Abstract
The nonlinearity and arousal hypothesis predicts that highly aroused mammals will produce nonlinear, noisy vocalizations. We tested this prediction by measuring faecal glucocorticoid metabolites (GCMs) in adult yellow-bellied marmots (Marmota flaviventris), and asking if variation in GCMs was positively correlated with Wiener entropy—a measure of noise. Contrary to our prediction, we found a significant negative relationship: marmots with more faecal GCMs produced calls with less noise than those with lower levels of GCMs. A previous study suggested that glucocorticoids modulate the probability that a marmot will emit a call. This study suggests that, like some other species, calls emitted from highly aroused individuals are less noisy. Glucocorticoids thus play an important, yet underappreciated role, in alarm call production.
Keywords: alarm calls, arousal, communication, noise, nonlinearity, Marmota flaviventris
1. Introduction
The vocalizations of highly aroused mammals often contain nonlinear vocal attributes that include chaotic noise. Indeed, noise and other nonlinearities increase with arousal in human infant cries [1], dog (Canis familiaris) barks [2], piglet (Sus scrufa) squeals [3], meerkat (Suricata suricatta) alarm calls [4] and highly aroused vocalizations from other taxa (e.g. [5]). These acoustic features are thought to result from ‘over-blowing’ the sound production system, and it is hypothesized that habituating to such nonlinearities is difficult [6,7].
Expanding on Fitch and his colleagues' idea, Blumstein et al. [8–10] proposed that these nonlinear sounds honestly communicate the sound of arousal and fear. To date, there have been three playback studies which have demonstrated that alarm calls with nonlinearities in them (noise, [9,11]; sub-harmonic intrusions, [12]) are more evocative than calls without nonlinearities or with less noise. A further prediction from the nonlinearity and arousal hypothesis is that glucocorticoids should be associated with acoustic characteristics. Specifically, individuals that have relatively high glucocorticoid levels should produce alarm calls that are relatively more noisy (or contain other forms of nonlinearities), because they should be more aroused than those with relatively low glucocorticoid levels.
We tested this hypothesis with yellow-bellied marmots (Marmota flaviventris), a 3–5 kg, facultatively social, ground-dwelling squirrel—a good model because much is known about the contexts under which this species emits alarm calls [13]. Previous research has demonstrated that the propensity of adult females to produce alarm calls is correlated with glucocorticoids [14]; females are more likely to call on an occasion when she has relatively higher levels. In addition, there is substantial variation in faecal glucocorticoid levels between individuals and within an individual on different occasions; thus there is the opportunity for variation in glucocorticoid levels to be associated with call structure. Finally, juvenile marmots recently emerged from their natal burrows occasionally produce fear screams that contain noise and other nonlinear vocal attributes [8], but the degree of noise in either juvenile or adult alarm calls has not been formally quantified. We predicted that glucocorticoids would be associated with the degree of noisiness in adult alarm calls.
2. Material and methods
(a). Subjects and data collection
Marmots were studied at the Rocky Mountain Biological Laboratory during their summer active seasons between 2003 and 2009. We aimed to trap individuals in our study population every other week during routine morning and early evening trapping sessions. When trapped, marmots occasionally called. We recorded alarm calls from marmots contained in live traps using Audix OM-3xb microphones (frequency response: 40 Hz–20 kHz) placed 20–40 cm from calling subjects; these calls were recorded onto digital recording gear (either a Sony PCM-M1 digital audiotape recorder, or a Marantz PMD 660 direct to disk recorder) sampling at 44.1 kHz with 16-bit resolution. Because yellow-bellied marmots communicate risk, not predator type [13,15], recording trapped subjects controlled the context (and presumably the degree of risk) that calling marmots experienced, which allowed us to focus on other potential drivers of acoustic variation.
We used recordings from 46 adult subjects (eight males and 38 females) and extracted three to five 100 ms duration segments that contained alarm calls for a total of 223 calls. We used Sound Analysis Pro (Ofer Tchernichovski, City College of New York) to automatically extract the Wiener entropy associated with each adult alarm call; additional analyses described in the electronic supplementary material quantified entropy in the background environmental noise. A sound with Wiener entropy = 0 would be pure noise; noise is reduced as Wiener entropy decreases (i.e. approaches negative infinity). It is important to note that Wiener entropy does not distinguish between turbulent noise created by wind or microphone movement, and the chaotic noise produced by asymmetrically vibrating vocal folds. Only the latter is relevant for the nonlinearity hypothesis. Thus, we were very careful to select calls with no obvious background noise in them (see the electronic supplementary material). The marmot alarm calls analysed were all whistles that had variable numbers of harmonics; thus, we also counted the number of energy bands in the call (i.e. the fundamental frequency plus all harmonics).
