Abstract
Courtship displays are often energetically and temporally costly as well as highly conspicuous to predators. Selection should therefore favour signalling tactics that minimize courtship costs while maintaining or increasing signal attractiveness. In fiddler crabs, males court females by waving their one greatly enlarged claw in a highly conspicuous and costly display. Here, we investigate whether courting males adjust their wave rate, and therefore the cost of courtship, to the current level of competition. We show that display rate increases as competition increases and that when competition is removed, males reduce their display rate by 30 per cent. These results suggest that male fiddler crabs actively reduce the cost of courtship by adjusting their wave rate in response to the immediate level of competition.
Keywords: animal communication, courtship competition, fiddler crabs, mate attraction, Uca annulipes
1. Introduction
Mate choice often takes place within a complex social environment, where numerous individuals simultaneously compete for the attention of a mate (e.g. choruses and leks) [1]. Information exchange during these communication events is rarely confined to that between a signaller and a receiver [2]. Instead, individuals other than the intended receiver, including neighbouring competitors, sometimes use the information transmitted by a signaller when making decisions about their own signalling strategy [3]. For instance, on detecting another male's courtship signal, competing males may alter specific properties of their own signals, such as the rate of production, so as to increase their relative attractiveness and thereby their chance of obtaining a mate [4]. For example, in the grey treefrog (Hyla versicolor), males increased their call rate with an increase in chorus size [5].
Several studies in acoustic signalling systems have shown that males alter call rate, duration and/or complexity in response to the presence and/or calling of other males [4–10]. Surprisingly, few studies have, however, examined how competing sexual signals influence male behaviour in a visual signalling context [11–13]. Nevertheless, plasticity in visual signalling behaviour during mate attraction is likely to be widespread. Courtship signals are often costly because of the increased expenditure of energy and time, and a greater risk of predation. Selection should therefore favour males that minimize these costs by adjusting their signalling behaviour according to the level of competition and/or likelihood of attracting a mate [13,14].
In fiddler crabs (genus Uca), males attract females by waving their one greatly enlarged claw in a conspicuous courtship display [15]. In Uca annulipes, females preferentially visit males that wave at a faster rate (waves per min) and produce more leading waves [16,17] implying that wave rate and wave leadership are targets of female choice. In the related species, U. mjoebergi, experiments show that leadership and wave rate are direct targets of female choice [18]. Waving is, however, extremely costly, in terms of energy, time and increased conspicuousness to predators. For example, wave rate in U. annulipes decreases with lower food availability [19], and predation rate by black-bellied plovers (Pluvialis squatarola) and ruddy turnstones (Arenaria interpres) on U. uruguayensis is greater for courting males [20]. Consequently, males should facultatively adjust their wave rate according to the level of male competition to increase their chance of attracting a mate while minimizing the costs of producing courtship signals. This was, however, not the case in a study of U. tangeri where large males reduced their wave rate when competitors were present. It was argued that this decline in wave rate increased signal effectiveness by producing more lagging waves. It should be noted, however, that U. tangeri does not wave synchronously and that the sample size was only six males [21].
Here, we investigate how the presence of competing males influences wave display rate in the synchronously displaying fiddler crab U. annulipes.
2. Material and methods
We studied U. annulipes on the Chukwani mudflats (6°13′19.37″S; 39°12′13.56″E), Zanzibar, Tanzania from August to October 2010. Uca annulipes occur in dense mixed sex colonies and both sexes defend territories that are centred on a burrow [22]. During the mating period, receptive females leave their territory and wander through the population in search of a mate [16]. Courting males cluster around the female and wave their one greatly enlarged claw. Males tend to produce waves that are in close synchrony with each other [23]. Female U. annulipes preferentially approach males with faster wave rates, greater wave leadership (slightly earlier waves during a synchronous bout) and larger claws [16,17,23]. In combination with studies on the closely related U. mjoebergi [18], we assume here that females show a direct preference for higher wave rates. Final mate choice is based on burrow quality (mating and incubation occurs within the male's burrow). Courting males occur at an average density of 19.6 males m−2 and females sequentially sample as many as 24 males and travel as far as 28 m in search of a mate [17].
