Abstract
Delphinids produce tonal whistles shaped by vocal learning for acoustic communication. Unlike terrestrial mammals, delphinid sound production is driven by pressurized air within a complex nasal system. It is unclear how fundamental whistle contours can be maintained across a large range of hydrostatic pressures and air sac volumes. Two opposing hypotheses propose that tonal sounds arise either from tissue vibrations or through actual whistle production from vortices stabilized by resonating nasal air volumes. Here, we use a trained bottlenose dolphin whistling in air and in heliox to test these hypotheses. The fundamental frequency contours of stereotyped whistles were unaffected by the higher sound speed in heliox. Therefore, the term whistle is a functional misnomer as dolphins actually do not whistle, but form the fundamental frequency contour of their tonal calls by pneumatically induced tissue vibrations analogous to the operation of vocal folds in terrestrial mammals and the syrinx in birds. This form of tonal sound production by nasal tissue vibrations has probably evolved in delphinids to enable impedance matching to the water, and to maintain tonal signature contours across changes in hydrostatic pressures, air density and relative nasal air volumes during dives.
Keywords: sound production, whistle, call, dolphin, toothed whale, communication
1. Introduction
Dolphins produce a rich acoustic repertoire, including clicks, burst pulses and tonal calls, usually referred to as whistles, for communication and echolocation. Tonal calls are used in social interactions and shaped by vocal learning so that a dolphin can produce individual signatures or imitate the signatures of conspecifics [1,2]. Unlike terrestrial mammals, dolphins produce sound in their nasal complexes [3], but little is known about how these sounds are generated. Most terrestrial vertebrates vocalize using an air-driven sound generator in the larynx, coupled to a vocal tract that may filter the produced sounds. While sounds are produced by two different organs, the syrinx in birds and the larynx in mammals, both groups are capable of producing long tonal calls for communication.
Vocalizations in terrestrial vertebrates are generally produced in two different ways; whistling, by which vortices from a fast air flow over an edge create fluctuations in the air pressure stabilized by the resonant properties of associated air spaces, or by air flow-induced vibrations of syringeal membranes or vocal folds, with membrane tension and mass determining the fundamental frequency [4]. Thus, the primary frequency modulation of vocal outputs can either happen by changing the resonance frequency of associated air spaces in the case of whistling, or by changing the driving air pressure or tension of vibrating vocal membranes [5]. So, while many birds and mammals produce long tonal signals that are coined whistles, these are strictly defined as tonal signals produced aerodynamically such as a human whistle [6].
While it is established that dolphin whistles, like clicks, are produced in the nasal complex, and that their production requires much more air and higher air pressure in the nasal cavity compared with clicks [7], only a few hypotheses have been advanced to account for how dolphin nasal structures may produce such tonal signals. Lilly [8] argued that dolphins produce true whistles generated aerodynamically and that the frequency of whistles thereby is given by resonating nasal air sac volumes. Mackay & Liaw [9] proposed that whistling involves ‘tissue vibrations [that] would excite resonances, rather than use of an evolved air vibration mechanism’ analogous to the operation of human vocal cords and the bird syrinx. So is a dolphin whistle a true whistle, in the sense that it is aerodynamically produced [6], or is the delphinid tonal sound source completely or partially decoupled from associated air volumes by which the resonance frequencies of these will have only some or no impact on the pitch of dolphin tonal calls? To address these questions, we trained a dolphin to produce tonal calls while breathing air and heliox mixtures with very different sound speeds which will change the resonance frequencies of the dolphins nasal air sacs.
