Abstract
The crustacean swimmeret system includes a distributed set of local circuits that individually control movements of one jointed limb. These modular local circuits occur in pairs in each segmental ganglion, and normally operate synchronously to produce smoothly coordinated cycles of limb movements on different body segments. The system presents exceptional opportunities for computational and experimental investigation of neural mechanisms of coordination because: a. The system will express in vitro the periodic motor pattern that normally drives cycles of swimmeret movements during forward swimming. b. The intersegmental neurons which encode information that is necessary and sufficient for normal coordination have been identified, and their activity can be recorded. c. The local commissural neurons that integrate this coordinating information and tune the phase of each swimmeret are known. d. The complete set of synaptic connections between coordinating neurons and these commissural neurons have been described. e. The synaptic connections onto each local pattern-generating circuit through which coordinating information tunes the circuit's phase have been discovered. These factors make possible for the first time a detailed, comprehensive cellular and synaptic explanation of how this neural circuit produces an effective, behaviorally-significant output.
This paper is the first comprehensive review of the system's neuroanatomy and neurophysiology, its local and intersegmental circuitry, its transmitter pharmacology, its neuromodulatory control mechanisms, and its interactions with other motor systems. Each of these topics is covered in detail in an attempt to provide a complete review of the literature as a foundation for new research. The series of hypotheses that have been proposed to account for the system's properties are reviewed critically in the context of experimental tests of their validity.
Keywords: locomotion, pattern generation, coordination, theoretical models
1. Introduction
Two fundamental goals of Neuroscience are to explain, in terms of the organization of their cellular components, how nervous systems work and how they generate overt behavior. Our progress toward these goals has often come from thorough study of specific nervous systems selected because under experimental conditions they continue to express behaviorally-related activity or because their orderly cellular organization and favorable anatomy permit critical and repeatable experiments. Cellular explanations of properties of particular systems have led to insights and general principles that apply widely across phyla, even if the links between these systems and other overt behaviors sometimes remain unclear. Locomotion is one behavior for which we have outlines of cellular explanations of the nervous system's performance. The subject of this review – the crustacean swimmeret system – is important to our understanding of locomotion because coordinated swimmeret movements are driven by a motor pattern generated by distributed local circuits within the central nervous system (CNS). It is also important in the history of neuroscience because the discovery of this centrally-generated motor pattern by Hughes and Wiersma (1960) was the first modern demonstration of fictive locomotion, and led to a fundamental change in thinking about what nervous systems do (Clarac, 2008; Mulloney and Smarandache, 2010).
The motive of this paper is to assemble a comprehensive review of published and previously-unpublished work on all aspects of the swimmeret system. To orient the reader, we begin with a succinct summary of the swimmeret system's features, and continue with more detailed descriptions of what has been learned about its neural organization, coordination, sensory modulation, and integration of swimmeret movements into the animal's overt behavior.
Summary of the swimmeret system's properties
Crustaceans are arthropods, like insects, and have paired jointed limbs on each body segment. In different segments of the body, these limbs are anatomically specialized for different functions (Fig. 1A, 1B). The limbs used for forward swimming are called swimmerets (or pleopods), and are located in pairs on each segment of the animal's abdomen. Whenever they swim forward, prawns, krill, small crayfish and lobsters, and their other large-tailed relatives use four or five pairs of swimmerets to generate forward thrust. During forward swimming, swimmerets move rhythmically through cycles of power-stroke and return-stroke movements that propel the animal through the water. Similar sequences of movements also occur when an animal excavates or ventilates its burrow, or aerates clusters of eggs that females sometimes carry attached to their swimmerets. A detailed description of coordinated swimmeret movements in the lobster Homarus americanus, made from high-speed motion pictures (Davis, 1968b), provides a quantitative behavioral framework for physiological and biomechanical analysis of the system.
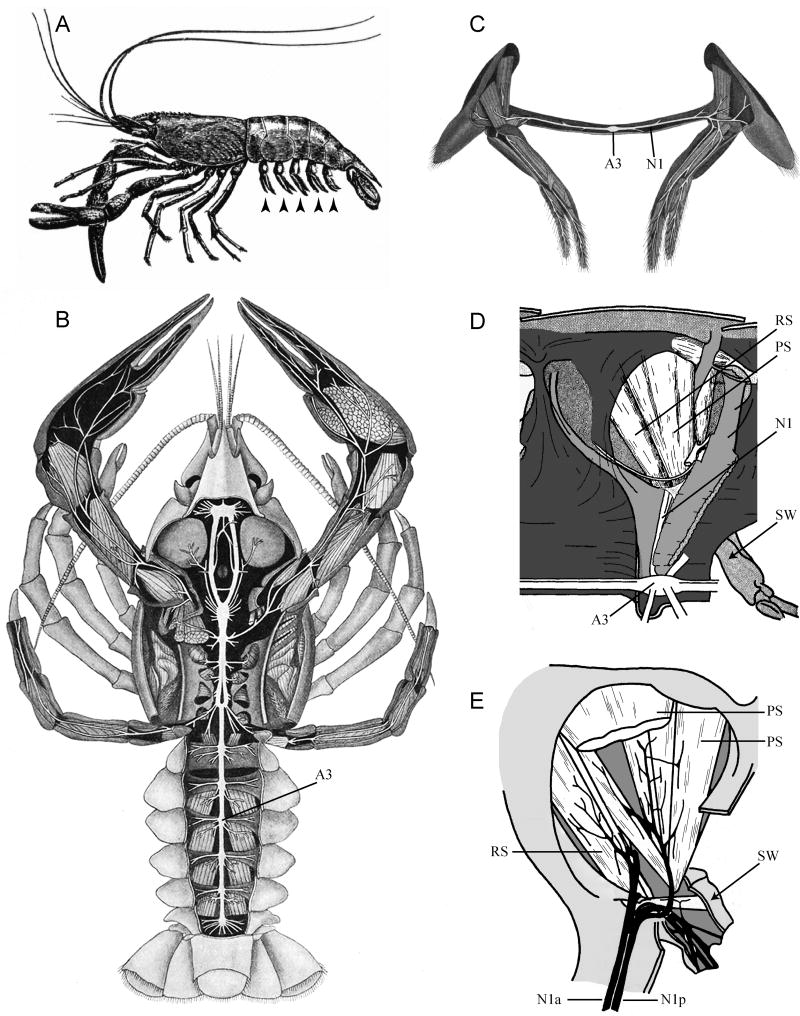
Fig. 1.
Anatomy of the crustacean CNS and swimmeret system. (A) Drawing of a prawn viewed from the left side that shows five pairs of swimmerets (arrowheads) on the ventral side of the abdomen (from Huxley, 1880). (B) Drawing of the crayfish nervous system, viewed from the dorsal side (modified from Keim, 1915). The third abdominal ganglion, A3, is labeled. (C) Drawing of a pair of swimmerets, viewed from the front, with their innervation and musculature exposed (modified from Keim, 1915). The nerve that innervates each swimmeret (N1) extends from the segmental ganglion (A3) to the swimmeret's muscles and sense organs. (D) A view from the midline of the right half of one segment of a dissected lobster abdomen (modified from Davis, (1968c). The main abdominal flexor and extensor musculature has been removed to expose some of the swimmeret muscles located in the lateral abdomen. A3: Abdominal ganglion 3. N1: Nerve projecting from A3 to the swimmeret (SW). PS: two power-stroke muscles. RS: a return-stroke muscle. Anterior is to the left; dorsal at the top. (E) A more detailed view of the innervation of a swimmeret, viewed from the same perspective as D. The anterior branch (N1a) and posterior branch (N1p) of the swimmeret nerve branch repeatedly to innervate individual PS and RS muscles and turn to enter the swimmeret itself (SW). In this drawing, modified from Davis (1968a), one large PS muscle has been cut away to reveal the nerves beneath it.
Quantitative properties
The period of a cycle is the interval from the start of the cycle to the start of the next cycle. The phase of an event in each cycle, e.g. a return-stroke movement, is the fraction of the period at which the event begins, and can range from 0.0 to 1.0. In adult crayfish, swimmerets can beat with periods ranging from less than 0.25 sec to more than 1.0 sec, and in smaller crayfish and larval lobsters, period can be less than 0.1 sec (Laverack et al., 1976). By our definition, each cycle of movements begins with a power-stroke that is followed by a return-stroke (Figs. 2B, 2C). When movements of different swimmerets are synchronized, each power-stroke by the most posterior pair of swimmerets begins a new cycle, and power-strokes of more anterior pairs follow their nearest posterior neighbor by about 0.25 of the cycle period (Fig. 2D). This coordinated alternation of power-strokes and return-strokes, and the posterior-to-anterior phase progression is maintained independent of changes in period (Laverack et al., 1976; Mulloney et al., 2006).
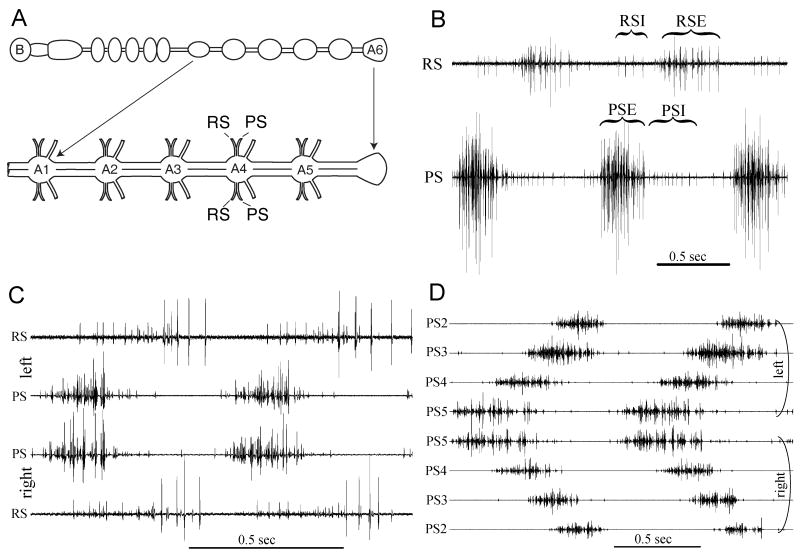
Fig. 2.
Features of the motor pattern that drives swimmeret movements. (A) Cartoon showing the positions of extracellular electrodes that recorded each trace in B and C. B: Brain. A1, A6: First and last abdominal ganglia. A2, A3, A4, and A5: abdominal ganglia that innervate unmodified swimmerets. (B) Simultaneous recordings from anterior (RS) and posterior (PS) branches of the nerve innervating one swimmeret that show two cycles of the normal swimmeret motor pattern. PSE, PSI: power-stroke excitor and power-stroke inhibitor units. RSE, RSI: return-stroke excitor and return-stroke inhibitor units. (C) Simultaneous bilateral recordings from power-stroke (PS) and return-stroke (RS) branches of the two swimmeret nerves that innervate the pair of swimmerets on the same abdominal segment. Left and right swimmerets are innervated by separate pools of motor neurons, but homologous neurons on both sides are active simultaneously. (D) Two cycles of spontaneous activity recorded simultaneously from left and right PS branches of swimmeret nerves from ganglia A2, A3, A4, and A5. These bursts of PS spikes in neighboring ganglia show the characteristic posterior-to-anterior progression of activity.
Motor innervation
Swimmerets are innervated through nerves that project from abdominal ganglia A1, A2, A3, A4, and A5 (Fig. 1B, 1C, 1D, 1E, 2A). Their movements are driven by alternating bursts of impulses in power-stroke (PS) and return-stroke (RS) motor neurons located in each of these ganglia. The complex motor pattern that drives their coordinated movements during swimming is produced in this chain of ganglia (Hughes and Wiersma, 1960; Ikeda and Wiersma, 1964). Experimental preparations of the isolated abdominal nerve cord of the crayfish CNS often express these motor patterns spontaneously (Fig. 2B, 2C, 2D), and the temporal structure of this fictive locomotion is quantitatively similar to active swimming in the intact animal. Two basic questions immediately arise: how does the CNS generate alternating PS and RS bursts in each ganglion (Fig. 2B, 2C), and how does it maintain this phase-constant coordination between ganglia (Fig. 2D) in the face of changing periods?
The swimmeret system is modular; each swimmeret is controlled by its own neural module, a micro-circuit located in each hemi-ganglion (Fig. 3A, 3B) separate from those controlling other swimmerets (Mulloney et al., 2003; Murchison et al., 1993). The function of each module is to produce the alternating bursts of spikes in its PS and RS motor neurons, which then activate muscles and cause the swimmeret to move. The synaptic kernels of these modules, the tangle of branches in which synaptic connections between motor neurons and pattern-generating neurons are made (Fig. 5), occur as bilateral pairs in the Lateral Neuropils (LN) of each ganglion that innervates swimmerets (Fig. 3A). When the swimmeret system is active, the two modules in each ganglion normally produce synchronous motor output (Fig. 2C), and the left and right swimmeret of that segment move in phase.
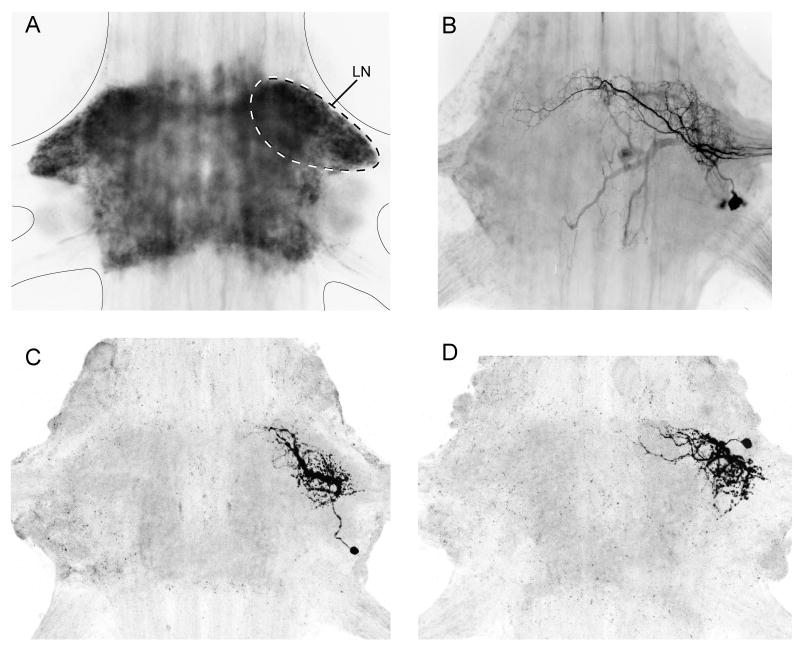
Fig. 3.
