Abstract
Arbuscular mycorrhizal (AM) fungi represent ubiquitous mutualists of terrestrial plants. Through the symbiosis, plant hosts, among other benefits, receive protection from pathogens. A meta-analysis was conducted on 106 articles to determine whether, following pathogen infection of AM-colonized plants, the identity of the organisms involved (pathogens, AM fungi and host plants) had implications for the extent of the AM-induced pathogen suppression. Data on fungal and nematode pathogens were analysed separately. Although we found no differences in AM effectiveness with respect to the identity of the plant pathogen, the identity of the AM isolate had a dramatic effect on the level of pathogen protection. AM efficiency differences with respect to nematode pathogens were mainly limited to the number of AM isolates present; by contrast, modification of the ability to suppress fungal pathogens could occur even through changing the identity of the Glomeraceae isolate applied. N-fixing plants received more protection from fungal pathogens than non-N-fixing dicotyledons; this was attributed to the more intense AM colonization in N-fixing plants. Results have implications for understanding mycorrhizal ecology and agronomic applications.
Keywords: arbuscular mycorrhizas, biocontrol, meta-analysis, organic farming, plant pathogens
1. Introduction
Terrestrial herbaceous plants typically associate with glomeromycotan fungi to form arbuscular mycorrhizas (AM). Not only does the symbiosis promote plant growth through facilitation of nutrient uptake, but it also protects the plant from pathogens. In intensively managed arable land or following disturbance, however, a shortage of AM propagules is commonly recorded [1]. As a consequence, AM-fungal-propagule-containing formulations have been proposed for boosting yield in organic farming. The plant growth-promoting effect induced through AM inoculation has been extensively studied and includes plant biomass increases of the order of 50 per cent [2]. Our understanding of the function of pathogen suppression is lagging behind by comparison. Despite a meta-analysis which confirmed that AM fungi are able to suppress fungal and nematode pathogens [3], there is a wide range of open questions. We have limited information on the effectiveness of AM-fungal pathogen suppression for different pathogens, and do not know if this varies depending on pathogen lifestyle or identity. Moreover, we lack conclusive evidence for divergent ability within the Glomeromycota to efficiently suppress pathogens: at the AM family level, there has been evidence that large differences occur [4], requiring further testing. Finally, given the large differences in the extent of colonization of terrestrial plants by AM fungi [5], it is possible that plant identity also matters.
Here, we have conducted a meta-analysis, based on an unprecedented amount of data, to test whether the identity of the organisms in the association following AM colonization and pathogen infection does affect the severity of pathogen infection. We hypothesized that (hemi)biotrophic (fungal) and sedentary (nematode) pathogens would be most responsive to modification of the AM status of the plant owing to their feeding on living tissue. It was further hypothesized that Glomeraceae would be more effective in suppressing pathogens than Gigasporaceae owing to their more extensive investment in intracellular growth [6]. Finally, we hypothesized that the level of protection N-fixing plants receive from AM fungi is lower because of their higher tissue N content, which could reduce the efficiency of pathogen-protection mechanisms [7].
2. Material and methods
(a). Sources of data
The dataset was compiled based on the following sources: (i) articles that had been included in Borowicz [3]—covering a period from 1978 to 1998; (ii) articles published after 1999 that could be retrieved from a Web of Knowledge search on 12 May 2011 using the search strings ‘arbuscular and (pathogens or verticillium or phytophthora or pythium or fusarium or rhizoctonia)’ (413 articles) and ‘arbuscular and (nematodes or meloidogyne or heterodera or pratylenchus)’ (164 articles). Retrieved articles were screened so that they met the following criteria: (i) experiments were conducted in a soil or a sand : soil mix substrate that had been sterilized before the experiment to completely eliminate AM propagules; (ii) a measure of disease severity was provided—for nematode pathogens, we preferentially used number of nematodes/galls per gram of root dry weight (if absent root wet weight/shoot dry weight); (iii) the effect of AM fungi on disease severity could be studied in the absence of interactions with organisms other than indigenous soil microbes and rhizobia; and (iv) in split root experiments, only the halves where AM fungi and pathogens coexisted were considered. In the analysis of Borowicz [3], many studies had been excluded because they had not reported a measure of variance. Here we used a non-parametric measure of weighting instead to conduct the meta-analysis as inclusively as possible. In total, 106 articles were included in the database; compared with 22 articles for the parametric meta-analysis of Borowicz [3].
