Abstract
Adaptations to suppress the viability of conspecifics may provide novel ways to control invasive taxa. The spread of cane toads (Rhinella marina) through tropical Australia has had severe ecological impacts, stimulating a search for biocontrol. Our experiments show that cane toad tadpoles produce waterborne chemical cues that suppress the viability of conspecifics encountering those cues during embryonic development. Brief (72 h) exposure to these cues in the egg and post-hatching phases massively reduced rates of survival and growth of larvae. Body sizes at metamorphosis (about three weeks later) were almost twice as great in control larvae as in tadpole-exposed larvae. The waterborne cue responsible for these effects might provide a weapon to reduce toad recruitment within the species' invaded range.
Keywords: alien species, Anura, Bufo marinus, competition, larva, pheromonal communication
1. Introduction
The damaging ecological impacts of invasive species have spawned a search for novel approaches to control [1,2]. One such opportunity involves the exploitation of species-specific competitive mechanisms, such as pheromones that suppress the reproduction, growth or survival of conspecifics [3–8]. We studied cane toads (Rhinella marina), bufonid anurans whose spread through Australia has killed many native predators [9–11]. High densities of toad tadpoles [12,13] result in intense competition, reducing survival, growth and size at metamorphosis [14–17]. Older tadpoles search out and consume eggs before they hatch, thereby reducing competition [17,18]. After they hatch, however, the mobile larvae are invulnerable to attack [17]. If it is difficult for cannibalistic tadpoles to find eggs in muddy weed-choked ponds, we might also expect pheromonal suppression to evolve. Anuran tadpoles use sophisticated chemical communication systems [19] and exhibit plastic developmental responses to chemical cues [20,21]. If toad tadpoles exploit those sensitivities to interfere with their competitors, larval chemical cues might offer novel approaches for targeted control of toads [8,22]. We conducted experiments to look for such effects.
2. Material and methods
(a). Tadpole husbandry and experimental treatments
We collected adult cane toads from Middle Point Village (12°34′ S, 131°18′ E) in the Northern Territory, and injected them with leuprorelin acetate to stimulate oviposition (see [23] for details). A 10-egg section of the egg string was placed into each of 20 containers (17 × 11 × 7 cm; with 750 ml non-chlorinated well water) in a shaded outdoor area. Containers were divided into half with 1 × 1 mm vertical flyscreen mesh. In half of the containers, we added three cane toad tadpoles collected from a local pond (snout–vent length (SVL) 8.75–10.15 mm; Gosner stage 34–36 [24]), to the opposite side of the mesh (no food was provided). Thus, eggs were exposed to waterborne cues from tadpoles, but no physical contact. The remaining 10 containers (randomly allocated) served as controls (no tadpoles).
After 72 h, when the eggs had developed into free-swimming tadpoles (Gosner stage 25), we tested water quality (dissolved oxygen and temperature using a YSI 85 meter (Yellow Springs, OH, USA); ammonia and pH using API test kits (Chalfont, PA, USA) and SSS Universal Indicator Paper (Murarrie, Queensland)). Each group of 10 newly hatched tadpoles was then transferred to a larger container (37 × 28 × 20 cm; 20 tubs total) containing a 2 cm deep sediment from a nearby pond, and filled to a depth of 15 cm with non-chlorinated water, and 1 g Hikari algae pellets (Kyorin Co. Ltd., Himeji City, Japan) per tub to provide additional nutrients.
Five days later, we randomly euthanased all tadpoles in five replicates per treatment (with MS-222; Argent Chemicals, Redmond, WA, USA), and counted and measured them (SVL, mass after blotting dry, Gosner stage). The remaining tadpoles were checked daily, and placed individually in moist paper-lined containers when they began to metamorphose. When the limbs had emerged and the tail was resorbed, metamorphs were measured (snout–urostyle length (SUL), blotted dry mass) and the length of the larval period was calculated.
(b). Data analyses
Data were analysed using MANOVA and t-tests, using mean values per container to avoid pseudoreplication. If necessary, data were log-transformed to assure normality and variance homogeneity. Survival data were arcsine transformed. Non-normally distributed ammonia data were analysed using the Kruskal–Wallis test.