Neuroanatomy of a swimmeret module. (A) Whole mount of an A3 ganglion, seen from the dorsal side, that shows the distribution of anti-synapsin labeling (Klagges et al., 1996), a protein associated with synaptic vesicles and so a marker for chemical synapses. Synapsin is restricted to the neuropils in the core of the ganglion, and absent from the interganglionic connectives and the peripheral nerves. The edges of the ganglion are marked with a solid line. The Lateral Neuropil (LN) on the right side is outlined with a dotted line. (B) Cobalt backfills of a subset of the PS motor neurons that innervate one swimmeret (Mulloney and Hall, 2000). The dendritic processes of these neurons are largely restricted to one LN, and their axons exit the ganglion through the right N1 to reach targets in the swimmeret musculature. (C) Whole mount showing a dye-filled Int 1A, one component of the module's pattern-generating circuit (Paul and Mulloney, 1985a,b). The entire integrative structure of Int 1A is restricted to one LN. (D) Whole mount showing a dye-filled Int 2A, another component of the module's pattern-generating circuit (Paul and Mulloney, 1985a). Its entire integrative structure is restricted to one LN. In each ganglion A2 through A5, Int 1A and Int 2 neurons occur as mirror-image pairs.
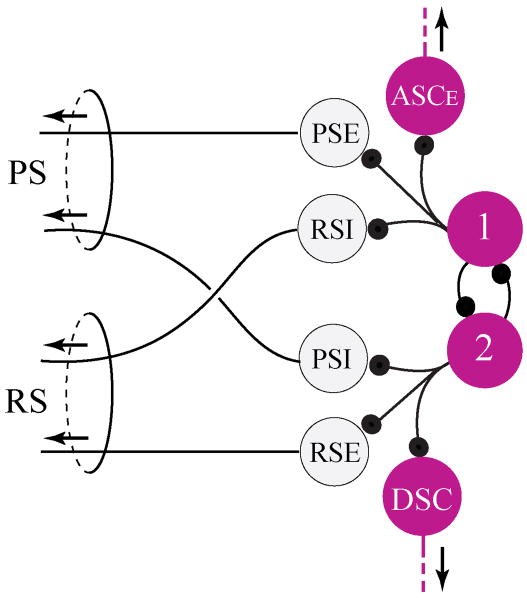
Fig. 5.
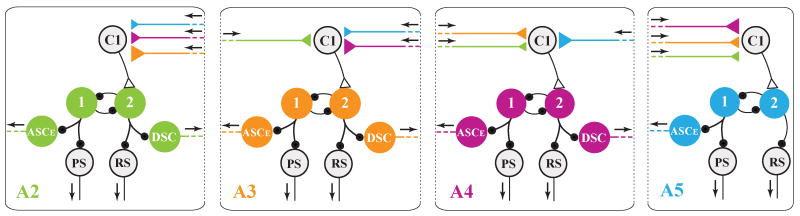
Model of the synaptic organization of one local pattern-generating circuit in each swimmeret module. The four types of motor neurons that innervate the swimmeret are driven by a pattern-generating kernel composed of reciprocally-inhibitory non-spiking local interneurons. Large circles symbolize individual neurons or functional groups of neurons. PSE: Power-stroke excitor. RSE: Return-stroke excitors. PSI: Power-stroke inhibitors. RSI: Return-stroke inhibitors. 1: Local neurons Int 1A and Int 1B. 2: Local neurons Int 2A. ASCE, DSC: Coordinating neurons that send axons anteriorly or posteriorly to other modules. Small solid black circles symbolize inhibitory synapses. Arrows mark the direction of impulse conduction.
This modular organization has important conceptual consequences. Because modules are distributed and can function even when isolated from one another (Mulloney et al., 1993a; Murchison et al., 1993), the task of describing and understanding a module's structure and dynamics can be separated from the task of describing and understanding the structure and dynamics of the circuit that coordinates modules.
2. Modular organization and local circuits in the CNS
The crayfish CNS consists of a brain (supraesophageal ganglion) anterior to the mouth, a subesophageal ganglion, five thoracic ganglia that innervate the large claws and four pairs of walking legs, and six abdominal ganglia that innervate the swimmerets and tail fan (Fig. 1B). In the nineteenth and early twentieth century, the golden age of comparative anatomy, crayfish received close attention from major figures in Biology. Huxley (1880) reviewed their neuroanatomy as a natural component of his discourse on all aspects of their biology. At a time when the cellular nature of nervous systems was controversial, Freud (1882) and Retzius (1890) described features of the cellular organization of the crayfish CNS. Keim (1915), in a beautifully-illustrated paper, wrote the best classical description of the gross neuroanatomy of crayfish. His colleague, Schmidt (1915) provided a detailed description of their musculature. Vogt (2002) provides a contemporary review of their functional anatomy.
The abdominal ganglia that innervate swimmerets are segmental components of the CNS. Each ganglion has about 630 neurons (Mulloney et al., 2003; Wiersma, 1957), and a well-organized core of tracts, commissures, and synaptic neuropils (Skinner, 1985a; 1985b). The positions and compositions of these anatomical structures are the same in each segmental ganglion (Mulloney et al., 2003). Movies of the structures of these ganglia are available at http://npb.ucdavis.edu/npbdirectory/mulloney_abdominal_ganglia/index.html and at http://www.science.smith.edu/departments/NeuroSci/courses/bio330/labs/LAanatomy.html . Features of particular importance for this review are the first pair of nerves, N1, that project from each ganglion (Fig. 1C, 1D), and the paired Lateral Neuropils, LN (Fig. 3A) located anterio-dorsal to the base of each N1 (Skinner, 1985b). Nerves N1 are the sole innervation of the segment's swimmerets, and each LN contains the neural circuit that controls movement of one swimmeret.
The intersegmental connectives that link these ganglia into a linear chain consist only of axons and a protective glial sheath. These connectives are bilaterally symmetrical (Fig. 6A), and contain about 2,500 pairs of axons (Sutherland and Nunnemacher, 1968). The early patterns of development of these ganglia follow the same pathways as does the development of the CNS of insects (Thomas et al., 1984), and the core of each ganglion is organized into the same patterns of tracts and commissures that occur in other arthropods (Skinner, 1985a,b; Mulloney et al., 2003).
Fig. 6.
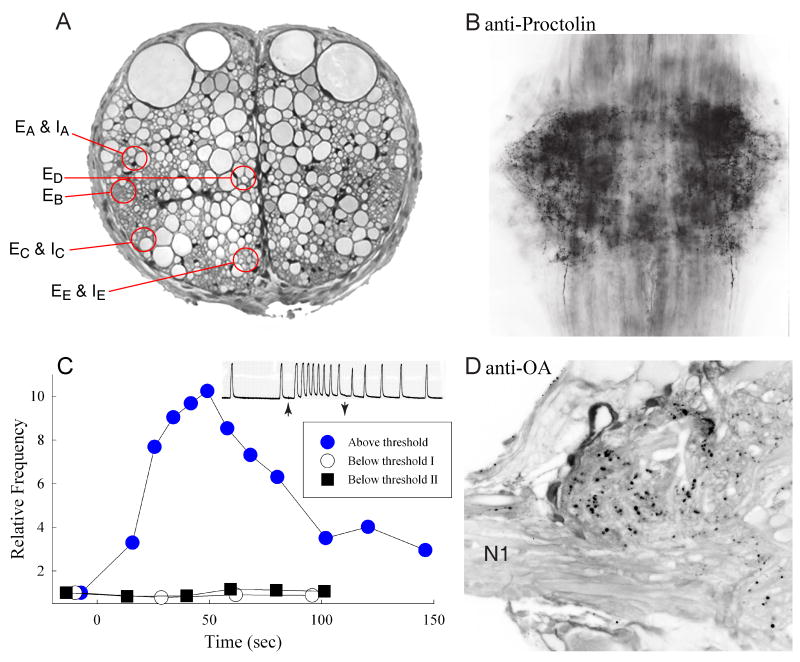
Swimmeret command neurons. (A) Cross-section of the intersegmental connective showing the locations of excitatory and inhibitory swimmeret command neurons (Acevedo, 1990; Acevedo et al., 1994; Wiersma and Ikeda, 1964). Dorsal is at the top. Colored circles show the five locations at which these axons occur. EA & IA: excitatory and inhibitory axons at Wiersma and Ikeda's position A. EB: excitatory axon at position B. EC & IC: excitatory and inhibitory axons at C. ED: excitatory axon at D. EE & IE: excitatory and inhibitory axons at E. (B) Whole mount of a ganglion A2, viewed from the dorsal side with anterior at the top, labeled with anti-proctolin antibody (Acevedo et al., 1994). Many processes and puncta in each LN are densely labeled. (C) Stimulation of certain excitatory command neurons both excites the swimmeret system and releases proctolin that can be recovered from the fluid bathing the nerve cord (Acevedo, 1990). The plot compares the proctolin recovered during a bout of above-threshold stimulation with that recovered during two bouts of below-threshold stimulation of the same command axon. Proctolin was detected using the frequency of spontaneous movements of a detached locust leg as a bioassay (O'Shea and Adams, 1981). The inset shows the increased frequency of movements caused by the above-threshold sample; arrows mark application and removal of the sample from the leg. (D) Cross-section through the left LN and the base of N1 of an abdominal ganglion labeled with anti-octopamine antibodies (Eckert et al., 1992; Schneider et al., 1993). The midline is to the right and dorsal is at the top. Within the LN, many puncta and fine processes are densely labeled.
2.1 Two modules in each ganglion
How is the swimmeret nervous system organized? Hughes and Wiersma (1960) suggested that “each swimmeret has a center of its own which is capable of controlling its rhythmic movements, …”. Ikeda and Wiersma (1964) severed the connectives between ganglia A4 and A5, and observed that periodic bursts of spikes persisted in recordings from swimmeret nerves on both A5 and A4, evidence of a local circuit in each ganglion.
Two later lines of evidence established the presence of two modules in each ganglion, one for each swimmeret. Within each ganglion A2 through A5, the major dendrites of swimmeret motor neurons and unilateral local interneurons are largely restricted to one LN (Mulloney and Hall, 2000). Each ganglion has two mirror-image LNs, so these restricted dendritic projections provide an anatomical locus for Hughes and Wiersma's “center” (Mulloney and Hall, 2000; Paul and Mulloney, 1985a). The observations that oscillations of membrane potentials in swimmeret neurons within one LN remained synchronized but oscillations in opposite LNs became unsynchronized when sodium spikes were blocked (Murchison et al., 1993) demonstrated that each LN held a local pattern-generating circuit that could in principle operate independently.
In summary, abdominal ganglia A2 through A5 include four pairs of neural modules, one for each swimmeret, and a coordinating circuit that synchronizes these otherwise independent modules (Murchison et al., 1993). Next we will consider the neuronal organization of a module.
2.1.1 Motor innervation of swimmerets
The neuromuscular basis of swimmeret movements have been well described in the lobster Homarus americanus, a crayfish relative (Davis, 1968c). Davis described and named 12 muscles that controlled each swimmeret and, from the positions of their origins and insertions in different parts of the abdomen and swimmeret, attributed to each a function. All these swimmeret muscles are striated (Atwood, 1976). Davis (1968c) also recorded junctional potentials (JPs) from some of these muscles while the lobster made sequences of coordinated movements. From the phase of each burst of JPs in the cycle of swimmeret movements, Davis found nine muscles that were active during power-stroke movements and three during return-stroke movements. He observed bursts of excitatory JPs that showed individual muscles received polyneuronal innervation from several excitatory axons. Another observation was hyperpolarizing inhibitory JPs in some muscles, which showed that these muscles also received inhibitory innervation (see 2.1.2). This complex innervation resembles the innervation of crustacean walking legs (Cattaert and Clarac, 1987a; Dudel and Kuffler, 1961; Van Harreveld and Wiersma, 1937). Some of these muscles are formed by more than one muscle bundle, but these bundles have a common innervation and so function as one muscle. Davis's description has been the basis of several studies that compared the differences between macruran (large-tailed) crustaceans like lobster and crayfish, anomuran crustaceans whose abdomens are modified to fit into snail shells (Bent and Chapple, 1977a; 1977b), and rock lobsters (Jasus) whose swimmerets deviate from the canonical crustacean biramous pattern (Cattaert et al., 1988).
The musculature of each swimmeret is innervated by its own population of about 70 motor neurons (Davis, 1971a; Mulloney and Hall, 2000). Almost all of these have similar shapes (Fig. 3B): a monopolar cell body located in one of two pairs of clusters on the ganglion's ventro-lateral margin, a process extending from the cell body into the LN, an arbor of branches within the LN, and an axon that projects from the LN into the N1 that innervates the swimmeret (Davis, 1970). As each N1 approaches the swimmeret, it divides into an anterior and posterior branch (Fig. 2A). As they reach the musculature, each of these branches divides further as particular axons find their specific target muscles (Fig. 1E) (Cattaert and Clarac, 1987b; Davis, 1969b; Keim, 1915). Since there are four pairs of swimmerets used for locomotion, each with its own set of motor neurons, coordinated swimmeret beating necessarily involves about 560 motor neurons that are active in a precisely organized pattern.
The set of motor neurons that innervates each swimmeret is anatomically separate from the set that innervates the other swimmeret in the same segment (Mulloney and Hall, 2000). The two exceptions to this segregation include the pair of GABAergic neurons with cell bodies on the ventral midline that together extend processes dorsally, and then enter the LN from its medial side (Mulloney et al., 2003; Mulloney and Hall, 1990; Mulloney and Hall, 2000). Both anatomical and immunocytochemical evidence suggests this pair is homologous with the thoracic ganglia's Common Inhibitory motor neurons that innervate muscles of each walking leg (Wiens and Wolf, 1993). There is also a pair of small neurons with axons in the anterior branch of each N1 whose cell bodies are located contralateral to the N1 (Mulloney and Hall, 2000).
Some swimmeret motor neurons also extend a process from the LN medially across the midline (Fig. 3B) through Dorsal Commissure 1 (DC1) to the opposite LN (Mulloney and Hall, 2000). In each ganglion, this subset with medial processes includes one pair of Return-Stroke Excitor neurons (RSE; see 2.1.2) that is dye-coupled through these processes (Paul and Mulloney, 1985b). These distinctive RSE neurons are also coupled by a rectifying synapse to one local pattern-generating neuron, Interneuron 1A (Int 1A), within each LN (Paul and Mulloney, 1985a); see 2.2.2.
The first abdominal ganglion, A1, innervates a pair of swimmerets that are not used for locomotion (Huxley, 1880) but are modified for sexual reproduction. In some species (e.g. Cherax destructor) and in females of additional species (e.g. Pacifastacus leniusculus), these swimmerets are altogether missing. Even in animals that lack swimmerets on the first abdominal segment, there are neurons with axons in the ganglion's N1 that look like swimmeret motor neurons. Drummond et al. (1998) used cobalt backfilling methods in Cherax destructor to compare the population of neurons in A1 with those in ganglia that innervate unmodified swimmerets and reported that the numbers of neurons in A1 were much reduced. Page (1985) used similar methods to compare the innervation of the sexually-dimorphic swimmerets of Homarus americanus.
