial and consistent reduction compared to the level of the last block of trials of the preceding condition. Infants' gaze shifts were necessary for a trial to be counted.
Differential reinforcement reinstatement (DR2)
This phase was identical to DR1 except for the fact that the prompting and shaping procedure aimed at reinstated reaching was conducted only during 25% of the trials of the first session of this phase. Thereafter, only the facial expressions cued the infant's reaching response toward the ambiguous object. Sessions continued until there was evidence of discriminated responding across positive and negative trials.
Interobserver Agreement
Interobserver agreement was calculated for 83% of all trials (for all participants) using the total count-per-trial method. We divided the number of trials in which an agreement was scored (i.e., the two observers coded the occurrence or nonoccurrence of gaze shifting or reaching responses) by the total number of trials (agreements plus disagreements), and this ratio was converted to a percentage. The mean and range of interobserver agreement scores across participants were 98% (range, 92% to 100%) and 96% (range, 89% to 100%) for gaze shifts and reaching responses, respectively.
RESULTS
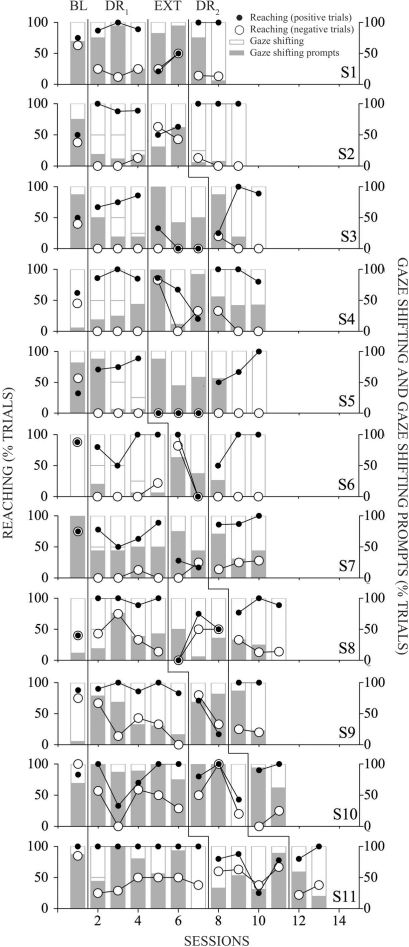
Figure 2 depicts the baseline, treatment (DR1, DR2), and extinction conditions for the 11 participants. None of the participants demonstrated the target discriminations in baseline. (Those who showed it would have been excluded.) The difference in infant reaching responses during the positive and negative trials was 10% (range, 0% to 25%) in baseline. Therefore, infants did not have the target discriminations in their repertoires. During DR, the joyful expression acquired discriminative properties for reaching for those who did not have a high rate of reaching during baseline (S2, S3, S4, S5, and S8), whereas reaching was clearly lower in the negative trials (fearful expression). The fearful expression became discriminative for not reaching during negative trials for participants who already had a high rate of reaching during the baseline condition (S1, S6, S7, S9, S10, and S11). It is important to note that prompted and unprompted instances of reaching during DR phases are combined in Figure 2.
Figure 2.
Percentage of trials with gaze shifting and reaching.
The pattern of change in scores between baseline and DR1, as well as the extinction condition and DR2 phases, reflected a learned discrimination. In the last session of both DR conditions, which were delivered without any prompting for reaching for most subjects (S5 through S11) in DR1 and with no prompting at all in DR2, all participants showed differential reaching across positive and negative trials. The mean difference in responding across positive and negative trials in the last session of DR1 and DR2 was 80% and 84%, respectively.
Extinction disrupted discriminated responding for all participants. Interestingly, although reaching dropped during positive trials for most participants during extinction sessions (S1, S2, S3, S4, S5, S6, S7, and S9), extinction obliterated differential responding without an extensive decrease in reaching for others (S8, S10, and S11).
Because of the prompting procedure in place, gaze shifts were also under the control of prompts and were not always under stimulus control of the ambiguous object. The prompting procedure ensured that gaze shifts occurred in all trials. All 11 infants looked at their mothers' faces to some extent during baseline. Mean percentages of trials with unprompted gaze shifts by phase across participants were as follows: baseline, 67%; DR1, 58%; extinction, 15%; and DR2, 69%. Most participants seemed to require more prompting for gaze shifting in the initial DR1 and DR2 sessions (S1, S3, S5, S6, S7, S8, and S9), whereas a few required prompting almost continuously during DR sessions (S10 and S11). Prompts for gaze shifting were needed frequently during both baseline and extinction (S1, S2, S3, S5, S7, S10, and S11). Unprompted gaze shifts dropped significantly during extinction, suggesting that the final reinforcer (baby music and lights) was indeed maintaining the whole behavior chain (gaze shifts and reaching). However, this finding was not consistent across all participants. For instance, S10 had independent gaze shifting on all trials in the final extinction session.
