Abstract
The presence of male siblings in utero influences female morphology and life-history traits because testosterone transferred among foetuses may masculinize females. Similarly, litter sex composition might alter the display of sexually dimorphic behaviour, such as play and allogrooming, since they are modulated by androgens. We explored whether masculinization alters the frequency of play and sociopositive behaviour in female yellow-bellied marmots (Marmota flaviventris). We found that masculinized juvenile females were more likely to initiate play and allogrooming, but yearling females exhibited higher levels of oestrogen-modulated sociopositive behaviours. Additionally, the more they interacted, the greater number of different partners they interacted with. Our results suggest that masculinization increases the rate of age-dependent social behaviour. This probably works by increasing exploration that predisposes individuals to higher encounter rates. Further support comes from previous findings showing that masculinized females were more likely to disperse. Our study stresses the importance of considering litter sex composition as a fitness modulator.
Keywords: masculinization, play, social interactions, yellow-bellied marmot
1. Introduction
Being born into a male-biased litter might have long-lasting effects on siblings, because males start producing testosterone in utero at an early stage [1]. Testosterone diffuses through the placenta and provokes organizational and activational changes in the neighbouring foetuses [2]. The effects are usually stronger for females, and apparent by masculinization of morphological, behavioural, physiological and life-history traits [3]. Several studies have shown that the anogenital (AG) distance in rodents is a proxy of masculinization [4,5]. Males have larger AG distances than females, and females treated with androgens have larger AG distances than non-treated females [6]. Some behaviours are triggered by the interaction of androgens with the androgen receptor in the amygdala [7,8], such as play fighting and allogrooming, and are dimorphic in polygynous species. Males usually initiate more play fights, whereas females are usually more engaged in other sociopositive behaviours where oestrogens play a leading modulatory role [8–10]. Behavioural modifications have not received much attention and might be important modulators of fitness [11]. Social play has physical, social and developmental consequences on the individual [12]. Its dimorphic nature suggests that one of the ultimate functions might be to hone skills that might increase later reproductive success [10,13,14] because the motor patterns observed are similar to those of adults during agonistic encounters [15].
Thus, social play might be an important behaviour to study because: (i) events during early life are important fitness modulators [16], and (ii) it is sexually dimorphic, so we might expect differences between masculinized and non-masculinized females. We examined the effects of masculinization on the frequency of play fighting, allogrooming and female-like sociopositive behaviour in female juvenile and yearling yellow-bellied marmots. We expected to find higher frequencies of male-type behaviour in masculinized females (those with larger AG distance indexes). Furthermore, we expected to find that non-masculinized females engage in female-like sociopositive behaviour. We explored whether the effects were long-lasting and if so, we would expect to find similar patterns in female juvenile and yearling marmots.
2. Material and methods
From 2002 to 2009, we regularly observed 10 social groups of yellow-bellied marmots in and around the Rocky Mountain Biological Laboratory, Colorado. Marmots are individually marked and we followed their fate from natal emergence to death. Natal emergence date was determined by regular observations. We trapped the juveniles with Tomahawk traps as soon as they emerged from the natal burrow, ear-tagged them and drew an individual symbol on their back with Nyanzol fur dye to facilitate behavioural observations. We weighed them and measured the AG distance (the distance from the centre of the genital papilla to the anus). We took a hair sample for later DNA parentage assignment (for further details about the procedure and the study population, see [17]). We calculated an AG distance index by extracting the standardized residuals of the relation between AG distance and body mass [3,5].
We observed all the colonies most days during the active season of the marmots (mid-April to mid-September). We continuously recorded the social behaviour of juveniles and yearlings [18]. We differentiated between play and other sociopositive behaviours, such as sitting in proximity, greeting and allogrooming (for further details about the sociogram, see [19]). We calculated the frequencies of play fighting initiated and received, allogroom initiated and received, and total sociopositive behaviour (excluding play). We calculated the frequency of occurrence of the different behaviours by relating the number of events to the total observation time of the colony. Moreover, we recorded the identity of the partners they interacted with.
For data analysis, we fitted generalized linear mixed models (GLMM) with a gamma distribution and an inverse link. We used the function glmmPQL from library MASS from the statistical package R, v. 2.13.0 [20]. For all the analyses, we included the AG distance index and litter size as fixed factors. For both age classes (and especially for juveniles) most of the recipients and/or initiators of the social interactions were siblings, so we incorporated litter identity as a random factor to control for any litter effect.
