Abstract
Understanding the effects of water temperature on the swimming performance of fishes is central in understanding how fish species will respond to global climate change. Metabolic cost of transport (COT)—a measure of the energy required to swim a given distance—is a key performance parameter linked to many aspects of fish life history. We develop a quantitative model to predict the effect of water temperature on COT. The model facilitates comparisons among species that differ in body size by incorporating the body mass-dependence of COT. Data from 22 fish species support the temperature and mass dependencies of COT predicted by our model, and demonstrate that modest differences in water temperature can result in substantial differences in the energetic cost of swimming.
Keywords: cost of transport, metabolism, climate change, biomechanics, ecomechanics
1. Introduction
Mounting evidence suggests that water temperatures have increased in marine and freshwater habitats around the globe and will probably continue to increase in the future [1]. While projections vary, some estimates indicate that sea surface temperatures, for example, may rise by as much as 2–4°C by the end of this century [1]. Water temperature has been linked to many features of fish swimming performance, including respiratory scope and escape swimming speed [2,3]. One feature in particular—the metabolic cost of transport (COT), which describes the amount of energy used to swim a given distance—appears to increase with increasing water temperature in several species [4–6]. However, it is not clear whether the temperature-dependence of COT observed in these species is a general phenomenon that applies across diverse fish species. Moreover, a quantitative model to describe the temperature-dependence of COT based on metabolism and swimming mechanics is still lacking, making it difficult to predict how changes in water temperature might affect fish swimming energetics.
To address this issue, we develop a model to describe the temperature-dependence of COT. The model facilitates comparisons among fishes that differ in body size by incorporating the body mass-dependence of COT. It builds on work addressing the relationship between temperature, body size and maintenance metabolism [7], and on work relating temperature and body size to swimming energetics [2]. We evaluate model predictions using data from swimming experiments on 22 diverse fish species.
2. Model development
The model developed here is based on the hypothesis that COT is governed by three variables: the rate of energy expenditure devoted to swimming (Rswim, W), the rate of energy expenditure devoted to maintenance (Rstd, W) and swimming speed (u, m s–1). This approach has been used to capture the principal features of swimming energetics in previous studies [4–6,8]. The COT (in J m−1) is given by:
| 2.1 |
COT is typically a u-shaped function of speed, with a minimum value (COTmin) occurring at speed, uopt, [6]. We focus on COTmin because, for fishes swimming at steady speeds and water temperatures, it sets a lower bound on the cost of swimming. It thus provides a conservative metric for evaluating effects of changing water temperature on swimming energetics.
From the hypothesis that Rstd and Rswim determine COTmin, it follows that differences in COTmin within and among species should be governed by the primary variables that determine Rstd and Rswim: temperature and body mass [6,8]. Local adaptations leading to changes in variables such as red versus white muscle fibre recruitment and muscle contractile properties have the potential to influence Rswim [6], but the relative importance of these variables is not well known. We hypothesize that the relationship between COTmin and water temperature is primarily determined by the well-documented relationships between temperature, maintenance metabolism and swimming mechanics. Rstd, can be described by the equation, 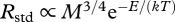 , where E is the average activation energy of the respiratory complex (eV), T is body temperature (K, assumed to equal water temperature), k is Boltzmann's constant (eV/K) and M is body mass (kg) [7]. This model posits that Rstd changes with temperature owing to the temperature-dependence of core metabolic processes [7]. The mass-dependence of Rstd has been hypothesized to result from physical constraints on the delivery networks that regulate energy supply to cells [7], but the origin of this relationship is still debated. In the electronic supplementary material, appendix, we derive an alternative model in which the relation
, where E is the average activation energy of the respiratory complex (eV), T is body temperature (K, assumed to equal water temperature), k is Boltzmann's constant (eV/K) and M is body mass (kg) [7]. This model posits that Rstd changes with temperature owing to the temperature-dependence of core metabolic processes [7]. The mass-dependence of Rstd has been hypothesized to result from physical constraints on the delivery networks that regulate energy supply to cells [7], but the origin of this relationship is still debated. In the electronic supplementary material, appendix, we derive an alternative model in which the relation 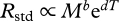 is assumed and empirical estimates of b and d are used. Such a model yields similar predictions to the model presented here.
is assumed and empirical estimates of b and d are used. Such a model yields similar predictions to the model presented here.