3. Results
The two treatments did not differ in mean dissolved oxygen concentration (control = 6.91 mg ml–1, conspecific exposure = 6.85 mg ml–1; t18 = 1.04, p = 0.31), temperature (27.1°C, 26.7°C; t18 = 1.78, p = 0.09) or pH (6.0 in all containers), but ammonia concentration increased (0.5, 1.0 mg ml–1; χ21 = 19.0, p < 0.0001).
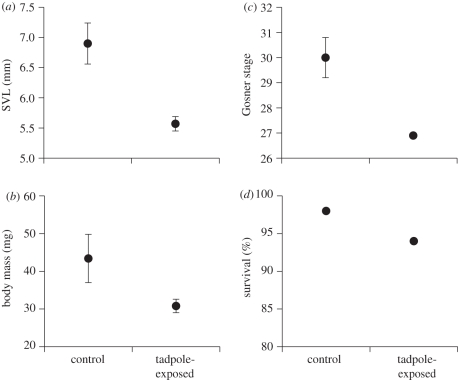
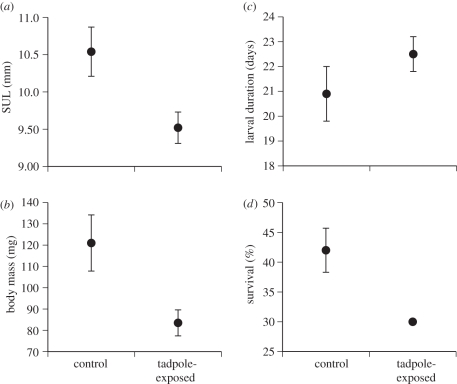
Exposure to chemical cues from conspecific tadpoles reduced the viability of larvae hatching from those eggs, at 5 days post-hatching (figure 1; MANOVA, F4,5 = 12.45, p < 0.001) and at metamorphosis (figure 2; MANOVA, F4,5 = 5.19, p < 0.05). When assessed after 5 days, exposure did not affect survival rate (t8 = 0.79, p = 0.45), but reduced the treatment tadpoles' body length (24% decrease, t8 = 4.06, p < 0.005), body mass (41% decrease, t8 = 3.40, p < 0.01) and developmental stage (t8 = 5.43, p < 0.001). The effects of embryonic exposure were evident more than 20 days later (figure 2). Larvae from tadpole-exposed eggs took greater than 8 per cent longer to complete development, but this difference was not significant (t8 = 1.20, p = 0.26). Metamorphs from the tadpole-exposure treatment averaged 11 per cent shorter (t8 = 2.270, p < 0.03) and 45 per cent lighter (t8 = 2.79, p < 0.03) than did unexposed siblings, and their survival rates were reduced by 40 per cent (t8 = 3.24, p < 0.05; figure 2).
Figure 1.
Phenotypic traits of cane toad tadpoles at 5 days post-hatching, as a function of exposure to chemicals from conspecific tadpoles during the egg stage. These chemicals reduced the younger tadpoles' (a) body length, (b) mass and (c) developmental stage, but (d) survival rates were unaffected. Graphs show means and standard errors.
Figure 2.
Phenotypic traits of metamorph cane toads as a function of exposure to chemical cues from conspecific tadpoles during the egg stage. Exposure to these cues reduced the (a) body length and (b) mass of metamorphs, (c) non-significantly extended the duration of the larval stage and (d) reduced larval survival. Graphs show means and standard errors.
4. Discussion
Embryonic exposure to chemical cues from cane toad tadpoles had devastating long-term consequences for conspecific larvae, even though water quality remained well within the range tolerated by cane toad tadpoles [25]. Larval survival, growth and development were substantially reduced, with metamorphosis at smaller body sizes. Delayed metamorphosis can impose a heavy fitness cost [26], and smaller tadpoles are more vulnerable to predation [27] and competition [28,29]. Smaller metamorphs are more vulnerable to desiccation [30], predation [31], cannibalism [32] and parasitism [33].
Bufonid tadpoles possess specialized epidermal secretory cells (‘riesenzellen’) that produce pheromones [34–36], possibly including these suppressors of the viability of conspecific larvae. Pheromonal production by the older tadpoles may be continuous, or may have been evoked by the presence of eggs (tadpoles can detect eggs from waterborne cues [17,18]) or by starvation [37]. These possibilities could be tested by exposing eggs to water from tadpoles that had or had not been exposed previously to conspecific eggs, or from fed versus unfed tadpoles. Chemical suppression of embryos might be owing to an adaptation of tadpoles, an adaptive plastic response of embryos and/or a fortuitous (unselected) effect. Future work could usefully explore these alternatives, as well as assessing the consistency of this response across pond conditions, sibships and the like.