2. Material and methods
Recordings were made in 1977, when a 12 year-old, resting male bottlenose dolphin (Tursiops truncatus) was given a mixture of 80 per cent helium and 20 per cent oxygen (heliox) through a US Navy Fenzy breather placed over the blowhole. Periodically, the breather cone was removed and the animal breathed air for several minutes. Sound recordings of stereotyped signature whistles [1,2] were obtained using an LC10 hydrophone in a suction cup on the melon. The hydrophone output was amplified by 40 dB with a Celesco 1364 amplifier and recorded at 38 cm s–1 on an Ampex FR 1300 instrumentation recorder, where a voice track was used to note when heliox was on or off. The frequency response of the recording chain was flat (±2 dB) between 3.2 and 75 kHz, which covers the full range of the harmonics (H0–H2) for the highest frequency vocalizations. The heliox mixture was tested on humans before the experiment, producing the expected upward shifts of formants to form the characteristic ‘Donald Duck’ voice. In 2010, the data were digitized using a 12-bit ADC sampling at 500 kHz, providing stereo wave files of the hydrophone recording and the voice track. Recordings were analysed using Matlab v. 7.5 (Mathworks) scripts. All whistles were identified by visually inspecting sound files displayed in a spectrogram format, and it was noted based on the voice track if the animal was breathing air or heliox. The sound speed in heliox is 1.74 times higher than in air, so the resonance frequency of a fixed air volume will also be 1.74 times greater if containing heliox [4,5]. Gain settings were constant across the recording and so was the hydrophone placement allowing for comparison of sounds produced in air and heliox. We computed relative root mean square sound level, duration and centroid frequency of each whistle. A custom program (K. Beedholm) was used to track and extract the harmonic frequency traces to compute the relative energy contents for each of these (figures 1 and 2).
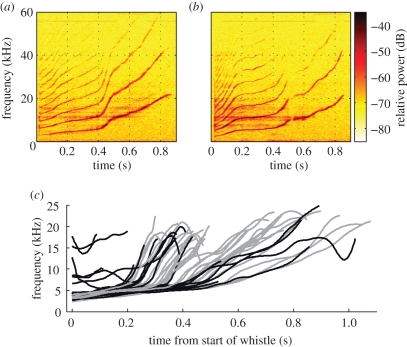
Figure 1.
(a) Tonal call produced breathing air. (b) Tonal call produced breathing heliox. (c) Fundamental frequency contours of tonal calls produced while breathing air (grey, n = 34) and heliox (black, n = 19).
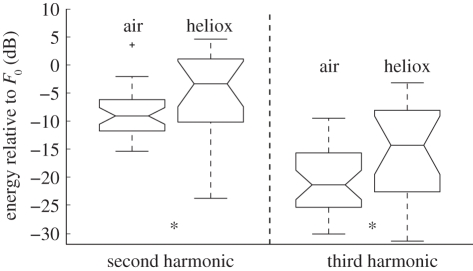
Figure 2.
Relative energy distribution between the fundamental contour (first harmonic) and the two following harmonics of tonal calls produced in air and heliox. Asterisks denote significant differences between air and helium (Kruskal–Wallis, p < 0.05).
3. Results
During a 1 h period, the vocal output was recorded from the dolphin with the blow-hole above the water line. The first 10 min of recordings were made while the animal was breathing air, during which it produced four whistles, and it subsequently breathed heliox for some 20 min, producing the first of 19 whistles after the 13th breath on heliox. Following this period, the animal was on air again for 30 min during which it produced 30 whistles. First, we tested the hypothesis that dolphins produce whistles aerodynamically and that the fundamental contour hence is given by the resonance frequency of the nasal air sacs. There were no significant changes in the fundamental frequency contour when changing from air to heliox (figure 1) in a manner consistent with a 1.74 times shift upwards in fundamental frequency [4,5].
All whistle fundamentals in air started around 4 kHz and underwent an upward sweep to about 20 kHz, and 70 per cent of the heliox whistles did the same. Six of the heliox whistles were shorter, weaker and had a starting frequency between 7 and 17 kHz, and were produced in between those of normal traces. The heliox whistles had a median received level 7 dB lower than those produced in air, but not significantly so (Kruskal–Wallis, p = 0.176). There were no significant differences between air and heliox whistles in duration, peak frequency, centroid frequency or mean contour frequency (Kruskal–Wallis, p > 0.05). We then tested whether air resonances changed filter properties of the nasal complex by quantifying the ratio of energy in the second and third harmonics relative to the energy in the fundamental contour. As seen from figure 2, compared with the fundamental, the second harmonic in heliox is 6 dB stronger than for air and the third harmonic is 7 dB stronger than in air (figure 2b).