The rate of energy expenditure, Rswim, required to swim at a constant speed is proportional to the product of u and the drag experienced at speed, u [6]. Increasing temperature decreases the viscosity of water [2]. Yet the corresponding reduction in friction drag appears to have a minimal effect on fishes in the size range of most juvenile and adult fishes (i.e. Re > 103–8.8 × 103) [2]. We therefore hypothesize that Rswim is independent of temperature. Body mass, by contrast, strongly affects Rswim. Rswim increases with increasing body mass because larger fishes experience greater drag at a given swimming speed. Pressure drag makes the largest contribution to total drag for swimmers in the size range of most juvenile and adult fishes, suggesting that Rswim is roughly proportional to body cross-sectional area, or 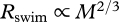 , where body cross-sectional area is assumed to be proportional to mass to the two-thirds power [2]. Combining these temperature and mass dependencies with the speed-dependence of Rswim [8] shows that
, where body cross-sectional area is assumed to be proportional to mass to the two-thirds power [2]. Combining these temperature and mass dependencies with the speed-dependence of Rswim [8] shows that 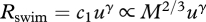 , where the speed exponent, γ, describes how Rswim increases with increasing swimming speed and the variable
, where the speed exponent, γ, describes how Rswim increases with increasing swimming speed and the variable 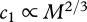 is introduced for notational convenience.
is introduced for notational convenience.
The effects of temperature and mass on the variables in equation (2.1) can be used to derive the temperature- and mass-dependence of COTmin. Equation (2.1) can be solved for COTmin by the standard technique of taking the derivative with respect to u, setting the result equal to zero, solving for uopt and substituting uopt for u in equation (2.1) ([6,8] and electronic supplementary material, appendix). Expressing Rstd and c1 in the resulting equation as functions of mass and temperature yields:
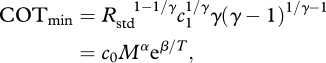 |
2.2 |
where c0 is a constant, α = 0.75–0.08γ−1 and β = −(E/k)[1 − 1/γ]. Expressing COTmin as dimensionless COT (J N−1 m−1) does not alter the predicted temperature-dependence (see the electronic supplementary material, appendix).
The constants, E, k and γ can be independently estimated from data or theory to yield quantitative predictions about the values of the α and β parameters. Equation (2.2) thus makes two predictions about the relationship between COTmin, temperature and body mass. First, the temperature exponent, β, is described by the equation β = −(E/k)[1 − 1/γ]. Rigid body hydrodynamic models suggest that γ should be in the range of 2.5–2.8 depending on flow conditions [8]. Boltzmann's constant, k, equals 8.62 × 10−5 eV K−1 [9] and empirical estimates of E suggest that E ≈ 0.41 eV [7] in fishes. The model therefore predicts that β is between –3.1 × 103 and –2.9 × 103. This means that fishes swimming in warmer waters should experience greater COTmin than fishes in colder waters, with the functional form of the temperature-dependence given by equation (2.2). Second, the mass-scaling exponent, #x03B1;, is given by, α = 0.75–0.08γ−1. Our model therefore predicts that α = 0.72. In other words, at a given water temperature, larger fishes should experience greater COTmin than smaller fishes, with COTmin ∝ M0.72.
3. Material and methods
To test our model, we compiled published studies that reported fish respiration rates over a range of swimming speeds from fishes using body-caudal fin gaits. We used only studies that acclimated fishes to test temperatures and maintained stable O2 concentrations (see the electronic supplementary material, appendix).
Data collection resulted in a total of over 800 observations from 22 species (electronic supplementary material, table S1 in appendix) ranging in mass from 8.3 × 10−3 to 1.2 kg. Swimming respiration rates were measured at water temperatures ranging from 5°C to 30°C. To identify COTmin for each species-by-temperature combination, we converted measurements of total respiration rate into W using standard oxycaloric values (20.1 J mlO2–1, 14.1 J mgO2–1; [6]) and used nonlinear least squares to estimate COTmin for each species at each temperature (see the electronic supplementary material, appendix).