2.1.2 Functional classes of swimmeret motor neurons
The motor neurons innervating each swimmeret can be partitioned into four functional groups: power-stroke exciters (PSE) and power-stroke inhibitors (PSI) that innervate PS muscles, and return-stroke exciters (RSE) and return-stroke inhibitors (RSI) that innervate RS muscles (Davis, 1968c; Davis, 1969b). In a gift from Nature to experimentalists, RSE and RSI axons segregate into the anterior branch of each N1 while PSE and PSI axons segregate into the posterior branch, so extracellular electrodes on each branch will record firing of the different functional groups (Fig. 2B). As excitation of the system increases, individual neurons within each group are recruited according to their size; small neurons have lower thresholds, are recruited earlier, and fire more spikes in each burst than do larger neurons (Davis, 1971a).
In each ganglion, the cell bodies of PSE neurons form a cluster posterior to the base of each N1, while the cell bodies of RSE neurons are clustered anterior to the base of N1 (Mulloney and Hall, 2000). Functionally-similar subsets of these neurons – e.g. small clusters of PSE or RSE neurons – are weakly electrically coupled through gap junctions that can be demonstrated by dye-coupling (Sherff and Mulloney, 1996). Our investigations of crayfish (Pacifastacus leniusculus, Procambarus clarkii) and Davis's description of lobster (Homarus americanus), have not progressed beyond these four groups of motor neurons, but Cattaert and Clarac (1987b) were able to identify rami-curler motor neurons in lobster Homarus gammarus whose firing did not fit neatly into these categories. In this review, we continue to classify motor neurons as PSE, RSE, PSI, or RSI neurons.
These functional distinctions were first based on correlations of bursts of spikes in these axons with swimmeret movements (Davis, 1969a; Ikeda and Wiersma, 1964), but immunohisto-chemistry for GABA, the probable transmitter in inhibitory motor neurons (Otsuka et al., 1967), revealed two labeled axons in the PS branch and three labeled axons in the RS branch of each N1 (Mulloney and Hall, 1990). Electron microscopy of motor innervation of swimmeret muscles in embryonic and juvenile lobsters has also found morphological evidence of excitatory and inhibitory synapses on the same muscle fibers (Kirk and Govind, 1992). The excitatory transmitter is quite likely glutamate (Otsuka et al., 1967; Sherff and Mulloney, 1996), but our attempts to demonstrate glutamatergic neurons using immunolabeling against glutamate have so far failed.
It is unfortunate that the cell bodies of swimmeret motor neurons are electrically-remote from their synaptic sites and spike-initiating zones, so intracellular recordings from cell bodies do not yield much information about their integrative properties or synaptic connections. Intracellular recordings from their processes within the LN are more difficult but more informative.
Despite the different functions these four groups of motor neurons perform, they do not differ much in their integrative properties. Their input-resistances (measured as voltage-responses to small currents injected through a sharp microelectrode in a process in the LN) varied systematically with the neuron's size (measured as the size of its spike recorded extracellularly in N1), but all had similar membrane time-constants: median 9.3 msec (Sherff and Mulloney, 1997). Chrachri (1995) described the voltage-gated membrane currents in acutely-dissociated cell bodies of motor neurons. The inward currents include a TTX-sensitive Na+ current and a nifedipine-sensitive Ca++ current. The outward currents include a transient, 4-AP-sensitive K+ current and a persistent TEA-sensitive K+ current (Chrachri, 1995). The relative densities of these currents in individual neurons have not been reported, but these currents are similar to the currents found in crayfish walking-leg axons (Connor, 1975; Connor et al., 1977), which are serial homologues of swimmeret axons.
2.2 A local pattern-generating circuit in each module
Each swimmeret has its own neural module with five types of components: 1) all the motor neurons and 2) primary sensory neurons that innervate the swimmeret, 3) a set of non-spiking local neurons, 4) coordinating neurons that project to neighboring ganglia, and 5) a local commissural neuron that receives coordinating information from other ganglia.
2.2.1 Motor neurons as pattern-generating elements in the module?
Which neurons form the local pattern-generating circuit? Stein (1977) and Heitler (1978) described swimmeret motor neurons whose membrane potentials oscillated in phase with swimmeret motor output. They also made the important observation of graded (subthreshold) synaptic interactions among motor neurons. Small currents injected into individual motor neurons affected not only that neuron's firing but also the firing of other synergistic and antagonistic motor neurons innervating the same swimmeret (Heitler, 1978; Heitler, 1981; Heitler, 1983). Pulses of current injected into individual motor neurons could reset the phase of the system's motor output (Heitler, 1978), an accepted criterion for a pattern-generating neuron (Friesen and Stent, 1978). To test the significance of direct connections between motor neurons for generating a module's PS-RS alternation, Sherff measured the distribution and strengths of monosynaptic and polysynaptic connections between excitatory and inhibitory motor neurons in the same module (see 2.1.2). She recorded the responses of identified motor neurons to puffs of glutamate or GABA, the two probable transmitters of excitatory and inhibitory motor neurons. Both transmitters inhibited all swimmeret motor neurons, as we would predict if peripheral excitors (PSE, RSE) used glutamate and peripheral inhibitors (PSI, RSI) used GABA (Sherff and Mulloney, 1996). Moreover, Neurobiotin injections into individual neurons revealed selective dye-coupling to small subsets of other motor neurons. However, microelectrode recordings from pairs of antagonist neurons within the same module, e.g. a PSE and a PSI neuron, showed that the strengths of their reciprocal inhibitory connections were comparatively weak, and that the synaptic delays of these connections were too long to attribute to monosynaptic connections (Sherff and Mulloney, 1996). From this evidence, we conclude that direct synaptic connections between motor neurons do not play a central role in generating PS-RS alternation (Fig. 2B).
2.2.2 Local interneurons form the pattern-generating circuit
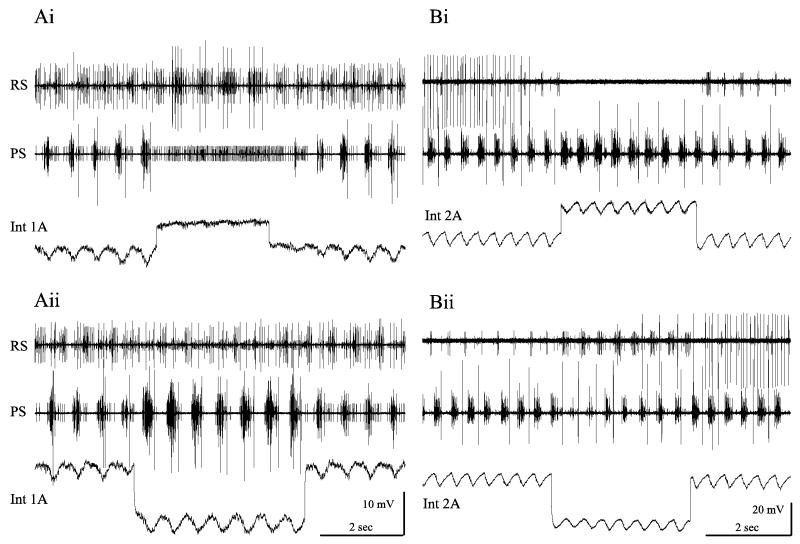
Local interneurons have structures limited to one segmental ganglion of the CNS. Unilateral local neurons are further restricted to one side of the ganglion, usually in mirror-image pairs (Fig. 3C, 3D). Heitler and Pearson (1980) described non-spiking, unilateral local interneurons in each LN whose membrane potentials oscillated in phase with the swimmeret system's output. Current pulses injected into these neurons reset the phase of the system. Longer current steps injected into them affected firing of entire classes of motor neurons (Fig. 4). For example, small depolarizations of one neuron might excite all PSE neurons but inhibit all RSE neurons in that module, while small hyperpolarizations of the same neuron had opposite effects on the same PSE and RSE motor neurons (Fig. 4). These non-spiking neurons proved to be the key elements of the local pattern-generating circuit.
Fig. 4.
Physiology of local pattern-generating neurons in each swimmeret module. (A) Simultaneous recordings of activity in PS and RS motor axons that innervate one swimmeret (see Fig. 2B) and intracellular recording from the Int 1A in the same module as those motor neurons. Int 1A is periodically depolarized in phase with bursts of spikes in RS motor neurons, and hyperpolarized in phase with bursts in PS motor neurons. (Ai) Depolarizing Int 1A inhibits the whole pool of PSE motor neurons but excites the pool of RSE neurons. The small unit in the PS recording that fires continually during the depolarization is a PS Inhibitor neuron. (Aii) Hyperpolarizing Int 1A has the opposite set of effects on these antagonist motor neurons. Current steps in Int 1A were ±0.5 nA. (B) Simultaneous recordings of activity in PS and RS motor axons that innervate one swimmeret and intracellular recording from an Int 2A in the same module. Int 2A is depolarized during each PS burst but hyperpolarized during each RS burst. (Bi) Depolarizing this Int 2A inhibits the whole pool of RS excitor motor neurons but excites the pool of PS excitors. (Bii) Hyperpolarizing this neuron has the opposite set of effects on these antagonist motor neurons. Current steps in Int 2 were ±1.5 nA.
Paul used dye-filled microelectrodes to explore the population of neurons within these modules (Paul and Mulloney, 1985a; 1985b). She identified two types of nonspiking neurons whose membrane potentials oscillated at different phases when the system was active and had opposite effects on the module's output. These neurons occurred in bilateral pairs, and were repeated in each ganglion that innervated swimmerets (Paul and Mulloney, 1985b). They can be identified physiologically using sharp microelectrodes inserted into their branches in the LN (Mulloney, 2003). Subsequent research has confirmed these original identifications and supported their roles as components of the local pattern-generating circuits.
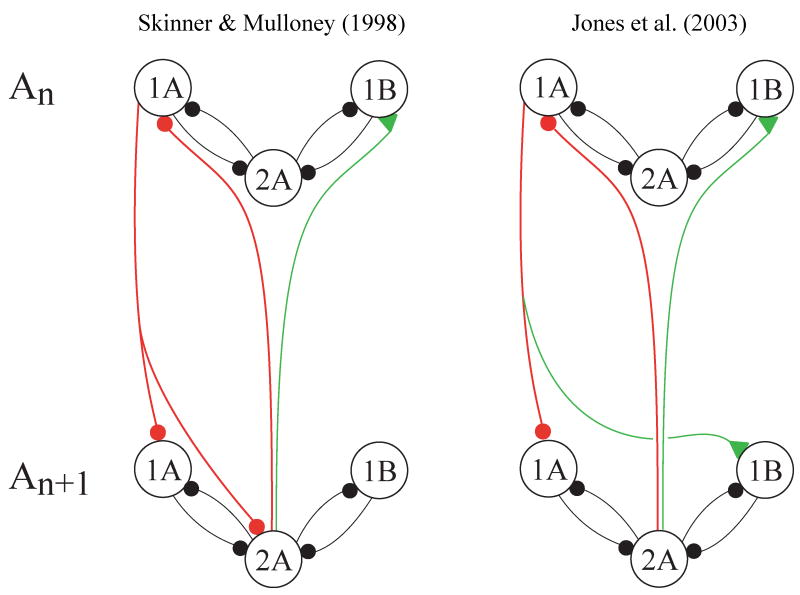
Four of these local pattern-generating neurons occur in each module (Mulloney, 2003; Skinner and Mulloney, 1998). Each kind has distinctive anatomy that can be used to confirm tentative physiological identifications. Two of them (Fig. 3D), a pair called Int 2A (Paul and Mulloney, 1985a), are depolarized during PS bursts but hyperpolarized during RS bursts. Int 2A neurons make an inhibitory monosynaptic connection onto RSE motor neurons (Smarandache-Wellmann, unpublished). Two other neurons, called Int 1A (Fig. 3C) and Int 1B (Paul and Mulloney, 1985a), are depolarized during RS bursts but hyperpolarized during PS bursts. Int 1A neurons make an inhibitory monosynaptic connection onto PSE motor neurons (Mulloney, 2003). Int 1A and Int 1B differ in their anatomy and in the patterns of PSPs they receive when the system is active (Paul and Mulloney, 1985a), but have the same effects on their module's motor output. The transmitters released by these non-spiking local neurons are unknown (they do not show GABA-like immunoreactivity), but both GABA and glutamate inhibit swimmeret motor neurons (Sherff and Mulloney, 1996) and other local non-spiking neurons (Nagayama, 2005). The evidence, still sparse, is that these pattern-generating neurons are not intrinsic oscillators, and that when the system is active, their membrane potentials oscillate in anti-phase because they are connected by reciprocal inhibitory synapses (Mulloney, 2003; Murchison et al., 1993; Skinner and Mulloney, 1998).
2.3 A model of the circuit that generates bursts in PS and RS motor neurons
Production of alternating bursts of spikes in PS and RS motor neurons is the essential function of each swimmeret module. Our current model of the module's organization proposes that the kernel of the pattern-generating circuit is formed by reciprocal graded inhibitory synapses between the Int 2A and the Int 1A-1B neurons (Fig. 5). When the system is excited, these synapses cause the membrane potentials of these non-spiking neurons to oscillate in antiphase (Skinner and Mulloney, 1998). These oscillations in potential modulate graded release of transmitter from these pattern-generating neurons onto specific subsets of the module's motor neurons. Whenever the system is active, the inhibitory currents caused by these transmitters shape the bursts of spikes in these motor neurons, and so orchestrate alternating bursts of spikes in PS and RS motor neurons (Fig. 2B).
In addition to these local pattern-generating neurons, four other kinds of unilateral non-spiking local interneurons also occur in each module (Paul and Mulloney, 1985a). Unlike the Int 1A, Int 1B, and Int 2A neurons, these others do not meet the criteria for pattern-generating neurons. They modulate firing of groups of motor neurons without affecting the timing of their firing. These other local interneurons have not been well-studied.
3. Command neurons and neuromodulation
In their original description of centrally-generated swimmeret motor output, Hughes and Wiersma (1960) also observed that stimulating bundles of axons dissected from the interganglionic connectives affected expression of these motor patterns. Some bundles elicited coordinated output from quiet preparations while others silenced preparations that were spontaneously active. Wiersma and Ikeda (1964) pursued this lead by mapping the positions in the connectives of five pairs of excitatory axons and three pairs of inhibitory axons. These axons occurred at specific, widely separated positions in the ventral nerve cord, and could consistently be found at these same positions in different animals (Fig. 6A). Above a frequency-threshold, tonic stimulation of one of the excitatory axons reliably elicited expression of coordinated swimmeret activity. The periods of bursts of spikes in motor axons decreased and the strengths of these bursts increased with stimulus frequency. Tonic stimulation of one of the three inhibitory axons promptly halted spontaneous motor output. Thus, the CNS appeared to be equipped with units that could control expression of this complex, behaviorally-relevant motor pattern. Wiersma and Ikeda introduced the term command neuron to describe the functions of these axons, and discussed explicitly that these axons were not parts of the intersegmental coordinating circuit. This idea, that firing of specific units within the CNS could elicit a complex behavior, stimulated many investigators to look for comparable units associated with other behaviors in other animals (Kupfermann and Weiss, 1978; Wiersma, 1978).