4. Discussion
The lack of frequency shift in the fundamental whistle contour while breathing heliox shows that a dolphin whistle is strictly speaking not a whistle [5,6], and it is as such a functional misnomer. Our findings do therefore not support the sound production model advanced by Lilly [8] who proposed that the frequency modulation in a dolphin whistle happens via changes in nasal air sac volumes and hence changes in resonance frequencies. Rather, our results suggest that the fundamental frequency of ‘whistles’ is set by the vibration frequency of a functional equivalent of mammalian vocal cords or the syringeal labia of birds, where the fundamental frequency is defined by the tension and mass of the vibrating source as well as the driving air pressure [4,9]. The supercranial airways of dolphins consist of a complex system of nasal passages, air sacs, nasal plugs and the phonic lips responsible for click production [3,7]. We propose that the phonic lips are good candidates for a vibrating source that can produce ‘whistles’. If so, they must be able to oscillate when air flows past them at frequencies between 4 and 20 kHz, where the fundamental contours of most dolphin ‘whistles’ are found. That dolphins with phonic lips much larger than human vocal cords produce tonal sounds about an order of magnitude above the fundamental frequency of human tonal calls may be explained by differences in stiffness of the sources: the vocal cords are of low mass and tension, whereas the posterior phonic lips are supported by a strong blowhole ligament, and the anterior phonic lips consist of a conglomerate of strong connective tissue and fat bursae [3]. This vibration model implies that dolphins produce frequency modulated ‘whistles’ by changing the configuration and tension of their phonic lips, rather than by changing the volume and resulting resonance frequency of their nasal air sacs as is also the case for click production [10]. That interpretation is consistent with a recent study by Jensen and co-workers [11], who found that the frequency of tonal calls of deep diving pilot whales do not change with depth as would be predicted if they were made as true whistles formed by diminishing resonating air volumes with depth. Given that clicks are also produced by pneumatic acceleration of phonic lips [3], it therefore seems that the production mechanisms for tonal and click sounds are not as different as they would be if the ‘whistles’ were true whistles, which may explain the apparent continuum between clicks, burst pulses and tonal calls of some delphinids [12].
Most mammals and birds have a vocal tract that will not affect the fundamental frequency of calls, but filter sounds produced at the vocal cords/syrinx to produce timbre [4,5]. We find that dolphin ‘whistles’ produced in heliox have less energy in the fundamental compared with the second and third harmonics (figure 2), indicating some air sac effects on timbre. Thus, while the fundamental frequency of a dolphin ‘whistle’ is apparently determined alone by tissue vibrations, it seems that the resonance frequencies of the nasal passages and air sacs or the different impedance of the heliox mixture may generate some vocal timbre. In line with that, Miller et al. [13] suggested that the differences in energy ratios of harmonics between male and female killer whales can be explained by different air sac volumes between sexes. However, if that is the case, the timbre of delphinid ‘whistles’ is predicted to change with nasal air sac volumes and hence by vocalization depth. This prediction is consistent with observations that ‘whistling’ belugas maintain their fundamental frequency, yet have relatively more energy in the harmonics at 100–300 m depth compared with the surface [14]. Since voice features in the form of timbre, thereby change with depth, they do not form a reliable cue for individual recognition, which may explain the finding of Janik et al. [1] that identity information in dolphin ‘whistles’ can be conveyed by the fundamental contour alone and does not require voice features. Here, we demonstrate that the fundamental frequency of dolphin tonal calls is produced by tissue vibrations rather than by resonating air volumes, by which dolphins, and perhaps all other ‘whistling’ toothed whales, can effectively couple tonal sounds into the water and convey individual identity information independent of their depth, air density and recycled air volumes.
Acknowledgements
The experiments were approved by the Institutional Animal Care Committee of the Naval Undersea Centre in San Diego, CA, USA.