After calculating COTmin values, we fitted equation (2.2) to COTmin data to estimate α and β parameters. Prior to fitting, we ln-transformed COTmin, and M to linearize the equation (i.e. 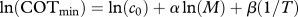 ). We then fitted the linearized model using generalized least squares to allow for correlation among observations taken from the same species at different temperatures.
). We then fitted the linearized model using generalized least squares to allow for correlation among observations taken from the same species at different temperatures.
4. Results and discussion
The model described by equation (2.2) provided a good fit to data, with mass and temperature explaining 83 per cent of the variation in COTmin (figure 1, best fit model: 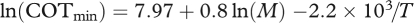 ). Data supported both quantitative predictions regarding effects of temperature and body mass on COTmin. The 95 per cent confidence interval (CI) for the β parameter (βest = –2.2 × 103, 95% CI = (–3.1 × 103, −1.3 × 103), p = 1 × 10−4) overlapped the predicted range (−3.1 × 103, −2.9 × 103), confirming that the observed effect of water temperature on COTmin matched that predicted by our model. The observed temperature-dependence is approximately equivalent to a Q10 of 1.3. The 95 per cent CI of the α parameter (αest = 0.80, 95% CI = (0.69, 0.92), p < 2 × 10−16) overlapped the predicted value of 0.72 (figure 1b), confirming that the observed mass-dependence also matched model predictions. COTmin depends on both temperature and mass; to display data in bivariate plots, COTmin values were normalized based on observed mass-dependence of COTmin (mass-corrected
). Data supported both quantitative predictions regarding effects of temperature and body mass on COTmin. The 95 per cent confidence interval (CI) for the β parameter (βest = –2.2 × 103, 95% CI = (–3.1 × 103, −1.3 × 103), p = 1 × 10−4) overlapped the predicted range (−3.1 × 103, −2.9 × 103), confirming that the observed effect of water temperature on COTmin matched that predicted by our model. The observed temperature-dependence is approximately equivalent to a Q10 of 1.3. The 95 per cent CI of the α parameter (αest = 0.80, 95% CI = (0.69, 0.92), p < 2 × 10−16) overlapped the predicted value of 0.72 (figure 1b), confirming that the observed mass-dependence also matched model predictions. COTmin depends on both temperature and mass; to display data in bivariate plots, COTmin values were normalized based on observed mass-dependence of COTmin (mass-corrected 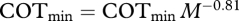 ; figure 1a) or observed temperature-dependence of COTmin (temperature-corrected