We analysed whether the number of different partners they interacted with was a function of the AG distance index and the frequency of interactions. Litter identity was included as a random factor. We fitted GLMM with a Poisson distribution and a log link, with the function lmer from the package lme4.
3. Results
We observed 202 female marmots from 89 different litters (110 juveniles and 92 yearlings). The average observation time for juveniles was 61.96 h (±47.92 s.d.) and for yearlings 130.98 h (±82.59 s.d.).
Female juveniles with a larger AG distance index tended to initiate more play (β = 0.015, t = 3.111, p = 0.003; figure 1), as well as to receive less (β = 0.016, t = 2.808, p = 0.007). In addition, the more they played, the more different partners they interacted with (initiated: β = 0.306, Z = 8.303, p < 0.001; received: β = 0.148, Z = 4.411, p < 0.001). Masculinized females initiated more allogrooming (β = 0.009, t = 2.148, p = 0.038). However, we did not find differences in the frequency of allogrooming received (β = 0.002, t = 0.926, p = 0.360) or total sociopositive behaviour (β = 0.021, t = 1.706, p = 0.096). In none of the cases was litter size significant (all p-values > 0.2).
Figure 1.
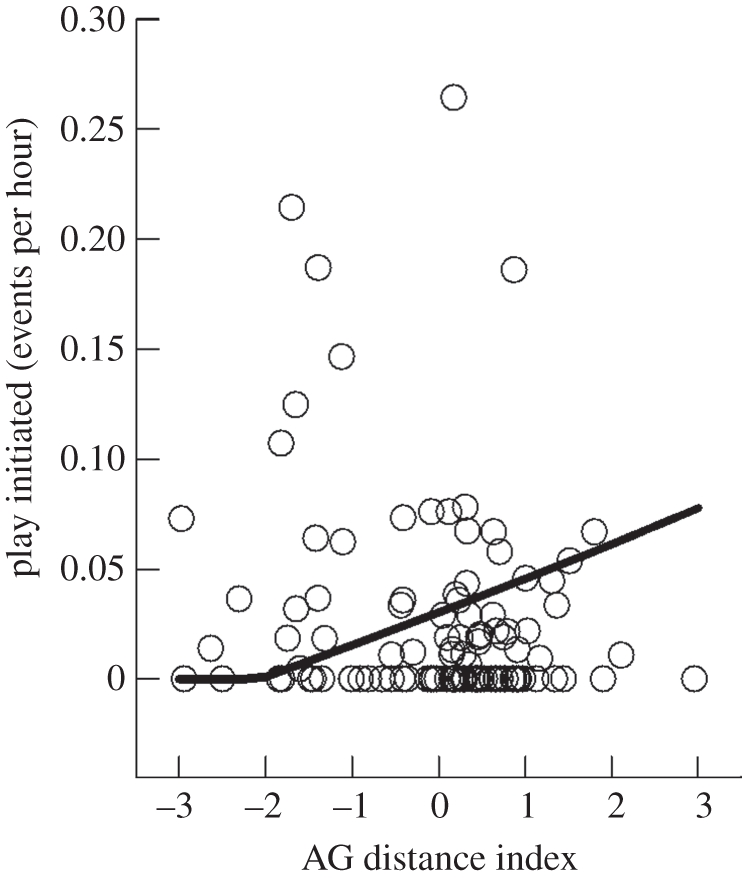
Frequency of play initiated in juvenile yellow-bellied marmots in relation to the AG distance index. The open circles represent the frequencies observed and the line represents the frequencies estimated after model selection. See text for statistics.
For yearlings, AG distance neither explained the frequency of play initiated (β = −0.009, t = −0.835, p = 0.411) nor received (β = −0.024, t = –1.568, p = 0.129). There were no effects of litter size on the frequency of play initiated or received (initiated: β = −0.006, t = −0.830, p = 0.410; received: β = −0.001, t = −0.016, p = 0.987). Yearling females with larger AG distances engaged in more sociopositive behaviour (β = −0.022, t = −2.123, p = 0.043; figure 2) and with more different partners (β = 0.028, Z = 6.445, p < 0.001). However, none of the variables considered significantly affected the frequency of allogrooming initiated (AG distance: β = 0.001, t = 0.420, p = 0.678; litter size: β = 0.001, t = 0.884, p = 0.380) and allogrooming received (AG distance: β = 0.002, t = 1.404, p = 0.172; litter size: β = 0.001, t = 0.718, p = 0.475).