3.1 Excitatory swimmeret command neurons
These first descriptions of locations and properties of the five excitatory command neurons have been confirmed repeatedly (Atwood and Wiersma, 1967). Stein (1971) used Wiersma and Ikeda's map to stimulate command axons during his experiments on the properties of coordinating fibers (see 4.3), and in the course of his experiments confirmed that the frequency at which each axon was stimulated affected the period of the resulting motor activity. West et al. (1979) also used command axons to activate swimmeret beating in semi-intact preparations in order to study influences of local proprioceptive feedback on intersegmental coordination. Acevedo et al. (1994) located each of these five axons sequentially in the same preparation, and demonstrated that stimulating any one of them elicited similar motor output. Although Wiersma and Ikeda first mapped these axons, they named them simply A, B, C, D, and E, and did not name the inhibitory command axons they mapped near axons A, C, and E. Acevedo et al (1994) introduced the names EA, EB, EC, ED, and EE for the five excitatory command neurons, and IA, IC, and IE for their inhibitory neighbors (Fig. 6A).
Davis and Kennedy (1972a; 1972b; 1972c) systematically investigated the effects on the swimmeret system of stimulating axons stripped from the connectives between ganglia A1 and A2. In their experiments, the CNS was severed between thoracic ganglion T5 and abdominal ganglion A1, and they carefully excluded effects of stimulating units that might have been primary sensory afferents, but their “methods permitted neither the confident identification of the same command interneuron from one animal to the next, nor any evaluation of whether the number of command fibers was the same in all preparations.” (Davis and Kennedy, 1972a). They did demonstrate clearly the increases in burst strengths and decreases in burst periods that followed increasing stimulus frequency, and they showed that simultaneous stimulation of two excitatory units caused greater excitation of the system than did stimulation of either axon alone. They interpreted these two-unit results as evidence of range fractionation among different command units. It is a great pity that the units that were studied in these experiments were not better mapped into Wiersma and Ikeda's description because it is very difficult, except in the most general way, to integrate Davis and Kennedy's results into the rest of the literature.
The full anatomy of these excitatory command neurons is still unknown. The more recent description of coordinating axons (Namba and Mulloney, 1999; Tschuluun et al., 2001) confirm that they are not the same units as the five pairs of command axons, but we do not know of any description of the locations of the cell bodies or input regions of these swimmeret command neurons.
3.2 Inhibitory command neurons
Wiersma and Ikeda (1964) also described the locations of three pairs of axons that inhibited expression of both spontaneous swimmeret activity and activity elicited by stimulating excitatory command axons. Given that there are about 2,500 pairs of axons in the intersegmental connectives (Somers and Nunnemacher, 1970), it is remarkable that each inhibitory axon runs close by one of three excitatory command axons. In her thesis research, Acevedo (1990) demonstrated these three inhibitory axons in the same preparation, and showed that their inhibitory effectiveness increased with stimulus frequency. She also introduced a useful nomenclature for these three inhibitory command neurons (see 3.1). Davis and Kennedy, in some of their two-unit experiments (1972b), stimulated an inhibitory unit simultaneously with an excitatory unit. Some of their inhibitory units reduced the strengths of bursts in motor axons without affecting burst periods, which suggests that those units inhibited the motor neurons directly but did not affect the local pattern-generating circuit. In other experiments, stimulating the inhibitory unit increased period and also decreased burst strength. Again, it is not possible to associate these differences with the inhibitory axons mapped by Wiersma and Ikeda and by Acevedo.
3.3 Neuromodulation
The experimental difficulties posed by using command neurons to elicit expression of the swimmeret motor pattern put a premium on alternative ways to control the state of the swimmeret system. Bath application of serotonin, L-DOPA, or amino acids were known to elicit coordinated motor output from leech and lamprey CNS (Cohen and Wallén, 1980; Poon, 1980; Willard, 1981), so Mulloney et al. (1987) surveyed many transmitters and neuropeptides to find similar pharmacological tools. In most of those experiments, drugs dissolved in normal saline were superfused over isolated abdominal nerve cords whose ganglia were not desheathed. They did not observe any responses from either active or quiet preparations to superfusion with dopamine, L-DOPA, DL-aspartate, glutamate, NMDA, GABA, histamine, acetylcholine (ACh), or serotonin. Some of these, notably glutamate, DA, GABA, and ACh were false (see 3.3.1) negatives attributable to experimental design, particularly to failure to open the ganglionic sheath. Two compounds – the pentapeptide proctolin and octopamine – had dramatic effects on the system, and became the pharmacological tools for many additional experiments.
3.3.1Low molecular-weight neurotransmitters
Acetylcholine
ACh is the transmitter used by many primary sensory afferent (Barker et al., 1972a; Florey, 1973; Hildebrand et al., 1971) and some motor neurons (Marder, 1974; 1976) in the crustacean CNS. In their initial screen for drugs that affected the swimmeret system, Mulloney et al. (1987) found that although ACh had no effect, pilocarpine -- a muscarinic analog of ACh -- sometimes elicited intermittent swimmeret activity that resembled normal intermittent activity. Chrachri and Neil (1993) reported that oxotremorine, another muscarinic ACh analog, excited spontaneously-active swimmeret preparations. Braun and Mulloney (1993) then found that pilocarpine elicited stable expression of swimmeret activity from silent preparations whose ganglia had been desheathed. Atropine, a competitive antagonist of ACh at some muscarinic receptors, had dose-dependent inhibitory effects on the swimmeret rhythm. As atropine concentrations increased, the period of the motor pattern increased and burst strength decreased, saturating at 20 μM atropine (Herrera and Mulloney, unpublished). Eserine, an inhibitor of acetylcholine esterase, has the opposite effect; bathing active preparations in eserine saline decreased period (Braun and Mulloney, 1993).
Braun and Mulloney (1993) also found that although nicotine, another ACh analog, could not elicit expression from silent preparations, it did increase the frequency of PS bursts in active preparations in a dose-dependent manner. Finally, they found that carbachol, an ACh analog with both nicotinic and muscarinic activity, could both elicit expression from silent preparations and increase excitation. In response to increasing concentrations of bath-applied carbachol (ED50 6.7 μM), periods change from more than 1.0 sec to less than 0.25 sec (Mulloney, 1997). Because of its low cost and stability in solution, carbachol has become the standard reagent for exciting swimmeret preparations. Chrachri and Neil (1993), Braun and Mulloney (1993) and Mulloney (1997) include dose-response curves for oxotremorine, pilocarpine, nicotine, and carbachol.
What are these cholinergic drugs doing? Tschuluun et al. (2009) voltage-clamped swimmeret motor neurons to measure changes in membrane currents in response to carbachol application. They found that as carbachol was introduced to the bathing solution and the system became active, two inward currents appeared in motor neurons. One current was a direct action of carbachol on the motor neuron. The second was due to synaptic input from another source; this second current was blocked by low-Ca++ high-Mg++ saline. They concluded that carbachol, and by extension ACh, acts directly both on swimmeret motor neurons and on unidentified components of the local pattern-generating circuit that are presynaptic to the motor neurons. The source of the inward currents that excite the pattern-generating neurons and motor neurons is unknown.
It is possible that muscarinic modulation of some neurons in the swimmeret system works by a different mechanism. Cattaert et al. (1994) studied responses of walking-leg motor neurons to cholinergic drugs. They observed evidence of short-term increases in an inward current in response to both nicotinic and muscarinic reagents, like those reported by Tschuluun et al. (2009). They also observed a long-lasting decrease in a small voltage-dependent outward K+ current in response to muscarinic modulation. They discussed modulation of this voltage-dependent current as a factor in cholinergic excitation of the walking system (Chrachri and Clarac, 1990). Muscarinic modulation of a similar outward current in swimmeret neurons, particularly the non-spiking local neurons, is a real possibility that has not been investigated.
What components of the swimmeret system use ACh as a transmitter? The cholinergic neurons in the system have not been identified. As a first step in identifying cholinergic neurons in abdominal ganglia, Braun and Mulloney (1994) mapped the distributions of neurons with high levels of acetycholinesterase (AChE), the degradative enzyme associated with cholinergic synapses. They found high levels of AChE in the LNs, in some neurons in the clusters of cell bodies that include both PS and RS motor neurons, and in neurons whose axons projected into the interganglionic connectives. None of these were identified in that paper as swimmeret motor neurons or other previously-described swimmeret neurons. The expression of AChE is not a trustworthy marker for cholinergic neurons, but repeated attempts to map the distribution of neurons containing choline acetyltransferase, the synthetic enzyme for ACh, using polyclonal antibodies have failed (Mulloney, unpublished).
Dopamine (DA)
DA occurs as a neurotransmitter in the crustacean nervous system (Barker et al., 1979; Tierney et al., 2003), and is known to modulate both intrinsic excitability of neurons and transmission at chemical synapses, (e.g. Ayali et al., 1998; Kloppenburg et al., 1999). In the lobster Homarus gammarus, Barthe et al (1989) described DA-like immunoreactivity in a pair of neurons in A5, apparently the only ganglion they studied, and also found that bath-application of 10-6– 10-5 M DA reliably elicited expression of swimmeret activity from desheathed preparations. Cournil et al. (1994; 1995) mapped the distributions of neurons with DA-immunoreactivity and tyrosine hydroxylase (TH), the synthetic enzyme for DA and related catecholamines. Their maps compared DA-immunoreactivity in sectioned ganglia with TH-immunoreactivity in whole mounts. Cournil et al. extended Barthe et al.'s description to a pair of DA/TH labeled neurons in each abdominal ganglion, and two larger unpaired immunoreactive neurons in A3 and A4 that extended axons posteriorly through A6 to innervate the hindgut. Because of problems with specificity of antibodies, the immunocytochemistry of DA and other catecholamines is difficult. Several authors have described differing numbers of neurons with DA-like immunoreactivity in swimmeret ganglia (reviewed critically by Tierney et al. (2003). Here, the important point is that Barthe et al. have demonstrated (contra Mulloney et al., 1987) that DA does elicit expression of swimmeret activity in lobster CNS. The dopaminergic neurons that do so in the intact animal remain to be described.
Octopamine (OA)
OA occurs as a neurotransmitter in the crustacean nervous system (Barker et al., 1972b; Kravitz et al., 1976). Bath-applied OA effectively inhibits expression of swimmeret motor activity (Mulloney et al., 1987), and does so quickly in a dose-dependent manner. The ED50 is about 50 μM OA. Two metabolites of OA, synephrine and norepinephrine, also inhibited the system but are not known to occur in crustacean CNS. An OA antagonist, phentolamine, partially blocked inhibition by OA. This pharmacology is significant because Mulloney et al. (1987) then stimulated inhibitory command neurons (see 3.2) and showed that this stimulation effectively inhibited the system, and that phentolamine also partially blocked this stimulus-dependent inhibition. This pharmacological block of inhibition by a command neuron implies that inhibitory command neurons might use OA as their transmitter.
The distribution of octopaminergic neurons in the CNS of lobster Homarus americanus has been mapped using polyclonal antibodies against OA (Schneider et al., 1993). In the CNS of crayfish (Pacifastacus leniusculus), the patterns of anti-OA labeling are similar to those in lobster (Mulloney unpublished). From the perspective of control of the swimmeret systems, two features stand out. First, two pairs of neurons originate in the subesophageal ganglion and extend prominent axons posteriorly through all segmental ganglia to end finally in A6. In crayfish, these axons project through each ganglion in the Dorsal Intermediate Tracts, DIT (Skinner, 1985a), and extend thin processes laterally into the LNs. These two pairs of axons might be inhibitory command axons. Second, within each abdominal ganglion A1 through A5 (in H. americanus, only in A4 and A5) there is a bilateral pair of tiny neurons that label brightly with anti-OA antibodies, and project axons anteriorly, perhaps in the Ventral Lateral Tracts (Mulloney et al., 1993b). Numerous varicosities are labeled within each LN, but whether these varicosities come only from the axons in DIT or also from the pairs of immunoreactive neurons in each ganglion is unknown. There are also labeled processes in the Horseshoe Neuropil, the Anterior Ventral Commissure, and in Dorsal Commissure 6 (Skinner, 1985a). Nonetheless, the positions and structures of labeled descending axons and the pharmacological block of command inhibition are consistent with the hypothesis that some inhibitory command neurons, probably neuron IA in Acevedo's terminology (Fig. 6), use OA as a transmitter.
3.3.2 Neuropeptides
Many neuropeptides serve both as transmitters and as modulators of neural circuits in crustacean nervous systems (Nusbaum et al., 2001). Two neuropeptides are known to elicit expression of the motor pattern from quiescent preparations, and immunolabeling shows that several more are present in neural processes within the LNs of each swimmeret ganglion.
Proctolin
Proctolin is a pentapeptide that occurs in crustacean and insect nervous systems (Kravitz et al., 1980; O'Shea and Adams, 1981; Schwarz et al., 1984), and elicits expression of the swimmeret motor pattern (Barthe et al., 1993; Mulloney et al., 1987). Antibody labeling shows proctolin is present in axons that project posteriorly from the subesophageal ganglion through all thoracic and abdominal ganglia to end finally in A6 (Fig. 6) (Acevedo et al., 1994; Siwicki and Bishop, 1986). These labeled axons occur in the same regions of the connectives that contain four excitatory command neurons: EA, EB, EC, and EE; they are absent from the region near ED. They traverse each ganglion in the Lateral Dorsal Tract, Dorsal Intermediate Tract, and Ventral Lateral Tract (Skinner, 1985a), and project branches into each LN as they pass by (Acevedo et al., 1994). The LNs of each abdominal ganglion have many thin processes and varicosities that label densely with anti-proctolin antibody (Fig. 6B).
Proctolin elicits active expression of the swimmeret motor pattern from silent preparations (Acevedo et al., 1994; Mulloney et al., 1987). When it is perfused through the ventral artery of an isolated nerve cord preparation, and so reaches the cores of all abdominal ganglia quickly, the ED50 is 1.6 μM. The motor output proctolin elicits is quantitatively similar to that expressed spontaneously or when excitatory command axons are stimulated, except that PS burst durations are significantly longer if higher concentrations of proctolin are used (Acevedo et al., 1994). The periods of proctolin-induced activity were limited to the middle of the normal behavioral range (0.596 sec ± 0.147), and did not change much as proctolin concentrations varied from 5 to 50 μM (Braun and Mulloney, 1993). Modules in each ganglion responded to local application of proctolin. When just one ganglion was exposed to proctolin, excitation always spread to the other ganglia in the system (Acevedo et al., 1994).