To determine whether the presence of competing males affects the waving display of a courting male, we conducted an experiment that manipulated the social environment of a focal male. We used a repeated-measures design with two treatments (high-competition and low-competition). We located naturally occurring groups of males and removed any wandering females within 50 cm of the group. A group of males was classified as two to five neighbouring males that were all waving at the same female at the time of initial sighting. For each trial, we tethered a female (superglued a 1 cm piece of cotton thread to the crab's carapace and attached it to a nail pushed into the sediment) and placed her 10 cm in front of a randomly designated focal male that could either see (high-competition) or not see (low-competition) his neighbours.
For the low-competition treatment, we startled all crabs into their burrows by standing up and approaching them. We then placed bottle caps over all burrows within 50 cm of the focal male to prevent the neighbouring crabs from emerging during the trial. Caps were removed immediately following the trial. We then waited approximately 3 min (up to 2 min to allow the male to surface and then 1 min to locate and start waving at the female) and measured the wave rate of the focal male by counting the number of waves produced over a 30 s period.
For the high-competition treatment, we scared all crabs into their burrows and randomly placed 10 or more bottle caps on the sediment surface within 50 cm of the focal male's burrow, without covering any neighbouring burrows. As described above, we then waited approximately 3 min (up to 2 min to allow all males to surface and then 1 min for the males to locate and start waving at the female) before recording the focal male's wave rate. The number of waving neighbours as well as the size (measured to the nearest 0.1 mm using dial callipers) and distance of the nearest male neighbour was also recorded.
The same male was designated the focal male for both treatments and the same group of males was used for both treatments. To avoid any bias owing to an order effect, the treatment order was random. We tested the wave rate of 50 males in 50 different groups of males.
We used Wilcoxon signed-rank tests to compare wave rate between the two treatments. All tests were two-tailed. Data are presented as median and upper and lower quartiles.
3. Results
Wave rate differed significantly between the high- and low-competition treatments (p < 0.001, z = −5.167). The median wave rate of the focal male during the high-competition treatment was 16.5 waves per minute (upper and lower quartile: 12.75–20.25). In the low-competition treatment, where all neighbouring males were enclosed in their burrow, the focal male's wave rate was 11.5 waves per minute (7.75–15).
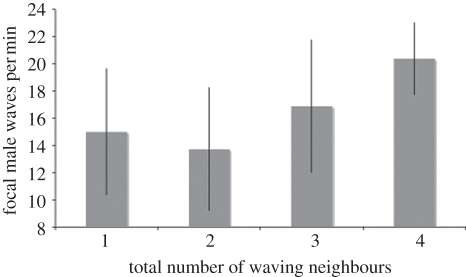
There was a positive relationship between the number of waving neighbours and the number of waves produced by the focal male during the high-competition treatment (rs = 0.43, p = 0.002). Males with more neighbours produced a greater number of waves (figure 1). There was no relationship between the distance to the nearest waving male neighbour and the number of waves produced by the focal male (rs = 0.112, p = 0.486). There was also no relationship between the size of nearest waving male neighbour and the number of waves produced by the focal male (rs = 0.21, p = 0.190).
Figure 1.
Focal male wave rate with 1, 2, 3 and 4 waving neighbours. Error bars indicate ±1 s.d.
4. Discussion
We have shown that male display rate in the fiddler crab U. annulipes is strongly influenced by the presence and number of nearby displaying males. Males whose male neighbours were not on the surface produced 30 per cent fewer waves than males whose male neighbours were present. Furthermore, males with many male neighbours displayed more often than those with fewer male neighbours. While a number of studies have shown similar alterations to signal rate in response to competition in acoustic signalling species [4–6,8,9], surprisingly few studies have empirically shown an increase in courtship rate in response to elevated mating competition in a visual signal context ([13] review). One example comes from guppies (Poecilia reticulata), where Farr [11] showed that males increased courtship rate in the presence of other males. Contrasting results were, however, reported by Jirotkul [12], who showed that with greater competition male courtship rate actually decreased.