Figure 2.
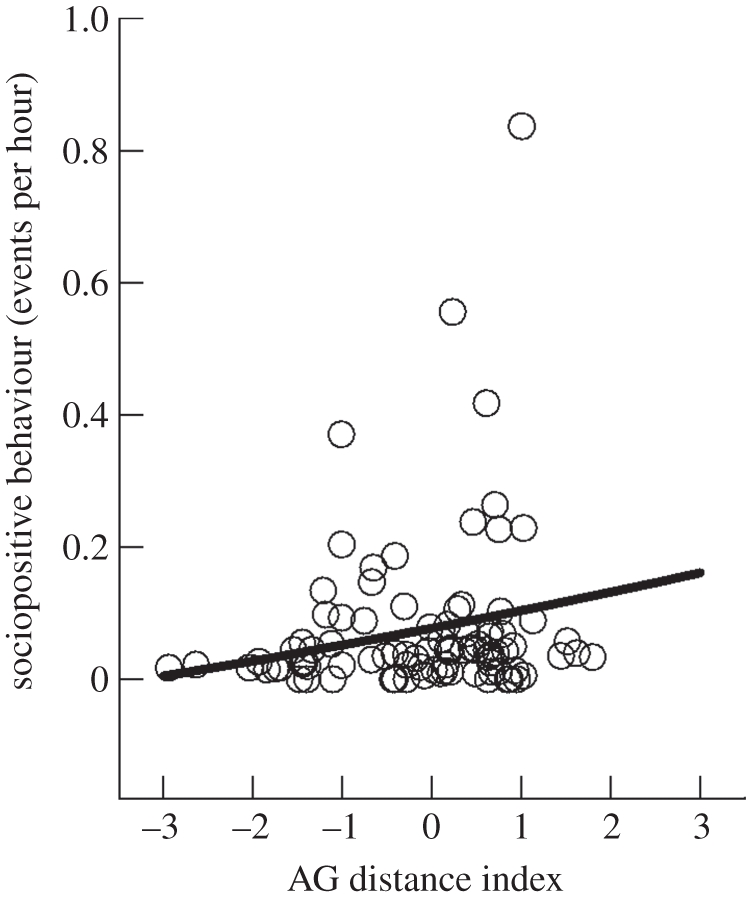
Frequency of total sociopositive behaviour (excluding play) in yearling yellow-bellied marmots in relation to the AG distance index. The open circles represent the frequencies observed and the line represents the frequencies estimated after model selection. See text for statistics.
4. Discussion
Litter sex composition has important masculinizing effects on the litter. The effects are usually stronger in females, apparent by morphological modifications and fitness consequences [4–6]. In this study, we showed that masculinized juvenile females, those with larger AG distances, engaged in more male-like behaviours, such as play fighting and allogrooming, while masculinized yearling females had higher rates of non-playful but nevertheless sociopositive behaviour. Also, the individuals that interacted more did so with a greater number of partners.
The development of allogrooming and play fighting are modulated by androgens, appear at very early ontogenetic stages, and are more frequent in males than in females [21]. In other species, females treated with testosterone show similar play frequencies as males, and these females play more than non-masculinized females [7]. The effect in marmots was apparent in juveniles. In marmots, play contains motor patterns similar to those observed in adult agonistic encounters and might hone competitive skills [15]. Under limiting conditions, masculinized females might have higher competitive abilities to monopolize resources [22]. Whereas the proximate mechanism could rely on testosterone, play fighting might hone the physical and social skills necessary to succeed later in life.
The higher frequencies of play fighting disappeared in masculinized yearlings, probably because of the ontogeny of the behaviour [14]. However, they engaged in more female-like behaviours, such as total sociopositive behaviour. Perhaps masculinized females simply engage in those behaviours that peak in each ontogenetic state, independently of the nature of the behaviour, and this might be driven by higher exploration rates: an animal that explores more will increase its rate of encounters with conspecifics, increasing the chances of social interactions. Higher rates of interactions might have several important consequences, from influencing disease dynamics [23] to indirectly increasing an animal's fitness [24]. Some evidence of increased exploration rates comes from the lack of selectivity of the interactions: females that interacted more did so with a greater number of different partners. Moreover, exploration is a male-like behaviour and masculinized females are more exploratory than non-masculinized females [25]. Exploration might trigger higher dispersal rates, and previous studies found that masculinized females are more likely to disperse [5,26]. This might have important consequences if investing in the dispersing sex has fitness benefits, as expected under a local resource competition scenario [27]. Individuals with higher testosterone levels might increase their chances of successful dispersal, settlement and competition for resources in the new environment.