Following Blumstein et al. [14], faeces found in traps when we reached them were collected in a plastic bag, immediately placed on ice, and frozen at −20°C within 2 h of collection. We assumed that faecal glucocorticoid metabolites (GCMs) reflect levels pooled over time, and, unlike concentrations in blood samples, are not influenced acutely by the capturing procedure. Faecal GCMs were measured with a double-antibody 125I radioimmunoassay kit (MP Biomedicals, Costa Mesa, CA, USA). Details of sample preparation and assay validation are found elsewhere [14].
(b). Statistical analysis
Faecal GCM levels were log10 transformed to normalize their distribution. We fitted a linear mixed effects model where we modelled alarm call entropy as a function of log10 GCM level on the occasion when the caller called, alarm call duration, the total number of energy bands in a call (calls are harmonically structured so this value was the number of harmonics + 1), the time of day in which the sample was collected (AM or PM), the caller's sex, along with the following two way interactions—sex × log10 faecal GCM, time of day × log10 GCM, sex × energy bands, sex × call duration and energy bands × call duration. We also fitted mixed effects models to study the effect of GCM on the total number of energy bands and on the call duration. In both models, we controlled for repeated individual observations.
3. Results
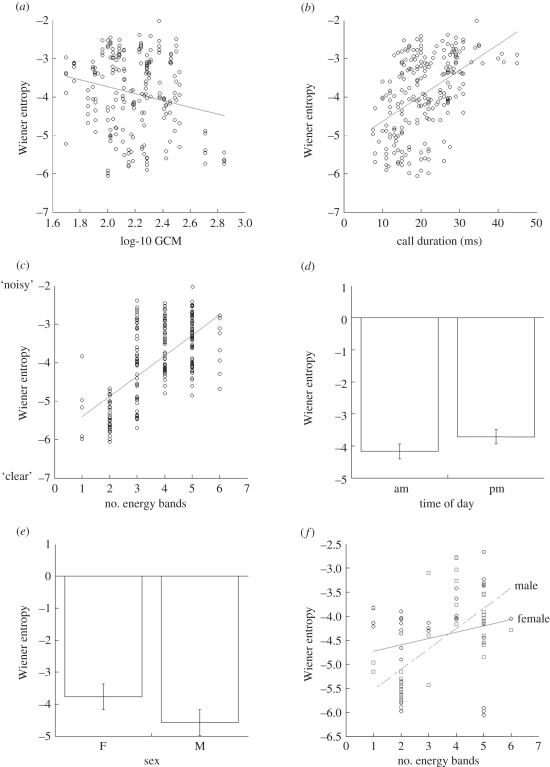
More negative values of Wiener entropy are ‘smaller’ and reflect sounds that are ‘clearer’ or more articulated. After controlling statistically for a number of other factors that could explain alarm call noisiness, Wiener entropy in calls decreased as faecal GCM levels rose—the calls became clearer as GCMs increased (table 1 and figure 1a). We also found significant effects by call duration (entropy increases as duration increases; figure 1b), the number of energy bands (entropy increases as the number of energy bands increase; figure 1c), time of day (entropy is greater in the afternoon; figure 1d) and the interaction between sex and energy bands (for males, as energy bands increased, entropy increased; figure 1f). Strictly, there was no sex effect (p = 0.05), but the marginal tendency (unlike the effect seen in the raw data; figure 1e) was for females to have noisier calls than males (table 1). Finally, in a mixed model, faecal GCM was not associated with the number of energy bands in a call (p = 0.532), but call duration decreased as faecal GCM increased (intercept estimate = 27.511 ms, GCM estimate = −3.457, p = 0.045).
Table 1.
Parameter estimates and p-values from linear mixed effect model predicting Wiener entropy contained in adult yellow-bellied marmot alarm calls.
| variable | estimate | p-value |
|---|---|---|
| GCM | −2.341 | <0.001 |
| call duration | 0.066 | 0.012 |
| energy bands | 0.468 | <0.001 |
| time of day (AM) | −2.148 | 0.007 |
| sex (female) | −3.215 | 0.050 |
| female × GCM | 0.983 | 0.146 |
| AM × GCM | 0.835 | 0.020 |
| female × energy bands | 0.197 | 0.016 |
| female × call duration | 0.0221 | 0.281 |
| energy bands × call duration | −0.010 | 0.068 |
Figure 1.