While we expected males to adjust their wave rate according to the current level of competition, there are several reasons why it might be beneficial for males to maintain high wave rates regardless of the level of competition. First, in fiddler crabs, the operational sex ratio is extremely male-biased with males infrequently encountering receptive females [24,25]. In the closely related species U. mjoebergi, there is approximately one sampling female to every 45 courting males [25]. Opportunities to attract a female are therefore infrequent. Second, wave rate is believed to be an indicator of male quality, and females appear to have a preference for wave rates that exceed a minimum threshold [18,19]. Third, in fiddler crabs, mate choice is often based on the sequential assessment of male traits. In U. annulipes, females often travel substantial distances and sample multiple males from multiple groups before selecting a mate [17]. Therefore, regardless of the level of immediate competition, one might expect that males will signal at their maximum intensity not only because females are rare, but also to increase the probability of exceeding the female's mating preference threshold. The latter will decrease the likelihood of the female rejecting the male and moving on to sample another male.
The above factors are likely to generate selection on males to court all receptive females at their maximum intensity regardless of the level of competition. Our results suggest, however, that the costs of excessive waving might override these potential benefits. We suggest that our results are best explained by the fact that waving is extremely costly and that males can reduce these costs by adjusting their wave rate according to the level of competition and/or the likelihood of attracting a female. By doing so, males effectively reduce the overall cost of courtship.
Acknowledgements
We thank Isobel Booksmythe, Jessica Bolton and Sophia Callander. We also thank John Christy and an anonymous referee for helpful comments. Research was funded by an Australian Postgraduate Award (to R.N.C.M.) and the Australian Research Council (P.R.Y.B. and M.D.J.).
References
- 1.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Akre K. L., Farris H. E., Lea A. M., Page R. A., Ryan M. J. 2011. Signal perception in frogs and bats and the evolution of mating signals. Science 333, 751. 10.1126/science.1205623 (doi:10.1126/science.1205623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peake T. M. 2005. Eavesdropping in communication networks. In Animal communication networks (ed. McGregor P. K.), pp. 13–37 New York, NY: Cambridge University Press [Google Scholar]
- 4.Martinez-Rivera C. C., Gerhardt H. C. 2008. Advertisement-call modification, male competition, and female preference in the bird-voiced treefrog Hyla avivoca. Behav. Ecol. Sociobiol. 63, 195–208 10.1007/s00265-008-0650-0 (doi:10.1007/s00265-008-0650-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz J. J., Buchanan B. W., Gerhardt H. C. 2002. Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav. Ecol. Sociobiol. 53, 9–19 10.1007/s00265-002-0542-7 (doi:10.1007/s00265-002-0542-7) [DOI] [Google Scholar]
- 6.Lopez P. T., Narins P. M., Lewis E. R., Moore S. W. 1988. Acoustically induced call modification in the white-lipped frog Leptodactylus albilabris. Anim. Behav. 36, 1295–1308 10.1016/S0003-3472(88)80198-2 (doi:10.1016/S0003-3472(88)80198-2) [DOI] [Google Scholar]
- 7.Hill P. S. M. 1998. Environmental and social influences on calling effort in the prairie mole cricket (Gryllotalpa major). Behav. Ecol. 9, 101–108 10.1093/beheco/9.1.101 (doi:10.1093/beheco/9.1.101) [DOI] [Google Scholar]
- 8.Jia F.-Y., Greenfield M. D., Collins R. D. 2001. Ultrasonic signal competition between male wax moths. J. Insect Behav. 14, 19–33 10.1023/A:1007893411662 (doi:10.1023/A:1007893411662) [DOI] [Google Scholar]