Unlike other toad pheromones [18,35], these effects reduce toad viability in the long term only after brief exposure. Thus, they have great potential as a species-specific pheromonal control for invasive cane toads. Australian native anurans do not respond to the alarm or attractant pheromones produced by cane toad tadpoles [18,38], and so may well ignore these development-suppressing toad pheromones. Sex pheromones have been used as attractants and mating disruptors for biocontrol [6,7,39]. Agricultural scientists exploit allelopathic effects to suppress weed growth [40]; and indeed, the effects we have documented would qualify as allelopathy under some but not all definitions of this term [41]. In a similar vein, anuran pheromones that reduce the viability of conspecifics may provide powerful weapons for the control of selected species.
Acknowledgements
The work was approved by the University of Sydney Animal Ethics Committee.
We thank Michelle Franklin and Nilusha Somaweera for assistance, and the Australian Research Council for funding.
References
- 1.Pimentel D., Lach L., Zuniga R., Morrison D. 2000. Environmental and economic costs of nonindigenous species in the United States. Bioscience 50, 53–65 10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2 (doi:10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2) [DOI] [Google Scholar]
- 2.Blaustein A. R., Kiesecker J. M. 2002. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol. Lett. 5, 597–608 10.1046/j.1461-0248.2002.00352.x (doi:10.1046/j.1461-0248.2002.00352.x) [DOI] [Google Scholar]
- 3.Drickamer L. C. 1984. Urinary chemosignals from mice (Mus musculus): acceleration and delay of puberty in related and unrelated young females. J. Comp. Physiol. 98, 421–431 [PubMed] [Google Scholar]
- 4.Gerlach G. 2006. Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Anim. Behav. 72, 1119–1124 10.1016/j.anbehav.2006.03.009 (doi:10.1016/j.anbehav.2006.03.009) [DOI] [Google Scholar]
- 5.Marsh R. E., Howard W. E. 1979. Pheromones (odors) for rodent control. Pest Control Technol. 7, 22–23 [Google Scholar]
- 6.Thomson D. R., Gut L. J., Jenkins J. W. 1999. Pheromones for insect control: strategies and successes. Methods Biotechnol. 5, 385–412 [Google Scholar]
- 7.Li W., Slefkes M. J., Scott A. P., Teeter J. H. 2002. Sex pheromone communication in the sea lamprey: implications for management. J. Gt Lakes Res. 29(Suppl. 1), 85–94 10.1016/S0380-1330(03)70479-1 (doi:10.1016/S0380-1330(03)70479-1) [DOI] [Google Scholar]
- 8.Wassersug R. J. 1997. Assessing and controlling amphibian populations from the larval perspective. In Amphibians in decline: Canadian studies of a global problem (ed. Green D.), pp. 271–281 (Herpetological conservation no. 1). Saint Louis, MO: Society for the Study of Amphibians and Reptiles [Google Scholar]
- 9.Lever C. 2001. The cane toad. The history and ecology of a successful colonist. Otley, UK: Westbury Scientific Publishing [Google Scholar]
- 10.Kraus F. 2009. Alien reptiles and amphibians: a scientific compendium and analysis. Dordrecht, The Netherlands: Springer Science and Business Media BV [Google Scholar]
- 11.Shine R. 2010. The ecological impact of invasive cane toads (Bufo marinus) in Australia. Q. Rev. Biol. 85, 253–291 10.1086/655116 (doi:10.1086/655116) [DOI] [PubMed] [Google Scholar]