DISCUSSION
In the present study, mothers' expressions acquired control over infants' approach toward an ambiguous object. These results add to a growing body of research that supports the role of operant conditioning in the development of social referencing. One of the highlights of the present study was a demonstration of the acquisition of social referencing using nonarbitrary (more natural) facial cues. Although we made use of several prompting strategies, we eventually faded all prompts for reaching, and facial cues continued to exercise stimulus control over reaching. Our results suggest that social referencing responses, and particularly reaching discriminated by maternal facial cues, can be shaped, maintained, and extinguished through operant contingencies. These results lend support to the notion that some portion of (if not the entire) social referencing process may be an outcome of incidental learning.
Although reaching was not under the control of mothers' facial stimuli at the beginning of the experiment, gaze shifting toward the mother in novel situations can be present as early as 4 months of age. Hagekull, Stenberg, and Bohlin (1993) also documented gaze shifting in a descriptive analysis of infant–mother interaction in a large sample of 10-month-old infants. They noted that 50% of infants looked immediately at their mothers after noticing the presence of a stranger in the room, and an additional 20% looked at their mothers within the next few seconds (see also Stenberg, 2003).
The use of shaping and prompts for reaching limits conclusions regarding the extent to which reaching was under the stimulus control of the mother's facial expression or was simply a function of the shaping and prompting procedure in place. Rapid discrimination in the first session of DR1 and DR2 by most subjects provides some evidence of stimulus control by maternal facial expressions. However, the evidence for stimulus control during DR1 is not convincing until prompts for reaching are limited to 25% of the trials or faded completely. S6 through S11 showed discrimination in DR1 after prompting was discontinued. In addition, stimulus control was reestablished during DR2 after prompts were delivered during 25% of the trials for just one session (Figure 2).
Several findings are consistent with the potential role of discriminative learning in the acquisition of social referencing under natural conditions. Naturalistic observation studies show that caregivers are highly responsive to their infant's looks (Power & Parke, 1983). Moreover, infants look at their caregiver more often during ambiguous situations as opposed to nonambiguous situations and show a higher level of item-related activity (e.g., playing with an ambiguous object) when adults are responsive to their looks (Stenberg, 2003). Infants turn to adults who are able to provide valuable information and not just to individuals to which they are attached (Stenberg, 2009). For instance, infants of mothers who are unresponsive or present delayed or noncontingent responses reference their mothers infrequently (Pelaez-Nogueras, Field, Cigales, Gonzalez, & Clasky, 1994). Similarly, the extent to which the caregiver's cues are predictive of later consequences rather than the specific facial expression presented by the adult seems to be critical to guide infant's behavior toward the ambiguous object (Gewirtz & Pelaez-Nogueras, 1991, 1992). Our demonstration that social referencing can be learned under conditions similar to the natural environment makes an operant account more probable. However, the possibility exists that social referencing would emerge at a later maturational point without social learning and that the reinforcement processes described in this study may not be representative of the incidental learning process by which social referencing typically develops.
Although developmental theorists have argued that social referencing does not occur before the 9th month of age (e.g., when the infant has already developed “supplementary cognitive abilities”; Feinman, 1983, 1985; Walden & Geunyoung, 2005), our results suggest that reaching discriminated by maternal facial cues can be acquired via discrimination training in infants as young as 4 months of age. Although it is true that components of social referencing were present (gaze shift and reaching) before DR, these responses were not under stimulus control of maternal cues. Newborns and infants demonstrate preference for face-like patterns, even a few hours after birth. For instance, they orient more frequently and for longer periods toward depictions of faces rather than other configurations of stimuli (Cassia, Turati, & Simion, 2004; Turati, Valenza, Leo, & Simion, 2005). Even though it is not clear how social referencing develops, some empirical findings indicate that adult facial expressions can influence infant behavior. Stenberg and Hagekull (1997) demonstrated that infants are more likely to approach an ambiguous object when the mother provides them with positive information (e.g., smiling while saying “what a funny thing!”). Conversely, infants tend to avoid the object if the given information is negative (e.g., mother says “nooo!” in a low voice tone while frowning). In addition, infants whose mothers did not provide them with information about an ambiguous object often turned to the experimenter for clues if he or she was more likely to provide them (Stenberg, 2003).