; figure 1a) or observed temperature-dependence of COTmin (temperature-corrected 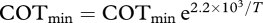 ; figure 1b).
; figure 1b).
Figure 1.
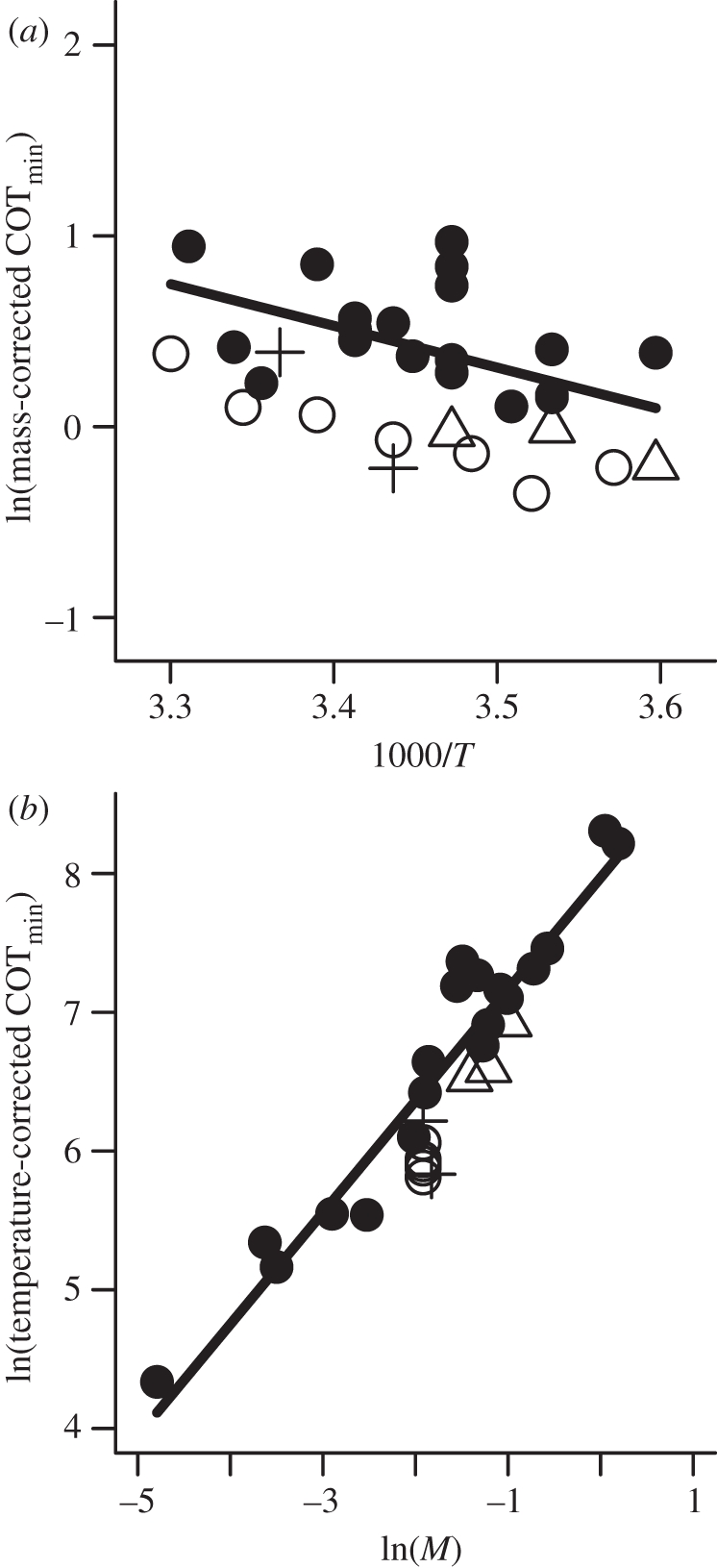
(a) Mass-corrected COTmin (see text) as a function of inverse temperature, 1000/T (K). (b) Temperature-corrected COTmin (see text) as a function of ln(M). Filled circles represent species for which a single measurement was used. Other symbols indicate species for which measurements at multiple temperatures were included (open circles, Dicentrarchus labrax; triangles, Gadus morhua; crosses, Scomber japonicus).
The consistent predictable increase in COTmin with increasing temperature demonstrates that COTmin increases with temperature among species as well as within species [5]. The strong agreement between predicted and observed values of the temperature exponent β, support our model, suggesting that the effect of temperature on COTmin may be primarily mediated by changes in Rstd; changes in Rswim are not necessary to produce the observed temperature-dependence. Further support comes from the direct observation that Rswim is independent of temperature in at least one species [5]. This finding contrasts sharply with the strong effect of temperature-mediated changes in viscosity on the swimming mechanics of small larval fishes, where increasing temperature can actually decrease COT [2]. Changes in water temperature thus appear to have opposing effects on the swimming performance of different life stages.
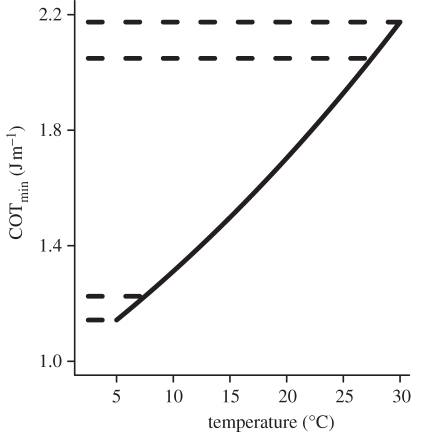
Our results lead to two general insights about the effect of water temperature on the cost of swimming. First, the model and empirical estimates of the α and β parameters can be used to predict the mean change in COTmin under particular climate change scenarios. For example, an increase in water temperature of 2–4°C [1] would lead to a 6–12% increase in the minimum amount of energy required to swim 1 m. Second, the data presented here support our assumption that COTmin changes exponentially with inverse temperature (1/K). If this is generally the case, a given increase in water temperature will elicit a larger absolute change in COTmin in warm water than in cold water. For example, an increase in temperature of 2.5°C, from 27.5°C to 30°C (figure 2, upper dashed lines), causes an increase in COTmin that is 1.5 times greater than that caused by an increase from 5°C to 7.5°C (0.12 J m–1 versus 0.08 J m–1; figure 2, lower dashed lines). It is thus plausible that major changes in the cost of swimming will occur in geographical regions where water temperatures are already high.