- 9.Byrne P. G. 2008. Strategic male calling behavior in an Australian Terrestrial Toadlet (Pseudophryne bibronii). Copeia 1, 57–63 10.1643/CE-05-294 (doi:10.1643/CE-05-294) [DOI] [Google Scholar]
- 10.Hobel G. 2011. Variation in signal timing behavior: implications for male attractiveness and sexual selection. Behav. Ecol. Sociobiol. 6, 1283–1294 10.1007/s00265-011-1142-1 (doi:10.1007/s00265-011-1142-1) [DOI] [Google Scholar]
- 11.Farr J. A. 1976. Social facilitation of male sexual behavior, intrasexual competition, and sexual selection in the guppy, Poecilia reticulata (Pisces: Poeciliidae). Evolution 30, 707–717 10.2307/2407811 (doi:10.2307/2407811) [DOI] [PubMed] [Google Scholar]
- 12.Jirotkul M. 1999. Population density influences male–male competition in guppies. Anim. Behav. 58, 1169–1175 10.1006/anbe.1999.1248 (doi:10.1006/anbe.1999.1248) [DOI] [PubMed] [Google Scholar]
- 13.Weir L. K., Grant J. W. A., Hutchings J. A. 2011. The influence of operational sex ratio on the intensity of competition for mates. Am. Nat. 177, 167–176 10.1086/657918 (doi:10.1086/657918) [DOI] [PubMed] [Google Scholar]
- 14.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 15.Crane J. 1975. Fiddler crabs of the world. Princeton, NJ: Princeton University Press [Google Scholar]
- 16.Backwell P. R. Y., Jennions M. D., Christy J. H., Passmore N. I. 1999. Female choice in the synchronously waving fiddler crab Uca annulipes. Ethology 105, 415–421 10.1046/j.1439-0310.1999.00387.x (doi:10.1046/j.1439-0310.1999.00387.x) [DOI] [Google Scholar]
- 17.Backwell P. R. Y., Passmore N. I. 1996. Time constraints and multiple choice criteria in the sampling behaviour and mate choice of the fiddler crab, Uca annulipes. Behav. Ecol. Sociobiol. 38, 407–416 10.1007/s002650050258 (doi:10.1007/s002650050258) [DOI] [Google Scholar]
- 18.Reaney L. 2009. Female preference for male phenotypic traits in a fiddler crab: do females use absolute or comparative evaluation? Anim. Behav. 77, 139–143 10.1016/j.anbehav.2008.09.019 (doi:10.1016/j.anbehav.2008.09.019) [DOI] [Google Scholar]
- 19.Jennions M. D., Backwell P. R. Y. 1998. Variation in courtship rate in the fiddler crab Uca annulipes: is it related to male attractiveness? Behav. Ecol. 9, 605–611 10.1093/beheco/9.6.605 (doi:10.1093/beheco/9.6.605) [DOI] [Google Scholar]
- 20.Ribeiro P. D., Iribarne O. O., Jaureguy L., Navarro D., Bogazzi E. 2003. Variable sex-specific mortality due to shorebird predation on a fiddler crab. Can. J. Zool. 81, 1209–1221 10.1139/z03-102 (doi:10.1139/z03-102) [DOI] [Google Scholar]
- 21.Burford F. R. L., McGregor P. K., Oliveira R. F. 1998. Chorusing by male European fiddler crabs, Uca tangeri: a study of visual communication networks. Acta Ethol. 1, 33–41 [Google Scholar]
- 22.Milner R. N. C., Booksmythe I., Jennions M. D., Backwell P. R. Y. 2010. The battle of the sexes? Territory acquisition and defence in male and female fiddler crabs. Anim. Behav. 79, 735–738 10.1016/j.anbehav.2009.12.030 (doi:10.1016/j.anbehav.2009.12.030) [DOI] [Google Scholar]
- 23.Backwell P. R. Y., Jennions M. D., Passmore N. I., Christy J. H. 1998. Synchronized courtship in fiddler crabs. Nature 391, 31–32 10.1038/34076 (doi:10.1038/34076) [DOI] [Google Scholar]
- 24.Murai M., Backwell P. R. Y. 2005. More signalling for earlier mating: conspicuous male claw waving in the fiddler crab Uca perplexa. Anim. Behav. 70, 1093–1097 10.1016/j.anbehav.2005.02.019 (doi:10.1016/j.anbehav.2005.02.019) [DOI] [Google Scholar]
- 25.Reading K., Backwell P. R. Y. 2007. Can beggars be choosers? Male mate choice in a fiddler crab. Anim. Behav. 74, 867–872 10.1016/j.anbehav.2006.09.025 (doi:10.1016/j.anbehav.2006.09.025) [DOI] [Google Scholar]