- 12.Alford R. A., Cohen M. P., Crossland M. R., Hearnden M. N., Schwarzkopf L. 1995. Population biology of Bufo marinus in northern Australia. In Wetland research in the wet–dry tropics of Australia (ed. Finlayson C. M.), pp. 173–181 (Supervising Scientist Report 101). Canberra, Australia: Supervising Scientist Commonwealth of Australia [Google Scholar]
- 13.Williamson I. 1999. Competition between the larvae of the introduced cane toad Bufo marinus (Anura: Bufonidae) and native anurans from the Darling Downs area of southern Queensland. Aust. J. Ecol. 24, 636–643 10.1046/j.1442-9993.1999.00993.x (doi:10.1046/j.1442-9993.1999.00993.x) [DOI] [Google Scholar]
- 14.Alford R. A. 1994. Interference and exploitation competition in larval Bufo marinus. In Advances in ecology and environmental studies (eds Mishra P. C., Behara N., Senapati B. K., Guru B. C.), pp. 297–306 New Dehli, India: Ashish Publishing House [Google Scholar]
- 15.Crossland M. R., Alford R. A., Shine R. 2009. Impact of the invasive cane toad (Bufo marinus) on an Australian frog (Opisthodon ornatus) depends on reproductive timing. Oecologia 158, 625–632 10.1007/s00442-008-1167-y (doi:10.1007/s00442-008-1167-y) [DOI] [PubMed] [Google Scholar]
- 16.Cabrera-Guzmán E., Crossland M. R., Shine R. 2011. Can we use the tadpoles of Australian frogs to reduce recruitment of invasive cane toads? J. Appl. Ecol. 48, 462–470 10.1111/j.1365-2664.2010.01933.x (doi:10.1111/j.1365-2664.2010.01933.x) [DOI] [Google Scholar]
- 17.Crossland M. R., Hearnden M. N., Pizzatto L., Alford R. A., Shine R. In press Why be a cannibal? The benefits to cane toad, Rhinella marina [=Bufo marinus], tadpoles of consuming conspecific eggs. Anim. Behav. 10.1016/j.anbehav.2011.07.099 (doi:10.1016/j.anbehav.2011.07.099) [DOI] [Google Scholar]
- 18.Crossland M. R., Shine R. 2011. Cues for cannibalism: cane toad tadpoles use chemical signals to locate and consume conspecific eggs. Oikos 120, 327–332 10.1111/j.1600-0706.2010.18911.x (doi:10.1111/j.1600-0706.2010.18911.x) [DOI] [Google Scholar]
- 19.Waldman B., Bishop P. J. 2004. Chemical communication in an archaic anuran amphibian. Behav. Ecol. 15, 88–93 10.1093/beheco/arg071 (doi:10.1093/beheco/arg071) [DOI] [Google Scholar]
- 20.Benard M. F. 2004. Predator-induced phenotypic plasticity in organisms with complex life-histories. Annu. Rev. Ecol. Evol. Syst. 35, 651–673 10.1146/annurev.ecolsys.35.021004.112426 (doi:10.1146/annurev.ecolsys.35.021004.112426) [DOI] [Google Scholar]
- 21.Touchon J. C., Warkentin K. M. 2008. Fish and dragonfly nymph predators induce opposite shifts in color and morphology of tadpoles. Oikos 117, 634–640 10.1111/j.0030-1299.2008.16354.x (doi:10.1111/j.0030-1299.2008.16354.x) [DOI] [Google Scholar]
- 22.Faragher S. G., Jaeger R. G. 1998. Tadpole bullies: examining mechanisms of competition in a community of larval anurans. Can. J. Zool. 76, 144–153 10.1139/z97-177 (doi:10.1139/z97-177) [DOI] [Google Scholar]
- 23.Hayes R. A., Crossland M. R., Hagman M., Capon R. J., Shine R. 2009. Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effect on predators. J. Chem. Ecol. 35, 391–399 10.1007/s10886-009-9608-6 (doi:10.1007/s10886-009-9608-6) [DOI] [PubMed] [Google Scholar]
- 24.Gosner K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 25.Crossland M. R., Brown G. P., Shine R. 2011. The enduring toxicity of road-killed cane toads (Rhinella marina). Biol. Invasions 13, 2135–2145 10.1007/s10530-011-0031-x (doi:10.1007/s10530-011-0031-x) [DOI] [Google Scholar]
- 26.Loman J. 1999. Early metamorphosis in common frog Rana temporaria tadpoles at risk of drying: an experimental demonstration. Amphib. Reptil. 20, 421–430 [Google Scholar]