We thank D. Ljungblad for operating the Fenzy breather and S. E. Moore for technical assistance. We thank M. Hansen, K. Beedholm, L. Saigh, C. Elemans, O. N. Larsen, M. Amundin and M. Johnson for comments and analytical assistance, and helpful reviewers for constructive critique.
References
- 1.Janik V., Sayigh L., Wells R. 2006. Signature whistle shape conveys identity information to bottlenose dolphins. Proc. Natl Acad. Sci. USA 103, 8293–8297 10.1073/pnas.0509918103 (doi:10.1073/pnas.0509918103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayigh L. S., Esch H. C., Wells R. S., Janik V. M. 2007. Facts about signature whistles of 5 bottlenose dolphins (Tursiops truncatus). Anim. Behav. 74, 1631–1642 10.1016/j.anbehav.2007.02.018 (doi:10.1016/j.anbehav.2007.02.018) [DOI] [Google Scholar]
- 3.Cranford T. W., Amundin M., Norris K. S. 1996. Functional morphology and homology in the odontocete nasal complex. J. Morphol. 228, 223–285 (doi:10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 4.Laje R., Mindlin G. B. 2005. The physics of bird song. Berlin, Germany: Springer [Google Scholar]
- 5.Nowicki S. 1987. Vocal tract resonances in oscine bird sound production. Nature 325, 53–54 10.1038/325053a0 (doi:10.1038/325053a0) [DOI] [PubMed] [Google Scholar]
- 6.Wilson T. A., Beavers G. S., DeCoster M. A., Holger D. K., Regenfus M. D. 1971. Experiments on the fluid mechanics of whistling. J. Acoust. Soc. Am. 1, 366–372 10.1121/1.1912641 (doi:10.1121/1.1912641) [DOI] [Google Scholar]
- 7.Ridgway S. H., Carder D. A. 1988. Nasal sound production and pressure in an echolocating white whale. In Animal sonar: processes and performance (eds Nachtigall P., Moore P.), pp. 53–60 New York, NY: Plenum Press [Google Scholar]
- 8.Lilly J. C. 1962. Vocal behavior of the bottlenose dolphin. Proc. Am. Phil. Soc. 106, 520–529 [Google Scholar]
- 9.Mackay R. S., Liaw H. M. 1981. Dolphin vocalization mechanisms. Science 212, 676–677 10.1126/science.212.4495.676 (doi:10.1126/science.212.4495.676) [DOI] [PubMed] [Google Scholar]
- 10.Amundin M. 1991. Helium effects on the click frequency spectrum of the harbour porpoise Phocoena phocoena. J. Acoust. Soc. Am. 90, 53–59 10.1121/1.401281 (doi:10.1121/1.401281) [DOI] [Google Scholar]
- 11.Jensen F. H., Marrero J., Aguilar Soto N., Johnson M., Madsen P. T. 2011. Calling under pressure. Proc. R. Soc. B 278 10.1098/rspb.2010.2604 (doi:10.1098/rspb.2010.2604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray S. O., Mercado E., Roitblat H. L. 1998. Characterizing the graded structure of false killer whale (Pseudorca crassidens) vocalizations. J. Acoust. Soc. Am. 104, 1679–1688 10.1121/1.424380 (doi:10.1121/1.424380) [DOI] [PubMed] [Google Scholar]
- 13.Miller P. J. O., Samarra F. I. P., Perthuison A. U. 2007. Caller sex and orientation influence spectral characteristics of ‘two-voice’ stereotyped calls produced by free-ranging killer whales. J. Acoust. Soc. Am. 121, 3932–3937 10.1121/1.2722056 (doi:10.1121/1.2722056) [DOI] [PubMed] [Google Scholar]
- 14.Ridgway S. H., Carder D. A., Kamolnick T., Smith R. R., Schlundt C. E., Elsberry W. R. 2001. Hearing and whistling in the deep sea. J. Exp. Biol. 204, 3829–3841 [DOI] [PubMed] [Google Scholar]