Significant (p < 0.05) factors that influence Wiener entropy in yellow-bellied marmot alarm whistles: (a) as faecal glucocorticoid metabolites increase, entropy decreases; (b) as call duration increases, entropy increases; (c) as the number of energy bands in the alarm calls increases, entropy increases; (d) entropy is greater in the afternoon; (e) sex may influence entropy directly (see text); (f) the effect of increasing energy bands on entropy is greater in males than in females.
4. Discussion
Contrary to a prediction of the nonlinearity and arousal hypothesis, adult yellow-bellied marmots with higher levels of faecal GCMs produced calls that had less, not more, noise. A possible mechanism for this is that highly aroused marmots pull their vocal folds taut while less aroused marmots relax their vocal folds. A functional consequence of this could be to ensure that receivers clearly received the message. Given that faecal GCM levels have already been demonstrated to be correlated with the probability of emitting calls in adult female marmots [14], we believe that our current correlative result is valid, but formal glucocorticoid manipulations would be required to demonstrate causality.
While juvenile yellow-bellied marmots may scream or emit alarm calls, adult marmots have three calls: a chuck, a whistle and a trill. Screams are full of a variety of nonlinear attributes [8] and chucks are extremely noisy (see fig. in Blumstein & Armitage [15]). Trills are formed by linking together a series of whistles.
Our previous studies that focused on marmot whistles had not specifically looked for noise or other nonlinear attributes. Here, we found that Wiener entropy, a measure of noise, varied, and this variation was explained by variation in GCM levels. Interestingly, there was no relationship between GCM levels and the number of energy bands. Such a relationship would suggest that scared marmots produced louder alarm calls. Since we also found that increased faecal GCM levels were associated with reduced call duration, we suspect that scared marmots are producing shorter and more defined calls, regardless of the number of energy bands produced.
Yellow-bellied marmots communicate risk by varying the rate and amplitude of their alarm calls [15]. When we recorded animals calling in traps, there was quite a bit of amplitude variation. This variation in amplitude sounds, to us, like what we hear naturally in the meadows; not all alarm calls are especially piercing.
Our results suggest that in marmots, and perhaps in other species, highly aroused animals produce piercing alarm calls that are probably the product of selection to have an immediate strong response in receivers. Piglets emit clearer, more piercing calls during castration compared with the less stressful periods before and after castration [16]. In addition, highly aroused isolated goats (Capra hircus) emitted tonal bleats [17]. Unhealthy or physically stressed dogs produce barks that either have lower-than-normal harmonic-to-noise ratios or very high harmonic-to-noise ratios [18]. These results suggest that stressed animals can either emit very noisy calls (low harmonic-to-noise ratio) or, like marmots, exceedingly clear, tonal calls (high harmonic-to-noise ratio). However, even in marmots, evocativeness can be produced by noisy vocalizations. Other vocalizations, such as screams, may be especially evocative because of their noise and other nonlinear attributes. Taken together, this research confirms that glucocorticoids play an important role in alarm call production [19], but risk can be communicated in multiple ways. Scared juveniles scream while scared adults produce less noisy calls.
Acknowledgements
We thank many marmoteers for help in the field; Raquel Monclús, Rebecca Nelson-Boot, Lynn Patton, Wendy Saltzman and Ofer Tchernichovski for help or advice in the laboratory; two anonymous reviewers for astute comments; and the U.C.L.A. Academic Senate and Division of Life Sciences, The National Geographic Society, The Rocky Mountain Biological Laboratory (Research Fellowship), and the N.S.F. (NSF-IDBR-0754247; to D.T.B.) and NSF-DBI 0242960, 0731346 (to the Rocky Mountain Biological Laboratory) for financial assistance.