(b). Meta-analysis
The effect size (ES) adopted was the natural log ratio of disease severity or nematode population with and without AM inoculation. Thus, a negative ES means suppressive action of AM additions to the pathogens. The non-parametric weighting method implemented was as in Adams et al. [8]. Meta-analysis was conducted using MetaWin v. 2.0 software [9] based on separate one-factor random-effects models to test each moderator. Testing for the significance of moderators was based on a randomization resampling procedure with 3999 iterations. Confidence intervals (95% CIs) were constructed according to the bootstrapping method integrated in MetaWin. Because the specific meta-analytical procedure does not make any assumption about data distribution [9], we did not test for normality. In agreement with other studies [1], more than one trial per study was included. A critical point in the analysis was whether trials with information on fungal pathogens and on nematode pathogens should be analysed together. While mycorrhizal protection from fungal pathogens is through a combination of systemic and local mechanisms [10], nematode suppression occurs exclusively through local mechanisms ([11]—although there is evidence that the suppression mechanism for migratory nematodes may be systemic [12]). Moreover, data on nematodes were based on measures of nematode populations, whereas data on pathogen suppression were measures of disease severity and were not directly comparable. Finally, preliminary analysis revealed significant differences in the AM-induced suppression effect for the two types of pathogens. Therefore, the analysis was conducted separately for fungal and nematode pathogens. Additional details on the methodology are provided in the electronic supplementary material.
3. Results
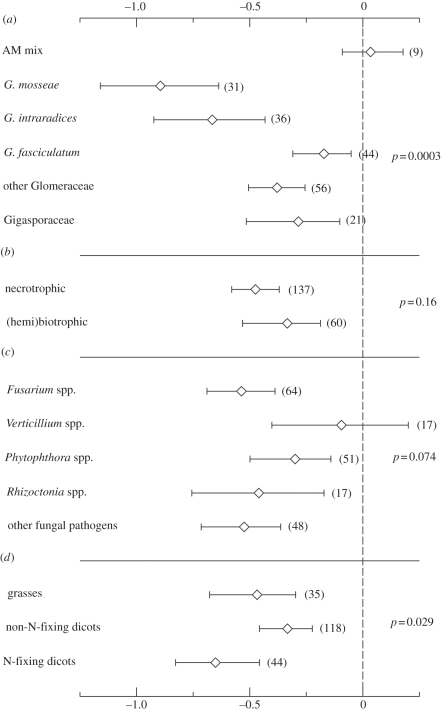
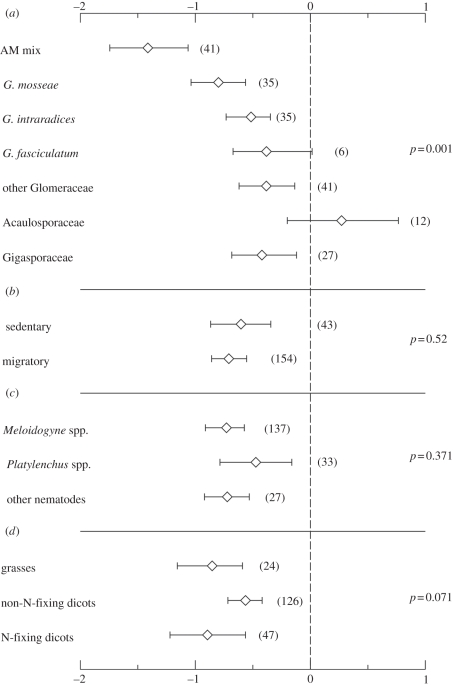
Overall AM colonization effects on fungal and nematode pathogens (and, respectively, 95% CIs; in both cases n = 197) were −0.44 (−0.54 to −0.36) and −0.69 (−0.82 to −0.56), respectively. For fungal pathogens, the effect of AM fungus identity was significant, and significant differences were recorded even for contrasts within the Glomeraceae (figure 1a). AM-induced disease suppression was not affected by the identity or lifestyle of the fungal pathogens, although there was a trend for Verticillium wilt to be more tolerant to AM fungal inoculation (figure 1b,c). N-fixing dicotyledons were better protected by AM fungi than their non-N-fixing relatives (figure 1d). Similar patterns were recorded for nematode pathogens. The identity of the AM fungi had a significant effect as well, although the differences were mainly between the trials where single and multiple AM inoculation had been carried out (figure 2a). In trials with Acaulosporaceae isolates, nematode stimulation was recorded (figure 2a). No differences were found with respect to the lifestyle or identity of the nematode pathogens (figure 2b,c). There was a trend for N-fixing dicotyledons to be better protected than non-N fixing dicotyledon plants (figure 2d). When the analysis was repeated to include a single trial per study, the probability of significance of moderators declined; however, the trends recorded were similar (electronic supplementary material).