Figure 2.
COTmin as a function of temperature for a 1 kg fish. Solid line based on equation (2.2) and parameter estimates presented in text. Dashed lines show change in COTmin owing to an increase in temperature from 5°C to 7.5°C (0.08 J m–1, lower dashed lines) and 27.5°C to 30°C (0.12 J m–1, upper dashed lines).
Unexplained variation about the fitted lines in figure 1 at least partially reflects variation in the speed exponent γ, which affects COTmin as illustrated by equation (2.2). The mean value γ among the species included in our analysis was 2.4, close to the values predicted by simple hydrodynamic models (γ = 2.5–2.8) [8]. However γ varied around this value (coefficient of variation = 0.44). One promising approach for improving predictions may be using more detailed models of fish swimming mechanics (e.g. [10]) to better understand variation in the γ parameter in the simpler model used here.
Our results suggest that rising water temperatures may increase the energetic cost of routine swimming behaviours such as foraging and migration. The precise impact of climate change will ultimately depend on the degree to which water temperatures rise. However, integrating our results with work on the thermal physiology of larval, juvenile and adult fishes [2,3] will produce a more comprehensive picture of the potential for climate change to affect fishes and the communities they inhabit.
Acknowledgements
We thank members of the Gillooly laboratory for helpful thoughts and comments. A.M.H. was supported by a National Science Foundation Graduate Research Fellowship under grant no. DGE-0802270.
References
- 1.IPCC 2007. IPCC fourth assessment report: climate change 2007. Cambridge University Press, Cambridge, UK [Google Scholar]
- 2.Hunt von Herbing I. 2002. Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J. Fish Biol. 61, 865–876 10.1111/j.1095-8649.2002.tb01848.x (doi:10.1111/j.1095-8649.2002.tb01848.x) [DOI] [Google Scholar]
- 3.Nilsson G. E., Crawley N., Lunde I. G., Munday P. L. 2009. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Change Biol. 15, 1405–1412 10.1111/j.1365-2486.2008.01767.x (doi:10.1111/j.1365-2486.2008.01767.x) [DOI] [Google Scholar]
- 4.Claireaux G., Couturier C., Groison A. 2006. Effects of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 209, 3420–3428 10.1242/jeb.02346 (doi:10.1242/jeb.02346) [DOI] [PubMed] [Google Scholar]
- 5.Ohlberger J., Staaks G., Holker F. 2007. Effects of temperature, swimming speed and body mass on standard and active metabolic rate in vendace (Coregonus albula). J. Comp. Physiol. B 177, 905–916 10.1007/s00360-007-0189-9 (doi:10.1007/s00360-007-0189-9) [DOI] [PubMed] [Google Scholar]
- 6.Videler J. J. 1993. Fish swimming, 1st edn. London, UK: Chapman & Hall [Google Scholar]
- 7.Gillooly J. F. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 8.Alexander R. M. 2003. Principles of animal locomotion. Princeton, NJ: Princeton University Press [Google Scholar]
- 9.Mohr P. J., Taylor B. N., Newell D. B. 2008. CODATA recommended values of the fundamental physical constants: 2006. Rev. Mod. Phys. 80, 633. 10.1103/RevModPhys.80.633 (doi:10.1103/RevModPhys.80.633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candelier F., Boyer F., Leroyer A. 2011. Three-dimensional extension of Lighthill's large-amplitude elongate-body theory of fish locomotion. J. Fluid Mech. 674, 196–226 10.1017/S002211201000649X (doi:10.1017/S002211201000649X) [DOI] [Google Scholar]