In conclusion, we showed how masculinization affects the frequency of social interactions in young yellow-bellied marmots, suggesting a link between prenatal androgens and behavioural styles. Endocrine-disrupting chemicals are now widespread in our environment and are known to impact neuroendocrine and neurobehavioural development in the laboratory. While we have no information on whether endocrine disruptors are present at our remote study site, these results further illustrate the diverse consequences that endocrine disruptors might have in wild animals.
Acknowledgements
We are very grateful to all the marmoteers who spent many hours observing marmots. R.M. was supported by post-doctoral fellowships from the Spanish Ministerio de Innovación y Ciencia and the Fulbright programme. D.T.B was supported by the National Geographic Society, UCLA (Faculty Senate and the Division of Life Sciences), a Rocky Mountain Biological Laboratory research fellowship, and by the NSF (IDBR-0754247 to D.T.B., as well as DBI 0242960 and 0731346 to the Rocky Mountain Biological Laboratory).
References
- 1.Ryan B. C., Vandenbergh J. G. 2002. Intrauterine position effects. Neurosci. Biobehav. Rev. 26, 665–678 10.1016/S0149-7634(02)00038-6 (doi:10.1016/S0149-7634(02)00038-6) [DOI] [PubMed] [Google Scholar]
- 2.vom Saal F. S., Dhar M. G. 1992. Blood flow in the uterine loop artery and loop vein is bidirectional in the mouse: implications for transport of steroids between fetuses. Physiol. Behav. 52, 163–171 10.1016/0031-9384(92)90447-A (doi:10.1016/0031-9384(92)90447-A) [DOI] [PubMed] [Google Scholar]
- 3.Vandenbergh J. G. 2009. Effects of intrauterine position in litter-bearing mammals. In Maternal effects in mammals (eds Maestripieri D., Mateo J. M.), pp. 201–226 Chicago, IL: The University of Chicago Press [Google Scholar]
- 4.Vandenbergh J. G., Huggett C. L. 1995. The ano-genital distance, a predictor of the intrauterine position effects on reproduction in female house mice. Lab. Anim. Sci. 45, 567–573 [PubMed] [Google Scholar]
- 5.Monclús R., Blumstein D. T. 2011. Litter sex composition affects life-history traits in yellow-bellied marmots. J. Anim. Ecol. 10.1111/j.1365-2656.2011.01888.x (doi:10.1111/j.1365-2656.2011.01888.x) [DOI] [PubMed] [Google Scholar]
- 6.Clark M. M., Galef B. G., Jr 1998. Effects of uterine position on rate of sexual development in female Mongolian gerbils. Physiol. Behav. 42, 15–18 10.1016/0031-9384(88)90253-3 (doi:10.1016/0031-9384(88)90253-3) [DOI] [PubMed] [Google Scholar]
- 7.Meaney M. J., McEwen B. S. 1986. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 398, 324–328 10.1016/0006-8993(86)91492-7 (doi:10.1016/0006-8993(86)91492-7) [DOI] [PubMed] [Google Scholar]
- 8.Pellis S. M., Pellis V. C. 1997. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus). Dev. Psychobiol. 31, 193–205 (doi:10.1002/(SICI)1098-2302(199711)31:3<193::AID-DEV4>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss A. K., Ostby J. S., Vandenbergh J. G., Gray L. E. 2003. An environmental androgen, vinclozolin, alters the organization of play behavior. Physiol. Behav. 79, 151–156 10.1016/S0031-9384(03)00093-3 (doi:10.1016/S0031-9384(03)00093-3) [DOI] [PubMed] [Google Scholar]
- 10.Chau M. J., Stone A. I., Mendoza S. P., Bales K. L. 2008. Is play behavior sexually dimorphic in monogamous species? Ethology 114, 989–998 10.1111/j.1439-0310.2008.01543.x (doi:10.1111/j.1439-0310.2008.01543.x) [DOI] [Google Scholar]
- 11.Fagen R., Fagen J. 2009. Play behaviour and multi-year juvenile survival in free-ranging brown bears, Ursus arctos. Evol. Ecol. Res. 11, 1053–1067 [Google Scholar]