Figure 1.
Effect sizes (means ± 95% CIs) of fungal pathogen disease response to arbuscular mycorrhizal (AM) colonization grouped according to (a) AM isolate identity, (b) pathogen lifestyle, (c) pathogen identity and (d) plant-host functional group. Numbers in parentheses refer to the number of trials that were present in the specific group. p-Values reported are those that are obtained for the Qb permutation test.
Figure 2.
Effect sizes (means ± 95% CIs) of nematode pathogen responses to arbuscular mycorrhizal (AM) colonization grouped according to (a) AM isolate identity, (b) pathogen lifestyle, (c) pathogen identity and (d) plant-host functional group. Numbers in parentheses refer to the number of trials that were present in the specific group. p-Values reported are those that are obtained for the Qb permutation test.
4. Discussion
Our analysis provides unprecedentedly strong evidence of the ability of AM fungi to suppress plant pathogens. Additionally, it was possible to determine the magnitude of the AM-induced decline in disease severity/nematode suppression that ranged from 30 to 42 per cent for fungal and 44–57% for nematode pathogens. The ability to deliver such statements represents one of the main advantages of using non-standardized ESs in meta-analyses. In contrast to our original hypothesis neither the lifestyle nor the identity of the pathogens mattered for the extent of the AM-induced pathogen suppression, despite the differences between nematode and fungal pathogens. This result suggests that through AM fungi, plants possibly receive similar protection from all pathogens rendering AM formulations a potentially broadly effective biocontrol agent.
The identity of the AM isolates applied was a key determinant of the extent of fungal pathogen suppression. Glomus mosseae was among the most effective inoculants (figure 1a). In the literature, G. mosseae has been demonstrated to be a fungus that pioneers colonization of new hosts (e.g. bait plants/greenhouse trials) but rarely occurs in undisturbed environments [13,14]. It is thus plausible to assume that it represents an AM fungus with a strategy equivalent to the ruderal strategy described for plants [15]. A characteristic example of a disturbed biome is arable land. Fast establishment of AM fungi in the field may be critical (as suggested from this study) to ensure efficient protection from pathogens; therefore, inclusion of AM fungi with aggressive plant-host colonization strategy in AM formulations would be advantageous.
By contrast, the main determinant of the protective effect of AM fungi on nematode pathogens was the simultaneous use of more than one AM species. AM fungi protect from sedentary nematodes mainly through local mechanisms [11]. Evidence is accumulating that colonization of plant hosts from multiple symbionts results in a complementarity effect with respect to P nutrition and plant growth stimulation [2,14,16] and potentially with per cent root colonization; this could increase protection of the plant host to sedentary nematode attacks. AM mixtures appeared to be less efficient for the suppression of fungal pathogens; this was unexpected based on the literature [17]. Although robustness of the result is questionable owing to the small sample size, the role of complementarity for fungal pathogen suppression is likely to be more limited owing to the systemic mode of action of AM fungi [10].
The most poorly performing AM ‘group’ in the nematode pathogen suppression trials was Acaulosporaceae. Unfortunately, no trials on fungal pathogen suppression were retrieved and we could not test whether there was a consistent inability of Acaulosporaceae to protect from pathogens (but see [18]). Contrary to our hypothesis, there was some limited evidence that N-fixing plants were better protected from fungal pathogens (a trend was also present for nematode pathogens) than non-N-fixing dicotyledons. We attribute this effect to the often high AM colonization of N-fixing plants [5]. The fact that grasses received moderate protection from pathogens may be agronomically important as many crops are grasses.
In conclusion, our study, based on an extended database of 106 articles, in addition to presenting robust and substantial evidence on the ability of AM fungi to protect from pathogens has demonstrated that the identity of the AM fungus may be crucial for pathogen protection. Our results suggest that the comparatively underappreciated AM function of pathogen protection should become the target of much more intense ecological and functional mycorrhiza research.