- 27.Alford R. A. 1999. Ecology: resource use, competition, and predation. In Tadpoles: the biology of anuran larvae (eds McDiarmid R. W., Altig R.), pp. 240–278 Chicago, IL: University of Chicago Press [Google Scholar]
- 28.Steinwascher K. 1978. Interference and exploitation competition among tadpoles of Rana utricularia. Ecology 59, 1039–1046 10.2307/1938556 (doi:10.2307/1938556) [DOI] [Google Scholar]
- 29.Morin P. J., Johnson E. A. 1988. Experimental studies of asymmetric competition among anurans. Oikos 53, 398–407 10.2307/3565542 (doi:10.2307/3565542) [DOI] [Google Scholar]
- 30.Child T., Phillips B. L., Shine R. 2008. Abiotic and biotic influences on the dispersal behaviour of metamorph cane toads (Bufo marinus) in tropical Australia. J. Exp. Zool. 309A, 215–224 10.1002/jez.450 (doi:10.1002/jez.450) [DOI] [PubMed] [Google Scholar]
- 31.Ward-Fear G., Brown G. P., Shine R. 2010. Factors affecting the vulnerability of cane toads (Bufo marinus) to predation by ants. Biol. J. Linn. Soc. 99, 738–751 10.1111/j.1095-8312.2010.01395.x (doi:10.1111/j.1095-8312.2010.01395.x) [DOI] [Google Scholar]
- 32.Pizzatto L., Shine R. 2008. The behavioral ecology of cannibalism in cane toads (Bufo marinus). Behav. Ecol. Sociobiol. 63, 123–133 10.1007/s00265-008-0642-0 (doi:10.1007/s00265-008-0642-0) [DOI] [Google Scholar]
- 33.Kelehear C., Webb J. K., Shine R. 2009. Rhabdias pseudosphaerocephala infection in Bufo marinus: lung nematodes reduce viability of metamorph cane toads. Parasitology 136, 919–927 10.1017/S0031182009006325 (doi:10.1017/S0031182009006325) [DOI] [PubMed] [Google Scholar]
- 34.Fox H. 1988. Riesenzellen, goblet cells, Leydig cells and the large clear cells of Xenopus, in the amphibian larval epidermis: fine structure and a consideration of their homology. J. Submicrosc. Cytol. Pathol. 20, 437–451 [PubMed] [Google Scholar]
- 35.Hagman M., Shine R. 2009. Larval alarm pheromones as a potential control for invasive cane toads (Bufo marinus) in tropical Australia. Chemoecology 19, 211–217 10.1007/s00049-009-0027-5 (doi:10.1007/s00049-009-0027-5) [DOI] [Google Scholar]
- 36.Hagman M., Shine R. 2009. Species specific communication systems in invasive toads versus Australian frogs. Aquat. Conserv. 19, 724–728 10.1002/aqc.1045 (doi:10.1002/aqc.1045) [DOI] [Google Scholar]
- 37.Voronezhskaya E. E., Glebov K. I., Khabarova M. Y., Ponimaskin E. G., Nezlin L. P. 2008. Adult-to-embryo chemical signaling in the regulation of larval development in trochophore animals: cellular and molecular mechanisms. Acta Biol. Hung. 59, 117–122 10.1556/ABiol.59.2008.Suppl.19 (doi:10.1556/ABiol.59.2008.Suppl.19) [DOI] [PubMed] [Google Scholar]
- 38.Hagman M., Shine R. 2008. Australian tadpoles do not avoid chemical cues from invasive cane toads (Bufo marinus). Wildl. Res. 35, 59–64 10.1071/WR07113 (doi:10.1071/WR07113) [DOI] [Google Scholar]
- 39.Birch M. C., Haynes K. F. 1982. Insect pheromones. London, UK: Edward Arnold [Google Scholar]
- 40.Putnam A. R., Defrank J., Barnes J. P. 1983. Exploitation of allelopathy for weed control in annual and perennial cropping systems. J. Chem. Ecol. 9, 1001–1010 10.1007/BF00982207 (doi:10.1007/BF00982207) [DOI] [PubMed] [Google Scholar]
- 41.Mallik A. U. 2008. Allelopathy: advances, challenges and opportunities. In Allelopathy in sustainable agriculture and forestry (eds Mallik A. U., Luo S. M.), pp. 25–38 New York, NY: Springer [Google Scholar]