We were interested only in trials in which the presentation of an ambiguous stimulus, a gaze shift, and an adult facial expression occurred in sequence. Therefore, our data are not an accurate portrayal of the actual prevalence of referencing behaviors in the natural environment (this was not a goal of the study, and it has been investigated in the past; see, e.g., D'Entremont, Hains, & Muir, 1997). In addition, we do not know what other contingencies may have been operating for either gaze shifts or reaching in the natural environment.
Further research is required to develop a finer behavior-analytic conceptualization of social referencing. Respondent processes cannot be ruled out based on the design of our protocol. For instance, fearful facial expressions (neutral stimuli) may have been classically paired with loud sounds (unconditioned stimuli) during the preexposure sessions and during the DR sessions with prompting and shaping for reaching. Eventually, reaching responses were maintained by operant contingencies alone, although classical conditioning of facial expressions during trials with prompted reaching may have affected responding.
More complex operant conceptualizations of social referencing are possible. Social referencing could be simply a response chain that is composed of two discriminated operants (as described above). Alternatively, as proposed by Dube et al. (2004) for joint attention, the unfamiliar object or event could function as a motivating operation (Laraway, Snycerski, Michael, & Poling, 2003) for gaze shifting, which establishes the reinforcing value of adult behavior toward the new object (Dube et al.). Additional research is warranted in this area, specifically to delineate the role of each of these potential processes.
On a methodological note, implementation of a longer baseline phase could have strengthened the design of the study by providing more compelling evidence of the absence of discriminated reaching on a subject-by-subject basis. However, long extinction-like baselines are associated with a high rate of emotional behavior (e.g., crying) and may alter the effectiveness of later contingencies (Lerman & Iwata, 1996; Sullivan, Lewis, & Alessandri, 1992). Nonetheless, 11 between-subject replications may compensate to some extent for our short baseline. Another methodological consideration has to do with the shaping and prompting protocol for reaching. Reporting prompted and unprompted reaching trials separately would have allowed a more immediate assessment of discriminated responding based solely on the stimulus control exercised by maternal facial cues.
In conclusion, the major findings of this study were that (a) even though infants look at their mothers in novel situations as early as 4 months of age, the facial expressions of joy and fear do not naturally cue differential infant responding in contexts of ambiguity; and (b) the discriminative properties of facial expressions of joy and fear can be established via operant conditioning in infants as young as 4 months of age. Social referencing frequently is lacking in children with an autism spectrum disorder and developmental disabilities, and this deficit has been associated with poor social and verbal performance later in development (Ozonoff & South, 2001). This study could provide the basis for training social referencing in infants at risk of developmental disabilities and very young children with autism spectrum disorders. Finally, our study is also relevant to normal development. Baer (1973) suggested a three-step process for studying child development. The first step is to determine if the “natural” behavioral process could be demonstrated to be sensitive to operant contingencies in a laboratory setting. Step 2 is to conduct naturalistic observations to determine if those operant principles appeared to operate in the natural environment. The final step was to manipulate those natural events within the natural environment. The present study could be considered to be part of Step 1. Future studies on the second and third steps are needed to improve our understanding of social referencing in typical and developmentally delayed children.
Acknowledgments
We thank Jennifer Hammond, Tiffany Field, Paul Fleming, Yalda Amir Kiaei, and Toby L. Martin for their thoughtful comments on earlier versions of this manuscript. We also are indebted to Claire Villate and Aida Sanchez for their assistance in data collection.