To test the idea that particular excitatory command neurons release proctolin as a transmitter that then excites the swimmeret system, Acevedo (1990) isolated small bundles of axons from the T5-A1 connective, showed by stimulation that a given bundle contained an excitatory command neuron, and demonstrated by immunolabeling that the same bundle contained an axon with proctolin-like immunoreactivity. She also demonstrated that proctolin appeared in the extracellular fluid when certain bundles that contained an excitatory command axon were stimulated just above threshold for excitation of the swimmeret system but was absent from that fluid when the bundle was stimulated just below this threshold (Fig. 6C). In these experiments, proctolin in the extracellular fluid was measured with a locust-leg bioassay sensitive to femtomolar quantities of the peptide (O'Shea and Adams, 1981). The above-threshold response was completely eliminated by pre-absorption of the above-threshold sample with a proctolin-specific antibody (Acevedo, 1990). Acevedo tested each of the five excitatory command axons (3.1) in several experiments. She concluded that EA, EC, and EE released proctolin, and that ED did not. There is a strongly-labeled axon near axon EB, but Acevedo did not usually detect stimulus-dependent release from EB, so it is unlikely that EB is proctolinergic. Thus, the bulk of evidence suggests that proctolin occurs as a neurotransmitter in three of five excitatory command neurons, and that firing of these neurons is one pathway through which the CNS can control expression of the swimmeret motor system.
Crustacean cardioactive peptide (CCAP)
CCAP is a cyclic nonapeptide that occurs in neurons in the crustacean CNS (Trube et al., 1994). In each swimmeret ganglion, three pairs of neurons in the cluster of cell bodies anterior to N1 label strongly with antibodies to CCAP. Each LN has processes with many intensely-labeled varicosities. No neurons known to be part of the swimmeret system are labeled by CCAP antibodies, but CCAP elicits expression of the swimmeret motor pattern from silent preparations and modulates the output of spontaneously active preparations (Mulloney et al., 1997). These effects are dose-dependent; the ED50 is 0.25 μM, and the responses saturate at about 3 μM CCAP. Like proctolin, CCAP concentration has little effect on the periods of the activity expressed.
Like carbachol, CCAP acts directly on swimmeret motor neurons and also on unknown components of the module's local pattern-generating circuit. However, CCAP's effects differ for different types of motor neurons (see 2.1.2). Bath-applied CCAP biases PS-RS alternation toward PSE bursts. PSE and RSI motor neurons are excited and depolarized when exposed to CCAP, while RSE and PSI neurons are hyperpolarized and partially inhibited (Mulloney et al., 1997). At concentrations above its ED50, the durations of PSE bursts increase significantly above normal spontaneous values, and durations of RSE bursts decrease. In some experiments, the orderly alternation of PSE and RSE bursts suddenly stopped, PSE neurons fired almost continuously and strong RSI bursts continued, but RSE and PSI neurons fell silent. These “burst deletions” have also been observed in other motor systems; remarkably similar episodes of burst deletions can occur in turtles during expression of complex periodic motor patterns that drive directed scratching movements of hind limbs (Stein, 2008). Intracellular recordings from PSE neurons show clearly the absence of the periodic inhibition that normally occurs in each cycle (Mulloney, 2003). When CCAP is again washed out, this inhibition returns at the same time as normal alternation of PSE and RSE activity. We interpret this RS-suppressed state as evidence that the normally alternating depolarizations of antagonist pattern-generating neurons caused by their reciprocal inhibition has failed because the circuit has locked with one side (Int 1) high, the other side (Int 2) low (Fig. 5). This differential action then implies that CCAP acts differently on the different types of local pattern-generating neurons.
Red Pigment Concentrating Hormone (RPCH)
RPCH is a peptide that occurs both as a circulating hormone and as a transmitter released by certain neurons in the crayfish and lobster CNS (Dickinson and Marder, 1989). Polyclonal anti-RPCH labels three small clusters of neurons in each abdominal ganglion, two axons in the Lateral Dorsal Tract, one in the Dorsal Intermediate Tract, and one in the Ventral Lateral Tract, VLT (Sherff and Mulloney, 1991). This VLT axon extends a branch into the LN, which contains many labeled varicosities. RPCH cannot activate silent preparations and does not alter intersegmental phases, but it does increase both periods and durations of PS bursts. Threshold for these changes is about 0.1 μM, and ED50 is about 1 μM RPCH (Sherff and Mulloney, 1991). Differential responses to RPCH by different types of motor neurons have not been described, but preparations bathed in 1 μM RPCH or more showed episodes of protracted PSE firing that might reflect RSE-suppression like that observed when high doses of CCAP are used.
Other neuropeptides
Other neuropeptides have been demonstrated using immunolabeling in crayfish abdominal ganglia. Antisera to FMRFamide (Sherff, unpublished results), Crustacean Hyperglycemic Hormone (Schwarz, unpublished), and Locusta tachykinin (Trinh, unpublished) all labeled neural structures in these ganglia. However, physiological experiments that looked for effects of these peptides on the output of the swimmeret system found none, and these observations have not been pursued.
4. Coordination of distributed modules
The characteristic progression within each cycle of power-stroke movements from posterior swimmerets to more anterior swimmerets persists in the fictive locomotion produced by isolated nerve cords (Fig. 2D), and so must be a property of the CNS. What organizational features of the CNS are responsible for this forward progression?
4.1 Single-timers, “Zeitgebers”, and excitability-gradient hypotheses
Ikeda and Wiersma (1964) tested the idea that a unique timer located in the more posterior ganglia of the CNS, A5 or A6, determined the start of each cycle. They simply severed the interganglionic connectives between ganglia A4 and A5 (Fig. 1B, 2A), and found that periodic, coordinated alternating PS-RS bursts that progressed forward from A4 persisted in the more anterior ganglia despite this cut. They concluded that ganglia A5 and A6 did not contain unique components required to produce or to coordinate swimmeret motor output in more anterior ganglia. They proposed instead that each ganglion contained a pacemaker connected to the next anterior pacemaker, and that within the series these pacemakers showed a progressively diminishing excitability. Accordingly, the pacemaker in A5 was most excitable and so fired first. Each more-anterior pacemaker (in A4, A3, and A2) then fired in order because they received excitation from their posterior neighbor.
Excitability-Gradient models
Ikeda and Wiersma's hypothesis is similar to hypotheses that propose gradients of excitation or excitability as the basis for anterior-to-posterior progression of motor patterns that drive swimming in fish and tadpoles (Matsushima and Grillner, 1992; Tunstall and Roberts, 1994). These models predict in crayfish that if you could somehow excite anterior and posterior ganglia separately and individually, and if you apply the same level of excitation to anterior and posterior ganglia, the posterior ganglia should respond more strongly. In particular, the period of PS bursts in posterior modules should be shorter than the period of PS bursts in anterior modules. Mulloney (1997) used the swimmeret system's responsiveness to carbachol (see 3.3.1) to test this prediction. When the same level of excitation, i.e. concentration of carbachol, was applied to both anterior (A2 and A3) and posterior ganglia (A4 and A5), while these ganglia were uncoupled by a tetrodotoxin block of impulse conduction on the A3-A4 connective, the model predicts that the posterior pair of ganglia should have the shorter period. On the contrary, the mean period of the anterior pair was shorter than the period of the posterior pair. Karlsson and Mulloney (unpublished results) repeated these experiments using the more readily-reversible “sucrose-knife” technique (Masino and Calabrese, 2002), and found a significant difference between anterior and posterior modules (paired t-test on A3 and A4 periods, P < 0.0005). An ANOVA test of the motor patterns elicited by uniform excitation in the presence and in the absence of a block between A3 and A4 showed that the significant effect of the block was to increase the period of the posterior ganglia; the periods of anterior ganglia did not change significantly. These results contradict the predictions of the excitability gradient models.
4.2 Asymmetric coupling of neighboring modules
The observations that stimulating command axons (see 3.1) anterior to A1 elicited coordinated swimmeret activity and that this activity always began with a PS burst in A5, although the command axons had traversed the more anterior ganglia to reach A5, led Ikeda and Wiersma (1964) to propose that “in the (coordinating circuit) there is absolute polarity, that is, conduction within the network is possible only from back to front.” Another possibility might be that the coordinating circuit is symmetric in the sense that either anterior or posterior segments might lead because the circuit itself is not inherently polarized, but some other aspect of the system normally prevents anterior segments leading. This possibility was tested by exciting individual swimmeret ganglia pharmacologically in an intact crayfish nerve cord to see if asymmetric excitation would drive a reversed motor pattern (Acevedo et al., 1994; Braun and Mulloney, 1995). In otherwise silent preparations, each of the four swimmeret ganglia (A2, A3, A4, and A5) responded to pharmacological excitation locally-applied. Even when just one ganglion was excited, the rest being bathed in normal saline but still connected together, all four ganglia responded. Once the initial transient passed, each cycle began with a PS burst in A5. The intensity of firing was far stronger in the ganglion bathed in the drug (Acevedo et al., 1994; Braun and Mulloney, 1993), but the period and phase of this firing was integrated into the normal coordination of the complete system. So, excitation of one anterior ganglion can activate the whole swimmeret system but cannot reverse the normal pattern of posterior-to-anterior coordination.
Split-bath experiments, in which anterior and posterior ganglia remained connected but were exposed to different levels of pharmacological excitation, yielded new evidence against a symmetric coordinating mechanism. If the bath surrounding ganglia A1 to A3 is separated from the bath surrounding A4 to A6 by a barrier across the A3-A4 connective, anterior and posterior ganglia can be excited differentially. When different levels of pharmacological excitation are applied, the phase between the two ganglia on opposite sides of the barrier, A3 relative to A4, changed (Braun and Mulloney, 1995; Mulloney and Hall, 2007b). If posterior excitation was higher than anterior, the onset of each anterior PS burst was delayed. If anterior excitation was higher, the onset of each anterior PS burst was advanced. The phases of activity in ganglia in the same bath – A5 and A4 or A3 and A2 – did not change despite this non-uniform excitation (Mulloney and Hall, 2007b). In some experiments while the anterior ganglia were more strongly excited, a local phase-reversal occurred at the boundary between high and low excitation, i.e. A3 led A4, but a general reversal of the motor pattern was never observed (Mulloney and Hall, 2007b). Stein (1973, 1974) obtained analogous results using command axons to excite individual modules.
In summary, these results are consistent with the view that each swimmeret module is equivalent to the others, and that these modules are coordinated by an intersegmental circuit that is asymmetric, or polarized, in the sense that it forces the system to begin each cycle in the most posterior swimmeret ganglion, A5. From this perspective, the normal phase differences in limb movements are properties of the coordinating circuit, not properties of the local pattern-generating circuits that control each limb.
4.3 Intersegmental coordinating neurons
In their seminal paper, Hughes and Wiersma (1960) described “activity fibres” located in the dorsal parts of the interganglionic connectives that “fire rhythmically in bursts when the swimmerets are active, but are not excited by passive movements of the swimmeret”. They speculated that these units might be activated by contractions of particular muscles, or might be collaterals of motor neurons that provided an efference copy of the motor output to neighboring modules (Von Holst and Mittelstädt, 1950). In their Discussion, Wiersma and Ikeda (1964) explicitly distinguished between command neurons that control activation or inhibition of the swimmeret system and coordinating neurons responsible for stable entrainment and phase differences between neighboring ganglia. In order to entrain independent modules to a common period and stable phase, the effect of a spike in a coordinating axon on the timing of a module's output must depend on the point in the module's cycle – the phase – at which the spike occurs (Hill et al., 2003; Kopell and Ermentrout, 1986). Stein (1971) provided the first demonstration that Hughes and Wiersma's activity fibers had the phase-dependent property required of coordinating neurons. In a remarkably difficult series of experiments, he severed most of the intersegmental connectives between ganglia A3 and A4 but left the region with the activity fibers intact. Then he separately stimulated command axons (see 3.1) above and below the cut. These command axons were interrupted at the cut between A3 and A4, so stimulating above the cut excited only A2 and A3, while stimulating below the cut excited only A4 and A5. By stimulating with different frequencies on opposite sides of the cut, he elicited swimmeret activity with different periods in A3 and A4. During these simultaneous bouts of stimulation, the periods of PS bursts in A3 were affected by the timing of PS bursts in A4 (PS4) relative to the A3 cycle. A PS4 burst early in the cycle delayed the start of the next A3 cycle, but PS4 bursts later in the cycle advanced the start of the next A3 cycle. This is the key property of a mechanism that entrains two oscillators to a common period and stable phase. Stein plotted an experimental Phase-Response Curve (PRC) that demonstrated this property. An assumption buried in these experiments is that each PS4 burst was accompanied by a burst of spikes in an ascending activity fiber, and Stein later demonstrated that this was so by showing that single PS4 bursts were accompanied by a burst of spikes in an ascending fiber. Whenever these ascending bursts occurred, the timing of the concurrent cycle in A3 was affected in a phase-dependent manner (Stein, 1974; 1976; 1977).
These results show that the “activity fibres” noticed by Hughes and Wiersma do project axons from a swimmeret module to other modules, and that they can entrain the output of their target modules to a common period and stable phase. Dye-fills of individual motor neurons and backfills of N1 and its branches have failed to reveal any axon collaterals from motor neurons that project into the interganglionic connectives (Mulloney and Hall, 2000), so Hughes and Wiersma's suggestion for an efference copy mechanism was not correct in detail. Moreover, the same anatomical methods failed to reveal any sensory afferents from the N1 that projected anteriorly into the connectives. The identification of the projection neurons whose axons are the activity fibers lagged until new experimental methods were developed.