References
- 1.Facchini A., Bellieni C. V., Marchettini N., Pulselli F. M., Tiezi E. B. P. 2005. Relating pain intensity of newborns to onset of nonlinear phenomena in cry recordings. Phys. Lett. A 338, 332–337 10.1016/j.physleta.2005.02.048 (doi:10.1016/j.physleta.2005.02.048) [DOI] [Google Scholar]
- 2.Tokuda I., Riede T., Neubauer J., Owren M. J., Herzel H. 2002. Nonlinear analysis of irregular animal vocalizations. J. Acoust. Soc. Am. 111, 2908–2919 10.1121/1.1474440 (doi:10.1121/1.1474440) [DOI] [PubMed] [Google Scholar]
- 3.Held S., Mason C., Mendl M. 2006. Maternal responsiveness of outdoor sows from first to fourth parities. Appl. Anim. Behav. Sci. 98, 216–233 10.1016/j.applanim.2005.09.003 (doi:10.1016/j.applanim.2005.09.003) [DOI] [Google Scholar]
- 4.Manser M. B. 2001. The acoustic structure of suricates' alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 10.1098/rspb.2001.1773 (doi:10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riede T., Owren M. J., Arcadi A. C. 2004. Nonlinear acoustics in pant hoots of common chimpanzees (Pan troglodytes): frequency jumps, subharmonics, biphonation, and deterministic chaos. Am. J. Primatol. 64, 277–291 10.1002/ajp.20078 (doi:10.1002/ajp.20078) [DOI] [PubMed] [Google Scholar]
- 6.Fitch W. T., Hauser M. D. 2002. Unpacking ‘honesty’: vertebrate vocal production and the evolution of acoustic signals. In Springer handbook of auditory research (eds Simmons A., Fay R. R., Popper A. N.), pp. 65–137 New York, NY: Springer [Google Scholar]
- 7.Fitch W. T., Neubauer J., Herzel H. 2002. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 63, 407–418 10.1006/anbe.2001.1912 (doi:10.1006/anbe.2001.1912) [DOI] [Google Scholar]
- 8.Blumstein D. T., Richardson D. T., Cooley L., Winternitz J., Daniel J. C. 2008. The structure, meaning, and function of yellow-bellied marmot pup screams. Anim. Behav. 76, 1055–1064 10.1016/j.anbehav.2008.06.002 (doi:10.1016/j.anbehav.2008.06.002) [DOI] [Google Scholar]
- 9.Blumstein D. T., Récapet C. 2009. The sound of arousal: the addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology 115, 1074–1081 10.1111/j.1439-0310.2009.01691.x (doi:10.1111/j.1439-0310.2009.01691.x) [DOI] [Google Scholar]
- 10.Blumstein D. T., Davitian R., Kaye P. D. 2010. Do film soundtracks contain nonlinear analogues to influence emotion? Biol. Lett. 6, 751–754 10.1098/rsbl.2010.0333 (doi:10.1098/rsbl.2010.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manser M. B., Bell M. B., Fletcher L. B. 2001. The information that receivers extract from alarm calls in suricates. Proc. R. Soc. Lond. B 268, 2485–2491 10.1098/rspb.2001.1772 (doi:10.1098/rspb.2001.1772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend S. W., Manser M. B. 2011. The function of nonlinear phenomena in meerkat alarm calls. Biol. Lett. 7, 47–49 10.1098/rsbl.2010.0537 (doi:10.1098/rsbl.2010.0537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumstein D. T. 2007. The evolution, function, and meaning of marmot alarm communication. Adv. Study Behav. 37, 371–400 10.1016/S0065-3454(07)37008-3 (doi:10.1016/S0065-3454(07)37008-3) [DOI] [Google Scholar]
- 14.Blumstein D. T., Patton M. L., Saltzman W. 2006. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol. Lett. 2, 29–32 10.1098/rsbl.2005.0405 (doi:10.1098/rsbl.2005.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumstein D. T., Armitage K. B. 1997. Alarm calling in yellow-bellied marmots: I. The meaning of situationally-specific calls. Anim. Behav. 53, 143–171 10.1006/anbe.1996.0285 (doi:10.1006/anbe.1996.0285) [DOI] [Google Scholar]
- 16.Puppe B., Schön P. C., Tuchscherer A., Manteuffel G. 2005. Castration-induced vocalization in domestic piglets, Sus scrofa: complex and specific alterations of vocal quality. Appl. Anim. Behav. Sci. 95, 67–78 10.1016/j.applanim.2005.05.001 (doi:10.1016/j.applanim.2005.05.001) [DOI] [Google Scholar]
- 17.Siebert K., Langbein J., Schön P., Tuchscherer A., Puppe B. 2011. Degree of social isolation affects behavioural and vocal response patterns in dwarf goats (Capra hircus). Appl. Anim. Behav. Sci. 131, 53–62 10.1016/j.applanim.2011.01.003 (doi:10.1016/j.applanim.2011.01.003) [DOI] [Google Scholar]
- 18.Riede T., Herzel H., Hammerschmidt K., Brunnberg L., Tembrock G. 2001. The harmonic-to-noise ratio applied to dog barks. J. Acoust. Soc. Am. 110, 2191–2197 10.1121/1.1398052 (doi:10.1121/1.1398052) [DOI] [PubMed] [Google Scholar]
- 19.Bercovitch F. B., Hauser M. D., Jones J. H. 1995. The endocrine stress response and alarm vocalizations in rhesus macaques. Anim. Behav. 49, 1703–1706 10.1016/0003-3472(95)90093-4 (doi:10.1016/0003-3472(95)90093-4) [DOI] [Google Scholar]