REFERENCES
- Baer D.M. The control of the developmental process: Why wait. In: Nesselroade J.R, Reese H.W, editors. Lifespan developmental psychology: Methodological issues. New York: Academic Press; 1973. pp. 187–193. (Eds.) [Google Scholar]
- Baldwin D.A. Understanding the link between joint attention and language. In: Moore C, Dunham P.J, editors. Joint attention: Its origins and rolein development. Hillsdale, NJ: Erlbaum; 1995. pp. 131–158. (Eds.) [Google Scholar]
- Brim D, Townsend D.B, DeQuinzio J.A, Poulson C.P. Analysis of social referencing skills among children with autism. Research in Autism Spectrum Disorders. 2009;3:942–958. [Google Scholar]
- Campos J.J, Anderson D.I, Barbu-Roth M.A, Hubbard E.M, Hertenstein M.J, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Campos J.J, Stenberg C.R. Perception, appraisal, and emotion: The onset of social referencing. In: Lamb M.E, Sherrod L.R, editors. Infant social cognition. Hillsdale, NJ: Erlbaum; 1981. pp. 274–313. (Eds.) [Google Scholar]
- Cassia V.M, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns' face preference. Psychological Science. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- D'Entremont B, Hains S.M.J, Muir D.W. A demonstration of gaze following in 3- to 6-month-olds. Infant Behavior Development. 1997;20:569–572. [Google Scholar]
- Dinsmoor J.A. A quantitative comparison of the discriminative and reinforcing functions of a stimulus. Journal of Experimental Psychology. 1950;40:458–472. doi: 10.1037/h0056266. [DOI] [PubMed] [Google Scholar]
- Dube W.V, MacDonald R.P.F, Mansfield R.C, Holcomb W.L, Ahearn W.H. Toward a behavioral analysis of joint attention. The Behavior Analyst. 2004;27:197–207. doi: 10.1007/BF03393180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Unmasking the face. Englewood Cliffs, NJ: Prentice Hall; 1975. [Google Scholar]
- Feinman S. How does baby socially refer? Two views of social referencing: A reply to Campos. Merrill-Palmer Quarterly. 1983;29:467–471. [Google Scholar]
- Feinman S. Emotional expressions, social referencing and preparedness for learning in infancy: Mother knows best but sometimes I know better. In: Zivin G, editor. The development of expressive behavior: Biology-environment interactions. New York: Academic; 1985. pp. 291–318. (Ed.) [Google Scholar]
- Feinman S, Lewis M. Social referencing at ten months: A second-order effect of infants responses to strangers. Child Development. 1983;54:878–887. doi: 10.1111/j.1467-8624.1983.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Gewirtz J.L, Pelaez-Nogueras M. The attachment metaphor and the conditioning of infant separation protests. In: Gewirtz J.L, Kurtines W.M, editors. Intersections with attachment. Hillsdale, NJ: Erlbaum; 1991. pp. 123–144. (Eds.) [Google Scholar]
- Gewirtz J.L, Pelaez-Nogueras M. Infant social referencing as a learned process. In: Feinman S, editor. Social referencing and the social construction of reality in infancy. New York: Plenum; 1992. pp. 123–144. (Ed.) [Google Scholar]
- Hagekull B, Stenberg G, Bohlin G. Infant-mother social referencing interactions: Description and antecedents in maternal sensitivity and infant irritability. Early Development and Parenting. 1993;2:183–191. [Google Scholar]
- Hall G. Perceptual and associative learning. Oxford, England: Clarendon; 1991. [Google Scholar]
- Holth P. An operant analysis of joint attention skills. Journal of Early and Intensive Behavioral Interventions. 2005;2:160–175. [Google Scholar]
- Hornik R, Risenhoover N, Gunnar M. The effects of maternal positive, neutral, and negative affective communications on infant responses to new toys. Child Development. 1987;58:937–945. [Google Scholar]
- Klinnert M, Campos J, Sorce J, Emde R, Svejda M. Emotions as behavior regulators in infancy: Social referencing in infancy. In: Plutchik R, Kellerman H, editors. Emotion: Theory, research and experience. Emotion in early development (Vol. 2, pp. 57–85) New York: Academic; 1983. (Eds.) [Google Scholar]
- Kuhn S.A, Lerman D.C, Vorndran C.M, Addison L. Analysis of factors that affect responding in a two-response chain in children with developmental disabilities. Journal of Applied Behavior Analysis. 2006;39:263–280. doi: 10.1901/jaba.2006.118-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36:407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman D.C, Iwata B.A. Developing a technology for the use of operant extinction in clinical settings: An examination of basic and applied research. Journal of Applied Behavior Analysis. 2006;29:345–382. doi: 10.1901/jaba.1996.29-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Feiring C. Indirect and direct effects and family interaction. In: Feinman S, editor. Social referencing and the social construction of reality in infancy. New York: Plenum; 1992. pp. 123–144. (Ed.) [Google Scholar]
- Lovaas O.I, Freitag G, Kinder M.I, Rubenstein B.D, Schaeffer B, Simmons J.Q. Establishment of social reinforcers in two schizophrenic children on the basis of food. Journal of Experimental Child Psychology. 1966;4:109–125. doi: 10.1016/0022-0965(66)90011-7. [DOI] [PubMed] [Google Scholar]
- Matson J.L, Sipes M. Methods of early diagnosis and tracking for autism and pervasive developmental disorder not otherwise specified (PDDNOS) Journal of Developmental and Physical Disabilities. 2010;22:343–358. [Google Scholar]
- Michael J. Implications and refinements of the establishing operation concept. Journal of Applied Behavior Analysis. 2000;33:401–410. doi: 10.1901/jaba.2000.33-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Gomes A. Individual differences in joint attention skill development in the second year. Infant Behavior and Development. 1998;21:469–482. [Google Scholar]
- National Institute for Occupational Safety and Health. Criteria for a recommended standard: Occupational noise exposure (NIOSH Publication No. 98-126) 1998. Retrieved from http://www.cdc.gov/niosh/docs/98-126/default.html.