4.3.1 Coordinating neurons link swimmeret modules together
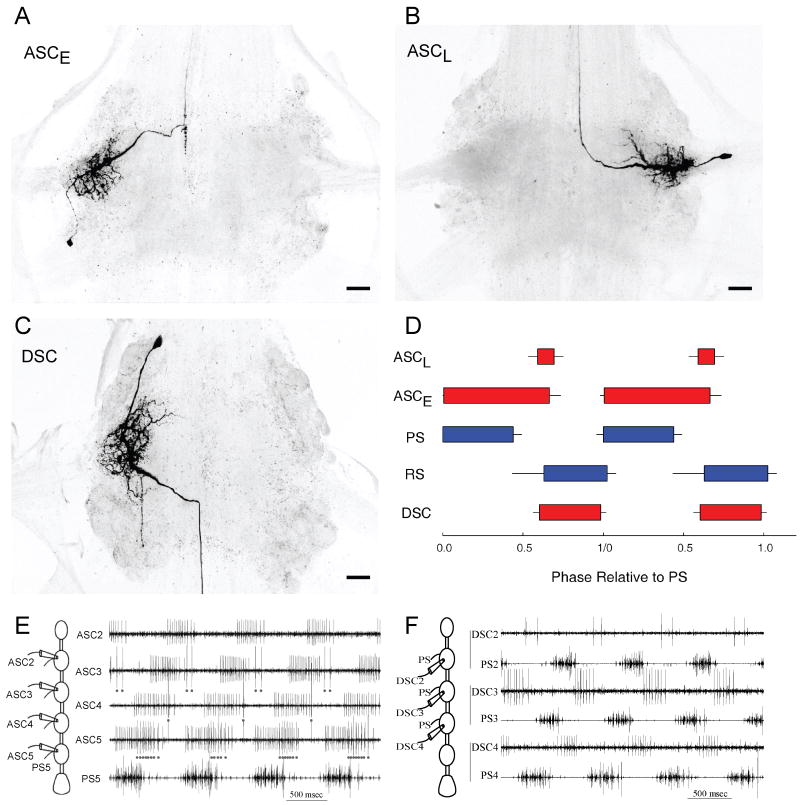
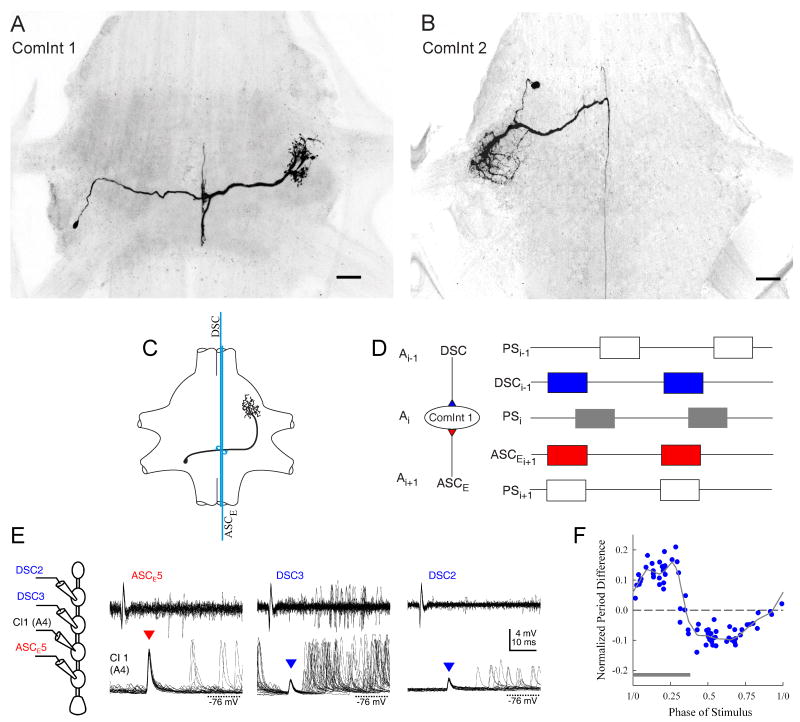
Coordination of swimmeret modules in different segments depends upon information encoded by neurons that arise in each module and project axons through the connectives to other modules. Stein (1971) tabulated three groups of central axons located at particular sites in the interganglionic connectives whose firing was correlated with different phases of swimmeret output. To our knowledge, Stein's was the first description of descending axons that fired bursts of spikes simultaneously with bursts in RS motor neurons. Namba and Mulloney (1999) used dye-filled microelectrodes to identify the neurons that gave rise to these coordinating axons. They described two neurons named ASCE and ASCL (for AScending Coordinating) in each module whose axons projected anteriorly in the “ascending direction” to targets in more anterior ganglia. They described a third neuron in each module named DSC (for DeScending Coordinating) whose axon projected posteriorly in the “descending direction” to targets in more posterior ganglia. Whenever their home module is actively producing activity, these neurons fire bursts of spikes at characteristic phases in each cycle of motor output and conduct those spikes to targets in other segments. These identified coordinating neurons are structurally and physiologically distinct from each other (Fig. 7), and from swimmeret command neurons (Acevedo et al., 1994; Wiersma and Ikeda, 1964).
Fig. 7.
Swimmeret coordinating neurons whose axons project dorsally from the LN toward the midline and then project into the interganglionic connective. Confocal images of whole mounts of three different ganglia, viewed from the dorsal side with anterior at the top. (A) An ASCE neuron in ganglion A4. (B) An ASCL neuron in ganglion A4. (C) A DSC neuron in ganglion A2. (D). Box plots that illustrate the timing of bursts of spikes in different neurons during two cycles of swimmeret motor output from one module (Mulloney et al., 2006). Each cycle begins with a burst of spikes in PS motor neurons. Each box shows the mean phase at which the neuron's burst began and its mean duration. Right error bars show Standard Deviation (SD) of these durations. Left error bars show SD of phases, except for PS bursts which show SD of normalized period. (E) Simultaneous extracellular recordings from ASCE and ASCL neurons originating in ganglia A2 through A5, and from the PS branch of N1 in ganglion A5 (Fig. 2). Four cycles of activity occur, marked by the four PS5 bursts. ASCE5 bursts begin simultaneously with each PS5 burst. ASCL5 start later in each cycle; each spike is marked by a dot. In each more anterior module, the two ASC neurons that arise there fire a burst that is delayed by the same amount as the PS burst in that module (Fig. 2). In more anterior ganglia, the numbers of ASCE spikes per burst are smaller than in A5 or A4. In this recording, ASCL in A2 was silent. (F) Simultaneous extracellular recordings from DSC neurons originating in ganglia A2 through A4 and from the PS branch of N1 from the same ganglia. In each ganglion, bursts of DSC spikes alternate with each PS burst. In more anterior ganglia, the numbers of DSC spikes per burst are smaller than in A4.
These coordinating neurons have measurable effects on motor output from their target modules (Jones et al., 2003; Mulloney and Hall, 2007a). Stimulating individual ASCE or DSC neurons through a microelectrode at different points in the output cycle affects the timing and strength of output from the neuron's target modules. To our knowledge, this is not true of individual coordinating neurons in lamprey, turtle, or rodent spinal cord, which makes these crayfish circuits uniquely valuable for studying intersegmental coordination at the cellular level.
When the whole swimmeret system is active, each module generates alternating bursts of spikes in PS and RS motor axons that drive movements of its swimmeret, and bursts of spikes in its coordinating axons that inform other modules of its activity (Fig. 7). The information carried by these coordinating axons is both necessary and sufficient for normal coordination of swimmeret activity in neighboring ganglia. In experiments in which the bulk of the connectives between two ganglia were severed, sparing only the regions that contained these axons, coordination was not affected. In contrast, when these regions were interrupted bilaterally but the rest of the connectives were left intact, coordination of PS bursts on opposite sides of the cut collapsed (Namba and Mulloney, 1999; Tschuluun et al., 2001). Stein (1971) also observed interactions between neighboring modules when the only connections remaining between them were in this medial region of one hemiconnective.
ASCE and ASCL neurons
Both ASCE and ASCL neurons occur as mirror-image pairs in modules in ganglia A2 through A5 (Fig. 7A, 7B) (Mulloney et al., 2006). Within each module, these two neurons occur as single copies, and have structures limited to one hemiganglion. The soma of ASCE neurons is commonly posterior to the base of N1 (Fig. 7A) while the soma of ASCL neurons is commonly anterior to this marker (Fig. 7B), but both send a process from their soma into the LN and extend many branches that are restricted to the LN. The axons of both neurons rise together from the LN through the Minuscule Tract (MnT) over the Lateral Giant (LG) axon before turning interiorly to enter a specific section of the connective, Area 78 (Skinner, 1985a; Wiersma and Hughes, 1961). Both neurons are silent except when their home module is active. Then, the ASCE neuron fires a burst of spikes simultaneously with each PS burst, beginning almost at the same time as the PS burst. The ASCL neuron also fires a burst, but starting somewhat later than the PS burst (Fig. 7D, 7E). It is not difficult to record their spikes with an extracellular suction electrode as their axons cross LG (Fig. 7E). The properties of these two neurons map onto Hughes and Wiersma's “activity fibres” and Stein's medial ascending coordinating fibers (Namba and Mulloney, 1999).
In isolated CNS preparations, bursts of spikes in PS motor axons recorded from left and right N1s of the same ganglion occur simultaneously and are similar in strength. Under these conditions, bursts in ASCE neurons recorded simultaneously on opposite sides of the same ganglion have similar numbers of spikes per burst (Mulloney et al., 2006). It is remarkable that the numbers of spikes per burst recorded simultaneously from ASCE neurons in different ganglia do differ significantly (Mulloney et al., 2006). ASCE neurons in A5 fire the most spikes per burst, and neurons in each more anterior ganglion fire fewer spikes (e.g. Fig. 7E). These differences in numbers of spikes form an orderly segmental gradient.
Ascending coordinating axons affect the phases of their target modules
In preparations that are producing stable periodic swimmeret motor output, ASCE and (sometimes) ASCL neurons fire bursts of spikes in each cycle (Fig. 7). Under these conditions, brief pulses of current injected into a single ASCE or ASCL neuron at different points in the cycle will change the timing and number of spikes the neuron fires. During the same cycle, these perturbations also affect output from the neuron's target modules in neighboring anterior ganglia (Jones et al., 2003; Mulloney and Hall, 2007a). Both timing and intensity of PS bursts change in a way dependent on the phase of the stimulus in the target module's cycle. The Phase-Response Curves (PRCs) of ASCE neurons (Mulloney and Hall, 2007a) cross the horizontal zero-change line with a negative slope near phase 0.4, and so have a stable fixed-point there (Schwemmer and Lewis, 2012). These PRCs resemble those originally constructed by Stein from median ascending fibers using different methods (1971, 1974).
Perturbing firing of an ASCE neuron with brief current pulses affects not only the neuron's targets in other modules but also the timing of output from its home module in a phase-dependent manner. These local effects are caused by direct interaction between the ASCE neuron and the local circuit within the module (Mulloney and Hall, 2007a), through connections that are still undescribed.
ASCE neurons encode multiple parameters of PS output from their home module
In isolated nerve-cord preparations that express the swimmeret motor pattern continuously, ASCE neurons fire a burst of spikes simultaneously with the bursts in PS motor neurons. These ASCE bursts begin just after the PS burst begins, and last as long as the PS burst does (Fig. 7D). If there is little variation in the strengths of PS bursts, there is also little variation in the numbers of ASCE spikes per burst. When the PS bursts are intermittent, or if they wax and wane in strength spontaneously or in response to sensory input (see 6), the numbers of spikes in each ASCE burst accurately tracks the strength of the simultaneous PS bursts (Mulloney et al., 2006). When strengths of PS bursts range widely, the numbers of ASCE spikes also range widely, and each additional spike represents a significantly stronger PS burst. So, each ASCE burst encodes the start, duration, and strength of the simultaneous PS burst in its home module, and these bursts adjust the phase differences between PS bursts in different ganglia.
Functions of ASCL neurons
Despite their many similarities with ASCE neurons, we think that ASCL neurons are not critical components of the intersegmental coordination circuit. The most significant difference between ASCE and ASCL neurons is that when the swimmeret system is actively producing coordinated motor output, ASCE neurons are always active, but ASCL neurons are often silent. Since intersegmental coordination is normal whether ASCL is bursting or silent, ASCL neurons cannot be essential coordinating neurons (Mulloney et al., 2006). ASCL neurons also affect timing of output from their target modules, but unlike the PRCs of ASCE neurons, the PRCs of ASCL neurons have two stable fixed points: near phases 0.05 and 0.75 (Jones et al., 2003). ASCE and ASCL neurons also synapse onto different target neurons in the ganglia to which they project (see 4.4.2), so the information ASCL neurons encode is decoded and interpreted differently than that encoded by ASCE neurons (Mulloney and Hall, 2003). This exclusion of ASCL neurons from the set of coordinating neurons is a revision of the analysis made by Namba and Mulloney (1999).
DSC neurons
DSC neurons also occur as mirror image pairs in the swimmeret modules of ganglia A2, A3, and A4. They have not been observed and we do not think they occur in A5 (Mulloney et al., 2006). Within each module, there is a single DSC neuron. DSC axons rise from the LN in MnT to cross dorsally over the LG axon (Fig. 7C). Before reaching the midline, they turn posteriorly into the interganglionic connective (Namba and Mulloney, 1999). Their position in the connectives and their firing patterns map onto Stein's (1971) description of medial descending coordinating fibers.
DSC neurons encode information about bursts of spikes in RS motor neurons
DSC firing can be recorded extracellularly in the MnT, and also in the same dorso-medial positions within the connectives where the axons of ASCE neurons run (Namba and Mulloney, 1999; Smarandache et al., 2009; Tschuluun et al., 2001). Except when their home modules are active, DSC neurons either fire tonically at low frequencies or are silent. When the system is active, each DSC neuron fires a burst of spikes simultaneously with each RS burst in its own module, beginning shortly after the preceding PS burst ends (Fig. 7D, 7F). The durations of DSC bursts are correlated with durations of concurrent RS bursts, and the numbers of DSC spikes per burst are correlated with the strengths of these RS bursts. These correlations are less strong than those of ASCE and PS bursts, but DSC bursts are negatively correlated with PS activity, and evidently encode information about each cycle of RS motor output (Mulloney et al., 2006).
DSC neurons affect the timing of motor output from modules to which their axons project. PRCs made by stimulating individual DSCs and measuring changes in timing of motor output from their target modules have the characteristic phase-dependent property required of a coordinating axon. Unlike ASCE neurons, DSCs have no apparent direct effect on the timing of output from their home modules (Mulloney and Hall, 2007a). Given these properties, and the observation that they are normally active whenever the system is active, we consider them to be essential coordinating neurons.
The firing of ASCE and DSC neurons conforms exactly to the definition of “corollary discharge” neurons (Crapse and Sommer, 2008; Von Holst and Mittelstädt, 1950), and it is not incorrect to consider them from that perspective. Unlike most other examples of corollary discharge units, however, we know more about these neurons than just their firing patterns. Given their demonstrated effects on the output of their target modules and the essential roles they play in organizing normal swimmeret activity, we purposely call them coordinating neurons.
Lateral descending neurons
In his original description of coordinating axons, Stein (1971) described a third category of “Lateral Ascending fibers” that fired in phase with PS bursts. In his experiments, these units were recorded in bundles of axons stripped from the interganglionic connective anterior to a ganglion that was actively expressing the swimmeret motor pattern. Their classification as “ascending” is based on the direction in which their spikes propagated in those experiments. These axons are not required for normal coordination because transecting these lateral regions of the connectives does not disrupt synchrony or intersegmental phase differences in active preparations (Namba and Mulloney, 1999; Stein, 1971; Tschuluun et al., 2001). Paul and Mulloney (1986) using Lucifer Yellow described bilaterally-projecting terminals of lateral descending axons that were repeated in ganglia A3, A4, and A5. During experiments on ComInt 1 neurons (see 4.4.1) using Neurobiotin to fill the recorded neuron, Mulloney and Hall (2003) discovered that these local neurons were dye-coupled through an electrical synapse to the terminals of a lateral descending axon. The anatomy of this terminal was restricted to the LN into which it descended, but its electrical synapse allowed Neurobiotin (and Lucifer Yellow) to also fill ComInt 1 and so replicate the bilateral projection originally described (Paul and Mulloney, 1986). Smarandache-Wellmann and Hall (unpublished) filled these terminals long enough to trace these axons back to the next anterior ganglion. Each Lateral Descending axon originates from a large cell body located anterior to N1 that extends a process into the local LN, and then projects its axon posteriorly to the next ganglion. We think it is possible that these neurons are the same as Stein's Lateral Ascending fibers, and that they have a spike-initiating zone in the LN where their axons terminate.