- 12.Bell H. C., Pellis S. M., Kolb B. 2010. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav. Brain Res. 207, 7–13 10.1016/j.bbr.2009.09.029 (doi:10.1016/j.bbr.2009.09.029) [DOI] [PubMed] [Google Scholar]
- 13.Held S. D. E., Špinka M. 2011. Animal play and animal welfare. Anim. Behav. 81, 891–899 10.1016/j.anbehav.2011.01.007 (doi:10.1016/j.anbehav.2011.01.007) [DOI] [Google Scholar]
- 14.Byers J. A., Walker C. 1995. Refining the motor training hypothesis for the evolution of play. Am. Nat. 146, 25–40 10.1086/285785 (doi:10.1086/285785) [DOI] [Google Scholar]
- 15.Jamieson S. H., Armitage K. B. 1987. Sex differences in the play behavior of yearling yellow-bellied marmots. Ethology 74, 237–253 10.1111/j.1439-0310.1987.tb00936.x (doi:10.1111/j.1439-0310.1987.tb00936.x) [DOI] [Google Scholar]
- 16.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348 10.1016/S0169-5347(99)01639-0 (doi:10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 17.Blumstein D. T., Lea A. J., Olson L. E., Martin J. 2010. Heritability of anti-predatory traits: vigilance and locomotor performance in marmots. J. Evol. Biol. 23, 879–887 10.1111/j.1420-9101.2010.01967.x (doi:10.1111/j.1420-9101.2010.01967.x) [DOI] [PubMed] [Google Scholar]
- 18.Martin P., Bateson P. 1986. Measuring behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Nowicki S., Armitage K. B. 1979. Behavior of juvenile yellow-bellied marmots: play and social integration. Z. Tierpsychol. 51, 85–105 10.1111/j.1439-0310.1979.tb00674.x (doi:10.1111/j.1439-0310.1979.tb00674.x) [DOI] [Google Scholar]
- 20.R Development Core Team. 2011. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org). [Google Scholar]
- 21.Field E. F., Whishaw I. Q., Pellis S. M. 2006. Play fighting in androgen-insensitive tfm rats: evidence that androgen receptors are necessary for the development of adult playful attack and defense. Dev. Psychobiol. 48, 111–120 10.1002/dev.20121 (doi:10.1002/dev.20121) [DOI] [PubMed] [Google Scholar]
- 22.Zielinski W. J., vom Saal F. S., Vandenbergh J. G. 1992. The effect of the intrauterine position on the survival, reproduction and home range size of female house mice (Mus musculus). Behav. Ecol. Sociobiol. 30, 185–191 10.1007/BF00166702 (doi:10.1007/BF00166702) [DOI] [Google Scholar]
- 23.Pulliam H. R., Caraco T. 1984. Living in groups: is there an optimal group size? In Behavioral ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 122–147 Sunderland, MA: Sinauer [Google Scholar]
- 24.Rödel H. G., Starkloff A., Bruchner B., von Holst D. 2008. Social environment and reproduction in female European rabbits (Oryctolagus cuniculus): benefits of the presence of litter sisters. J. Comp. Psychol. 122, 73–83 10.1037/0735-7036.122.1.73 (doi:10.1037/0735-7036.122.1.73) [DOI] [PubMed] [Google Scholar]
- 25.Laviola G., Alleva E. 1995. Sibling effects on the behavior of infant mouse litters (Mus domesticus). J. Comp. Psychol. 109, 68–75 10.1037/0735-7036.109.1.68 (doi:10.1037/0735-7036.109.1.68) [DOI] [PubMed] [Google Scholar]
- 26.Ims R. A. 1989. Kinship and origin effects on dispersal and space sharing in Clethrionomys rufocanus. Ecology 70, 607–616 10.2307/1940212 (doi:10.2307/1940212) [DOI] [Google Scholar]
- 27.Silk J. B., Brown G. R. 2008. Local resource competition and local resource enhancement shape primate birth sex ratios. Proc. R. Soc. B 275, 1761–1765 10.1098/rspb.2008.0340 (doi:10.1098/rspb.2008.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]