References
- 1.Lekberg Y., Koide R. T. 2005. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 168, 189–204 10.1111/j.1469-8137.2005.01490.x (doi:10.1111/j.1469-8137.2005.01490.x) [DOI] [PubMed] [Google Scholar]
- 2.Hoeksema J. D., et al. 2010. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407 10.1111/j.1461-0248.2009.01430.x (doi:10.1111/j.1461-0248.2009.01430.x) [DOI] [PubMed] [Google Scholar]
- 3.Borowicz V. A. 2001. Do arbuscular mycorrhizal fungi alter plant–pathogen relations? Ecology 82, 3057–3068 [Google Scholar]
- 4.Sikes B. A., Powell J. R., Rillig M. C. 2010. Deciphering the relative contributions of multiple functions within plant–microbe symbioses. Ecology 91, 1591–1597 10.1890/09-1858.1 (doi:10.1890/09-1858.1) [DOI] [PubMed] [Google Scholar]
- 5.Pawlowska T. E., Blaszkowski J., Rühling Å. 1996. The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza 6, 499–505 10.1007/s005720050154 (doi:10.1007/s005720050154) [DOI] [Google Scholar]
- 6.Hart M. M., Reader R. J. 2002. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 153, 335–344 10.1046/j.0028-646X.2001.00312.x (doi:10.1046/j.0028-646X.2001.00312.x) [DOI] [Google Scholar]
- 7.Mitchell C. E., Reich P. B., Tilman D., Groth J. V. 2003. Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Glob. Change Biol. 9, 438–451 10.1046/j.1365-2486.2003.00602.x (doi:10.1046/j.1365-2486.2003.00602.x) [DOI] [Google Scholar]
- 8.Adams D. C., Gurevitch J., Rosenberg N. J. 1997. Resampling tests for meta-analysis of ecological data. Ecology 78, 1277–1283 10.1890/0012-9658(1997)078[1277:RTFMAO]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1277:RTFMAO]2.0.CO;2) [DOI] [Google Scholar]
- 9.Rosenberg N. J., Adams D. C., Gurevitch J. 2000. MetaWin: statistical software for meta-analysis version 2.0. Sunderland, MA: Sinauer Associates [Google Scholar]
- 10.Pozo M. J., Cordier C., Dumas-Gaudot E., Gianinazzi S., Barea J. M., Azcon-Aguilar C. 2002. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 53, 525–534 10.1093/jexbot/53.368.525 (doi:10.1093/jexbot/53.368.525) [DOI] [PubMed] [Google Scholar]
- 11.de la Pena E., Rodriguez-Echeverria S., van der Putten W. H., Freitas H., Moens M. 2006. Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol. 169, 829–840 10.1111/j.1469-8137.2005.01602.x (doi:10.1111/j.1469-8137.2005.01602.x) [DOI] [PubMed] [Google Scholar]
- 12.Elsen A., Gervacio D., Swennen R., de Waele D. 2008. AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza 18, 251–256 10.1007/s00572-008-0173-6 (doi:10.1007/s00572-008-0173-6) [DOI] [PubMed] [Google Scholar]
- 13.Sýkorová Z., Ineichen K., Wiemken A., Redecker D. 2007. The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18, 1–14 10.1007/s00572-007-0147-0 (doi:10.1007/s00572-007-0147-0) [DOI] [PubMed] [Google Scholar]
- 14.Jansa J., Smith F. A., Smith S. E. 2008. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 177, 779–789 10.1111/j.1469-8137.2007.02294.x (doi:10.1111/j.1469-8137.2007.02294.x) [DOI] [PubMed] [Google Scholar]
- 15.Grime J. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194 10.1086/283244 (doi:10.1086/283244) [DOI] [Google Scholar]
- 16.Koide R. T. 2000. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol. 147, 233–235 10.1046/j.1469-8137.2000.00710.x (doi:10.1046/j.1469-8137.2000.00710.x) [DOI] [Google Scholar]
- 17.Wehner J., Antunes P. M., Powell J. R., Mazukatow J., Rillig M. C. 2010. Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53, 197–201 10.1016/j.pedobi.2009.10.002 (doi:10.1016/j.pedobi.2009.10.002) [DOI] [Google Scholar]
- 18.Maherali H., Klironomos J. N. 2007. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316, 1746–1748 10.1126/science.1143082 (doi:10.1126/science.1143082) [DOI] [PubMed] [Google Scholar]