These neurons are unmistakably different from DSC neurons. The function and normal direction of impulse traffic in these neurons is still unknown, but there is one outstanding observation that attracts our attention. Heitler (1985) observed that in spontaneously active preparations, current injected into a neuron with anatomy like these lateral descending neurons could elicit a reversal of the normal intersegmental phase-progression, that is, power-stroke bursts occurred in the sequence … A3-A4-A5-A3 … rather than the normal … A5-A4-A3-A5 … (Heitler's Figures 3 and 9 do not show A2 activity). While this preparation expressed normal motor output, this neuron fired a burst of spikes simultaneously with each RS burst in its own ganglion (contra Stein 1971). Given the failure of non-uniform excitation to disrupt posterior-to-anterior phase progression (see 4.1), this result suggests these lateral descending neurons might have significant effects on coordination.
Fig. 9.
How this coordinating circuit is organized. Each module (A2, A3, A4, A5) contains a local circuit that generates alternating bursts of spikes in PS and RS motor neurons, and simultaneously drives bursts in ASCE and DSC coordinating neurons (Fig. 5). Coordinating axons project to other modules, where they make excitatory synapses (triangles) onto ComInt 1 neurons (C1). In each module, the ComInt 1 neuron integrates these EPSPs and excites one half of the local pattern-generating circuit (2). Thus, coordinating neurons and ComInt 1 neurons form a concatenated circuit that entrains each module to the same period and determines the phase-differences between them. In the diagram, arrows indicate the direction of impulse propagation, colors indicate the origin of each coordinating axon, and the different sizes of triangles indicate the relative strengths of different excitatory synapses. Other symbols are the same as in Figure 5.
4.4 Local commissural neurons as targets of coordinating axons
Local commissural interneurons are critical links in the swimmeret coordinating circuit. These neurons have structures restricted to one ganglion, and extend processes across the midline. As the axons of ASCE, ASCL, and DSC neurons leave their home ganglion, they join a bundle of homologous axons from other ganglia located in Area 78 of the interganglionic connective (Skinner, 1985a; Wiersma and Hughes, 1961). These axons stay together as they run the length of the ventral nerve cord (Tschuluun et al., 2001). Within each ganglion through which they project, coordinating axons do not extend branches laterally into the LN. Instead, they extend a few stubby branches that synapse onto local commissural interneurons where these commissural neurons cross the ganglion's midline (Mulloney and Hall, 2003). Commissural neurons integrate information from coordinating axons and transmit it to the synaptic kernel of the ganglion's two modules.
4.4.1 Commissural Interneuron 1 (ComInt 1)
ComInt 1 neurons occur as bilateral pairs in each ganglion A2 through A5. Their small cell bodies are located posterio-laterally in the ganglion and extend a thin process dorsally to cross the midline in the MnT. As they cross, they extend a tuft of branches along the bundle of coordinating axons, and then continue laterally to enter the LN on the side opposite their cell body (Fig. 8A). In that LN they branch locally to synapse onto specific targets within the swimmeret module. ComInt 1 neurons do not fire spikes, and relay information to just one module (Mulloney et al., 2003).
Fig. 8.
Commissural interneurons (ComInt) of the swimmeret system. (A) Whole mount of an A4 ganglion, viewed from the dorsal side with anterior at the top, that shows a dye-filled ComInt 1 neuron with its small cell body on the left side posterior to the base of N1. A thin process extends from the cell body to the midline, where it sprouts a tuft of small branches, and then continues on to the right LN where it branches repeatedly. The tuft of branches at the midline is aligned with the axons of coordinating neurons that project through the ganglion, and is the principal input region of each ComInt 1. The branches contralateral to the cell body are the neuron's principal output region. (B) Whole mount of an A3 ganglion (same orientation) that shows a dye-filled Commissural Interneuron 2 (ComInt 2). (C) Drawing of a ComInt 1 neuron receiving synaptic contacts from ASCE and DSC axons that originate in neighboring modules. (D) Diagram of the convergence of two coordinating axons (ASCE, DSC) from different ganglia onto the same ComInt 1 neuron. The box plots describe two cycles of swimmeret activity in three ganglia (Ai-1, Ai, Ai+1). Spikes in coordinating axons travel anteriorly from the next posterior ganglion (ASCE i+1) and posteriorly from the next anterior ganglion (DSC i-1) to converge simultaneously on the ComInt 1 neuron in ganglion Ai. The PS boxes show the posterior-to-anterior progression of PS bursts in neighboring ganglia (Fig. 2D). (E) Simultaneous intracellular recordings of EPSPs in a ComInt 1 neuron in A4 (CI1 (A4)) and extracellular recordings of spikes in ASCE and DSC neurons from ganglia A5, A3, and A2. Each panel shows multiple sweeps triggered by spikes in one coordinating axon (Smarandache et al., 2009). (F) Phase-response curve of a ComInt 1 neuron, generated by injecting pulses of depolarizing current into the neuron at different phases and measuring the change in period of the module to which the neuron projects. Positive changes mean longer periods, a phase-delay. The grey bar indicates when in each cycle the module's PS burst occurred.
Coordinating information from several modules converge onto each ComInt 1 (Figs. 8C, 8E, 9) (Mulloney and Hall, 2003; Smarandache et al., 2009). Which coordinating axons synapse onto a particular ComInt 1 depends on the segment in which the ComInt 1 lies. In A2 ComInt 1 is postsynaptic to three ASCE axons but no DSC axons, while in A5 ComInt 1 is postsynaptic to three DSC axons but no ASCE axons. ComInt 1 neurons in A3 and A4 are targets of both ASCE and DSC axons (Fig. 9). Whenever the swimmeret system is active, bursts of spikes in ASCE and DSC axons cause excitatory postsynaptic potentials (EPSPs) that sum to depolarize the ComInt 1 in phase with the arriving bursts (Fig. 8C, 8D, 8E). Because of the approximately 0.25 phase difference between neighboring segments (Fig. 2D) and the 0.5 phase difference between ASCE and DSC bursts from any one module (Fig. 7D), ASCE and DSC bursts from neighboring segments arrive at the ComInt 1 neurons in A3 or A4 simultaneously.
Gradient of synaptic strengths in ComInt 1 neurons
In each ComInt 1 neuron, the strengths of EPSPs caused by different coordinating neurons differ systematically (Smarandache et al., 2009). Taking the size of the EPSP as a measure of strength, in A2, A3, and A4 the strongest EPSP is caused by the ASCE axon from the nearest posterior ganglion. In A5 it is caused by the DSC originating in A4. Comparing the relative strengths of EPSPs caused by the same axon in different postsynaptic ComInt 1s, e.g. by the ASCE axon originating in A5, the strongest synapse is in the next adjacent ganglion, and synapses in more distant segments are progressively weaker (Smarandache et al., 2009). This segmental gradient implies that strengths of these synapses are regulated locally by an interaction between the presynaptic axon and each ComInt 1 neuron, like the strengths of PSPs made by abdominal Stretch Receptor axons with different targets in A6 (Nakagawa and Mulloney, 2001).
Between any two neighboring swimmeret ganglia, e.g. A5 and A4 or A4 and A3, the ASCE and DSC axons that connect the two modules and the ComInt 1 neurons that connect to each module form a closed loop (Fig. 9) that synchronizes the periodic output of the two modules. The coordinating axons make similar connections with more distant modules, so these loops are concatenated, and form a robust coordinating circuit.
4.4.2 Commissural Interneuron 2 (ComInt 2)
A second type of commissural interneuron, ComInt 2, integrates information from ASCL axons, but not ASCE or DSC axons. ComInt 2 neurons also have a distinctive structure (Fig. 8B), and send a branch to the midline to intercept information from ASCL axons. This branch runs in a different commissure than does the ComInt 1 neuron. We know less about ComInt 2's properties than about ComInt 1 because we have recorded from it less often. However, it is significant that this neuron seems to provide a relay for information conducted by ASCL axons separate from the relay used by information from ASCE and DSC.
4.4.3 Other local commissural neurons
In their original survey of neural components of the swimmeret system, Paul and Mulloney (1985a) described two more local commissural neurons that affected the local swimmeret activity but differed in structure from ComInt 1 and ComInt 2. Both had processes in MnT that projected into the LN. One neuron, named 6D, had a midline cell body and sent processes bilaterally into both of the ganglion's LNs. In the original description of this 6D neuron, the authors described it as firing spikes based on bursts of fast-rising depolarizations. In retrospect, these bursts closely resemble the bursts of EPSPs that occur in ComInt 1 neurons when the system is active, so we suggest that 6D neurons are also postsynaptic targets of coordinating axons, and both their function and their status as spiking neurons remains to be determined.
5. Analysis and models of the swimmeret system
The swimmeret system's robust, behaviorally-relevant motor output has stimulated thinking at several levels about how this system works. The goals have been to understand how periodic motor output arises in each module and to explain how motor activity in neighboring segments of an animal's CNS can be coordinated to ensure useful behaviors. Davis and Murphey (1969) constructed what appears to have been an integrate-and-fire model of a motor neuron with variable partial refractory periods to investigate the structures of bursts of spikes. They used sinusoidal voltage stimuli with different amplitudes and periods to test the plausibility of Davis's hypothesis about the periodic synaptic drive to these motor neurons.
The original attempts to describe the mechanism that coordinated swimmerets on different segments (Stein, 1973; 1974) introduced the use of Phase-Response Curves (PRCs) as analytical tools to describe the system's properties and the effects of ill-timed bursts of spikes in “coordinating fibers” on intersegmental phase differences. Stein (1976; 1977) demonstrated experimental PRCs made by plotting changes in the periods of swimmeret cycles caused by bursts of spikes in coordinating axons that arrived at different phases in the cycle. He discussed the properties required of coordinating axons if a periodic system of oscillatory circuits was to maintain a stable phase difference. His experimental PRCs had these necessary properties, and he concluded that these fibers could coordinate swimmeret movements effectively.
Tatsumi and Suzuki (1983) extended this approach by using periodic changes in a train of stimuli applied to excitatory command fibers (see 3.1), rather than to coordinating fibers. In essence, this experimental design created either brief gaps or brief increases in the otherwise-regular excitation of the system, and caused phase-dependent changes in the period of the motor pattern. Tatsumi and Suzuki constructed Phase Transition Curves (Winfree, 2001), not PRCs, and paid particular attention to the effects of different durations of their perturbations. They also introduced a limit-cycle model of a swimmeret oscillator and analyzed their experimental results in terms of trajectories on a two-dimensional phase-plane. This pioneering effort, however, did not attempt to discover the cellular and synaptic organization that endowed the system with these properties.
When the isolated swimmeret system is active, its output can be subtly modulated on a time-scale hundreds of times longer than the cycle period. Olsen and Murray-Smith (1993) used Fourier analysis and Karhunen-Loeve transforms of a long intracellular recording to isolate the principal components of the variance of slow oscillations in membrane potential and of numbers of spikes per burst. For the unidentified neuron whose activity they analyzed, slow modulation of oscillations was uncorrelated with cycle-by-cycle fluctuations in numbers of spikes per burst. This analysis did not include information about simultaneous changes in motor output from the same module or from the whole system, so these slow modulations remain largely unstudied.
5.1 Coupled-Oscillator Theory
Anything that generates a periodic output, or simply evolves periodically through a cycle of different states, can be viewed as an oscillator. Features of its activity can be described in terms of its cycle period and the phase in each cycle at which these features occur. If there is more than one oscillator and there is some mechanism that informs each oscillator about the states of the others, then they can form a system in which the periods of different oscillators are the same, and phase relations relative to one another are precisely maintained. These features define a system of coupled oscillators. Coupled-Oscillator Theory is a formal mathematical approach to describing the properties of systems of coupled oscillators (Kopell and Ermentrout, 1988; Schwemmer and Lewis, 2012). Coupled-Oscillator Theory is not concerned with the cellular details of neural circuits or patterns of synaptic connections, but only with key features of information passing between oscillators in the system. Skinner et al. (1997) used this approach to explore the properties of information needed to “couple” a chain of four oscillators together so that they expressed the same period and the same phase differences as do active swimmeret modules in different segments. They found that if both ascending and descending coupling linked neighboring oscillators, and both were relatively strong but not identical, then phase-differences of 0.18 to 0.25, posterior module leading, could be achieved. They also observed that within this range of parameters, either ascending coupling or descending coupling alone could maintain phase differences between oscillators. Moreover, these phase differences were not much altered by changes in the intrinsic periods of the oscillators, changes equivalent to changes in excitation in the real system (Mulloney et al., 1998)
Analyses developed using PRCs, phase-planes, and Coupled-Oscillator Theory have revealed aspects of the swimmeret system's properties that were not readily apparent by inspection of the system's output, but shed no light on how the system's neural components combine to produce this output. Cellular and synaptic explanations require a different level of analysis.
5.2 Conductance-based cellular models of the local pattern-generating circuit
To explore how specific neurons might combine to produce periodic motor patterns and contribute individually to normal coordination, Skinner and Mulloney (1998) constructed a model of the local pattern-generating circuit using conductance-based models of individual cells. They reduced the local circuit to its essential components, the non-spiking local interneurons (see 2.2.2). Each local neuron was represented as a single compartment that had two voltage-gated currents and as many synaptic currents as the circuit required: a modified Morris-Lecar (ML) cell (Rinzel and Ermentrout, 1998). These ML cells were not inherently oscillatory; in the absence of synaptic input their membrane potentials were stable, and did not oscillate even when depolarized by steady depolarizing currents. In the local circuit, one pair of ML cells represented local neurons Int 1A and Int 1B. The third cell represented the pair of Int 2A neurons, which the model combined because there are no known differences between the members of the pair. To form the pattern-generating circuit, the Int 1A and Int 1B cells inhibited the Int 2A cell, and Int 2A inhibited both Int 1A and Int 1B (Fig. 10). These reciprocal synapses were described as a membrane conductance in the postsynaptic neuron whose activation was a function of the presynaptic neuron's membrane potential (Skinner and Mulloney, 1998). Jones et al. (2003) and Jones and Kopell (2006) used this same model in their later analysis of the dynamics of the coordinating circuit.
Fig. 10.
Diagrams of two intersegmental circuits whose dynamics have been explored using conductance-based models (Skinner and Mulloney, 1998; Jones et al., 2003). Each circuit shows two local pattern-generating circuits in two neighboring segments (An, An+1). An is an anterior segment, An+1 is the next posterior segment. In these models of local circuits, circles labeled 1A and 1B represent Int 1A and Int 1B neurons while the circle labeled 2A represents the pair of Int 2A neurons (see 2.2.2). Red-filled circles symbolize intersegmental inhibitory connections. Green-filled triangles symbolize intersegmental excitatory connections. These intersegmental connections are active whenever the local cell from which they originate is depolarized. Small black filled circles symbolize local graded inhibition. In both models, oscillations of potentials in these local cells depend on the kinetics of these graded synapses.