- Ozonoff S, South M. Early social development in young children with autism: Theoretical and clinical implications. In: Bremner G, Fogel A, editors. Blackwell handbook of infant development. Malden, MA: Blackwell; 2001. pp. 565–588. (Eds.) [Google Scholar]
- Pelaez M. Joint attention and social referencing in infancy as precursors of derived relational responding. In: Rehfeldt R.A, Barnes-Holmes Y, editors. Derived relational responding: Applications for learners with autism and other developmental disabilities. Oakland, CA: New Harbinger; 2009. pp. 63–78. (Eds.) [Google Scholar]
- Pelaez M, Gewirtz J.L, Wong S.E. A critique of stage theories of human development: A pragmatic approach in social work. In: Thyer B.A, editor. Comprehensive handbook of social work and social welfare: Vol. 2: Human behavior in the social environment. New York: Wiley; 2008. pp. 503–518. (Ed.) [Google Scholar]
- Pelaez-Nogueras M, Field T, Cigales M, Gonzalez A, Clasky S. Infants of depressed mothers show less “depressed” behavior with their nursery teachers. Infant Mental Health Journal. 1994;15:358–367. [Google Scholar]
- Pelaez-Nogueras M, Gewirtz J.L. The context of stimulus control in behavior analysis. In: Baer D.M, Pinkston E.M, editors. Environment and behavior. Boulder, CO: Westview; 1997. pp. 30–42. (Eds.) [Google Scholar]
- Power T.G, Parke R.D. Patterns of mother and father play with their 8-month-old infant: A multiple analyses approach. Infant Behavior and Development. 1983;6:453–459. [Google Scholar]
- Repacholi B.M. Linking actions and emotions: Evidence from 15- and 18-month-old infants. British Journal of Developmental Psychology. 2009;27:649–667. doi: 10.1348/026151008x354564. [DOI] [PubMed] [Google Scholar]
- Stenberg G. Effects of maternal inattentiveness on infant social referencing. Infant & Child Development. 2003;12:399–419. [Google Scholar]
- Stenberg G. Selectivity in infant social referencing. Infancy. 2009;14:457–473. doi: 10.1080/15250000902994115. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Hagekull B. Social referencing and mood modification in 1-year-olds. Infant Behavior and Development. 1997;20:209–217. [Google Scholar]
- Stone W.L, Yoder P.J. Predicting spoken language in children with autistic spectrum disorders. Autism. 2001;5:341–361. doi: 10.1177/1362361301005004002. [DOI] [PubMed] [Google Scholar]
- Sullivan M.W, Lewis M, Alessandri S.M. Cross-age stability in emotional expressions during learning and extinction. Developmental Psychology. 1992;28:58–63. [Google Scholar]
- Turati C, Valenza E, Leo L, Simion F. Three-month-olds' visual preference for faces and its underlying visual processing mechanisms. Journal of Experimental Child Psychology. 2005;90:255–273. doi: 10.1016/j.jecp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Uchiyama I, Anderson D.I, Campos J.J, Witherington D, Frankel C.B, Lejeune L, et al. Locomotor experience affects self and emotion. Developmental Psychology. 2008;44:1225–1231. doi: 10.1037/a0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden T.A, Geunyoung K. Infants' social looking toward mothers and strangers. International Journal of Behavioral Development. 2005;29:356–360. [Google Scholar]