To test the performance of alternative patterns of intersegmental connections, Skinner and Mulloney (1998) used four of these local-circuit models to construct a family of alternative intersegmental circuits. These alternatives linked neighboring modules by connections that fired a “spike” whenever a particular local neuron was depolarized. The numbers and directions of these connections were constrained by Stein's (1971) description of ascending and descending coordinating fibers, and whether each connection made an excitatory or an inhibitory synapse with its target defined the different intersegmental circuits. Only one of these alternatives (Fig. 10) had intersegmental phase differences at all like those of the swimmeret system itself. The performance of this model was surprisingly robust, and not very sensitive to variations in synaptic strength or to changes in period. Why do these connections permit the phase of two neighboring modules to remain unchanged when period changes?
Jones et al. (2003) examined the contributions of the ascending and descending intersegmental connections separately, using a second pattern of connections (Fig. 10) that also produced the characteristic phase differences. Neither ascending connections alone nor descending connections alone could maintain a constant phase at different periods, but the systematic change of phase with period for ascending connections alone was in a direction opposite to that for descending connections alone. When both ascending and descending connections were present, these opposite tendencies cancelled, and phase did not change despite changes in period. Jones et al. (2003) also analyzed the effects of individual connections in this circuit on intersegmental phase, and compared them with the experimental PRCs of individual ASCE and ASCL axons. In their models, the PRCs of the ascending excitatory and inhibitory connections had different shapes and different zero-crossings, and in these points resembled the PRCs of ASCE and ASCL axons in the coordinating circuit (see 4.3.1).
These efforts have increased understanding of the properties an intersegmental circuit must have in order to coordinate limbs on different segments effectively, but they did not incorporate recently discovered features of the coordinating circuit (Fig. 9) like integration of both ascending and descending information in ComInt 1 neurons and gradients in synaptic strength (see 4.3). These features have yet to be studied in computational models.
6. Proprioceptive reflexes and sensorimotor integration
Proprioceptive reflexes and integration of exteroceptive information about the environment are necessary if the activity of different central pattern-generating circuits is to be integrated to form sequences of coherent, adaptive behaviors (Wilson, 1968). In different motor systems, the importance of these sensory influences can differ. At one extreme, coordinated stepping during walking by stick insects is completely dependent on sensory signals that report the actual movement from the periphery (Büschges, 2005). The swimmeret system is not dependent on sensory feedback to generate a stable and well-coordinated motor output (Hughes and Wiersma, 1960; Mulloney and Smarandache, 2010), but nonetheless there are many different proprioceptors and other sensory inputs that can shape and adjust its output. We begin with a general review of how proprioceptive feedback changes swimmeret activity in semi-intact preparations, where several types of receptors are stimulated by swimmeret movements.
6.1 Proprioceptive feedback
Davis (1969b; 1969c) studied reflex organization in the lobster swimmeret system (Homarus americanus). He characterized for the first time reflex responses to imposed movements of a swimmeret, and attempted to localize where those receptors that caused reflexive motor firing were located on the swimmeret. In semi-intact preparations where the swimmerets were not actively beating, mechanical retraction (an imposed power-stroke) of a swimmeret to its maximal posterior position caused an increase in ongoing RSE firing and a decrease in RSI firing; responses that qualify as a resistance reflex. The same movements at the same time also increased PSE activity, a response similar to the reflex reversal seen in stick insects (Bässler, 1986; Bässler, 1988). Receptors in the coxa of each swimmeret are activated during natural movements and so act as proprioceptive sensors, but Davis did not describe the sensory neurons themselves in these papers.
When lobsters and crayfish move their swimmerets in water, the hair-like setae and the distal rami on each swimmeret are bent by the resistance of the water. Davis (1969b) used jets of water directed at a swimmeret to mimic this natural stimulation while recording efferent motor discharge from selected swimmeret muscles. Water flowing from the anterior direction, as if during a return-stroke movement, did not elicit a reflexive motor discharge. Water flowing from the posterior direction, as if during a power-stroke movement, caused a marked increase in PSE firing but inhibited RSE firing. Davis (1969b) suggested that positive feedback from the rami to PSE is most likely load compensation during movements in water; so swimmerets can automatically adjust their output to different loads. In his discussion, Davis noted that sensory feedback has the same strength in each segment and that he could not detect a segmental gradient in strength. He also pointed out that RS activity could be activated as a positive reflex loop by a power-stroke movement, but PS activity is not activated by a return-stroke movement. In a companion paper (1969c), Davis described the frequency range through which imposed movements affected motor discharges. Reflex discharges could follow sinusoidal movements throughout the swimmeret system's working frequency range, and these reflexes were strongest within the same frequencies as natural swimmeret beating.
West et al. (1979) used a semi-intact crayfish preparation to study the effects of proprioceptive feedback on swimmeret beating activated by command neuron stimulation. In these experiments, different excitatory command neurons were stimulated until the preparation produced a stable swimmeret rhythm. Then, movements of one swimmeret were perturbed by holding it in either a retracted or protracted position while stimulation continued and the motor output was recorded. In these experiments, the authors obtained three distinct results. Mechanical interference either had no effect on the period, or decreased it, or stopped the rhythm completely. These results imply that excitation of the system by some command neurons can change the effects of sensory input on the local CPGs. West et al. (1979) suggested that in those cases where they saw no proprioceptive effect on the period of the motor output, the command neuron they were stimulating gated the proprioceptive information or directly inhibited terminals of sensory afferents. In the other cases, they always saw a clear effect on period and they interpreted this difference to stimulating a different command neuron. Similar effects can be observed in the crustacean stomatogastric system, where sensory feedback interacts with projecting neurons to produce different styles of motor output (Blitz and Nusbaum, 2011).
In the preceding papers, either one swimmeret or just the stump of a swimmeret was manipulated during the experiment, and although some effects on period were observed, there was no evidence of proprioceptive entrainment of the swimmeret motor pattern. Deller and MacMillan (1989) built an apparatus to which they attached one, two, three or four ipsilateral swimmerets and so could impose sinusoidal movements on these limbs. With this device they could entrain the swimmeret motor output to the imposed frequency after the ventral nerve cord was cut anterior to A1. Logically, entrainment was more effective when more limbs were moved. If only one swimmeret was moved, no entrainment was detected. It is interesting that the most profound entrainment occurred when proprioceptive feedback from the unrestrained swimmerets on the contralateral side was removed.
All these results suggest that proprioceptive feedback can influence aspects of the movements of swimmerets in intact, freely-swimming animals. Nevertheless, static stimulation of a single swimmeret does not have a strong effect on period or intersegmental phase. Only when most sensory feedback and information from more anterior neuronal centers was abolished did imposed periodic movements affect the period of the centrally-produced motor pattern (Deller and MacMillan, 1989).
6.2. Non-spiking stretch receptors (NSSRs)
Two classes of sensory afferents have been described in the swimmeret system: non-spiking stretch receptors (NSSRs) and spiking primary afferents of several types. Each swimmeret has two NSSRs that are stimulated by rotation of the basi-coxal joint (Heitler, 1982). These neurons are homologues of the NSSRs found in walking legs and uropods of crustaceans (Paul, 1972; Ripley et al., 1968). Unlike most sensory afferents in arthropods, NSSRs have a centrally-located cell body and project an axon through the anterior branch of N1 to reach a strand of connective tissue, S1, at the base of the swimmeret's basipodite (Heitler, 1982). In all crustaceans that have them, the two NSSRs in each hemiganglion have distinct morphologies. One has its soma located anterior to the base of N1 within the cluster of RS neuron cell bodies (see 2.1.2) and is called NSSR-A. The second has its soma posterior to the base of N1 in the cluster of PS motor neurons and is called NSSR-P. Both NSSRs have extensive branches within the LN above the base of the N1 into which their axons project.
When swimmerets are in their resting positions (retracted), the S1 strand is not stretched and each NSSR is at its resting potential. During a power-stroke movement (protraction), S1 is stretched and the NSSRs depolarize, but do not fire spikes (Heitler, 1982; Heitler, 1986; MacMillan and Deller, 1989). During a return-stroke movement (retraction) , the NSSRs repolarize. Periodic sinusoidal movements of a swimmeret from retracted to protracted positions cause periodic depolarizations the NSSRs that track the swimmeret's position. NSSR-A and NSSR-P depolarizations do not differ in phase relative to imposed movements (MacMillan and Deller 1989). Paul (1989) observed that when the swimmeret system is active, periodic oscillations of the membrane potentials of NSSRs with the same period as the system's output occur in the central processes of NSSRs even if N1 is cut. She suggested that NSSRs receive direct synaptic input from the local pattern-generating circuit.
Heitler's (1986) intracellular stimulations of individual NSSRs in Pacifastacus leniusculus had strong effects on PS motor neurons in the same module. Depolarization of an NSSR reduced PS activity; hyperpolarization increased PS burst strength. His findings also showed that NSSRs tracked movements of a swimmeret precisely, but he was unable to entrain the swimmeret rhythm with sinusoidal currents injected into one NSSR. He concluded that sensory feedback to just one module cannot entrain the activity of a chain of four coupled oscillators. MacMillan and Deller (1989), working with Cherax destructor, used a Vaseline gap method to show that periodic sinusoidal depolarization of a cut N1 could entrain the swimmeret rhythm very well. They attributed this difference from Heitler's results to species differences, but to us it seems more likely to have resulted from simultaneous depolarization of both NSSR axons in the cut N1.
In the Norway Lobster Nephrops norvegicus, Miyan and Neil (1986) described a sensory strand associated with a muscle that twists the swimmeret at its base. This strand is similar to S1-S2 described in crayfish (Heitler, 1982), but has somewhat different insertions. Two sensory neurons with central cell bodies are associated with this strand, but their homology with the NSSRs is uncertain. Miyan and Neil named them twisting-muscle receptors, and demonstrated that they fire spikes in response to retraction of the swimmeret. Stretching the sensory strand excites the motor innervation of the twisting muscle (see 6.4), and so forms a positive feedback loop.
6.3 Spiking proprioceptors
In addition to the NSSRs, each swimmeret has numerous sensory afferent neurons whose anatomy is more typical of arthropod primary sensory neurons. These neurons have cell bodies located in the periphery, usually associated with specializations of the cuticle or connective tissue that allow the neuron to transduce physical deformation of the cuticle or changes in the angle of a joint. Different primary afferent neurons respond to different sensory modalities.
Killian and Page (1992a; 1992b) described three categories of mechanosensory afferents on the rami of the swimmerets of Homarus americanus. Two of these were activated by deformation of the cuticle of the rami (Killian and Page, 1992a). They distinguished “Wide-Field” afferents (WF) and “Cuticular-Ridge” mechanoreceptors (CR). The WF receptors responded to mechanical deformation of the soft, flexible cuticular areas on the posterior surface of each ramus and at the joint between the coxa and the abdominal wall. WF receptors have multipolar cell bodies with extensive branches in the hypodermis, and large receptive fields on the posterior surface of the ramus. They fire in response to deformation of the cuticle, initially at high frequency and then tonically for the duration of the stimulus. The authors suggest that WF receptors are activated during contraction of power-stroke muscles, especially the rami curlers, and might be spiking proprioceptors that monitor the rate, strength and duration of the muscle contraction.
Cuticular-ridge (CR) mechanoreceptors have bipolar cell bodies in the hypodermis beneath the small cuticular ridges that line the edges of each ramus. These ridges are calcified, and much stiffer than the cuticle on the posterior surface of the ramus. Apparently, each ridge has one CR receptor. The receptive field of each CR receptor is comparatively small, restricted to one ridge and the immediately adjacent soft cuticle. CR receptors respond to mechanical deformation of the ridge with a phasic high-frequency spike discharge. They also respond to curling of the rami because the ridges are then compressed, but do not fire as the rami uncurl. Killian and Page therefore suggested that these mechanosensors might monitor the degree and velocity of rami curling during strong power-stroke movements.
In a companion paper, Killian and Page (1992b) described the mechanosensory innervation of the feathery hairs that fringe each ramus. In H. americanus, these hairs are 2-4 mm long, oriented orthogonal to the long axis of the body, and significantly increase the effective surface area of each ramus. From differences in their locations that corresponded to a major difference in their innervation, Killian and Page distinguished Distal-Feathered hairs (DF) and Proximal-Feathered hairs (PF). DF hairs fringe the distal third of each ramus and are attached to sockets on the corners of cuticular ridges. They are not themselves directly innervated. Water-flow during power-stroke movements deflects them, and this directional deflection activates CR receptors in the ridges to which they are attached. This mechanical coupling increases the receptive field of each CR receptor. Large deflections of a hair, >25 °, were required to elicit firing from the CR receptor in the ridge to which a hair was attached.
Proximal-feathered (PF) hairs fringe the proximal 2/3 of each ramus and are also mounted on sockets on the corners of cuticular ridges. Unlike the DF hairs, each PF hair is innervated by several sensory afferents with cell bodies at the base of the hair, one of which responds to mechanical stimulation. Killian and Page described the properties of this PF mechanosensory afferent; the modalities of the other afferents are unknown. PF mechanosensory afferents are sensitive to displacement of the hair. They respond transiently to high-frequency deflections as small as 0.2°, and to slow deflections of 4° or less. They effectively track repeated deflections up to 5 Hz. Because their fringes overlap, they are also mechanically coupled, and large displacements of one PF hair will elicit responses from PF mechanoreceptors that innervate neighboring hairs. The sensitivity of these PF hairs to high-frequency displacements makes them sensitive to water-borne vibrations.
Joints in the limbs of arthropods are often equipped with sensory structures that encode joint angle and its derivative. Heitler (1982) described briefly a sensory strand, S2, innervated by small axons that lies near the S1 strand innervated by the NSSRs. He was unable to locate the cell bodies of these axons, and it seems probable that their cell bodies are displaced more centrally in the anterior branch of N1, like those of the cuticular receptors described by Pabst and Kennedy (1967). He demonstrated that bending the basi-coxal joint of the stub of a swimmeret caused an afferent volley of spikes, which he attributed to this mechanoreceptor. Other sensory discharges in Heitler's recordings are attributable to the afferents later described by Killian and Page.
In addition to the receptor described above (6.2), Miyan and Neil (1986) described a second spiking proprioceptive system associated with the base of the swimmeret in Nephrops. Two bipolar spiking neurons innervate a second sensory strand that spans the gap between the sternal rib and the basipodite. Due to the orientation of this strand, it is stretched during protraction. Recordings demonstrated that these neurons fire tonically during full retraction of the swimmeret, and this firing caused an increase in firing of power-stroke motor neurons. This stretch receptor then is part of a negative feedback loop that increases power-stroke activity and so opposes return-stroke activity responsible for the full retraction of the swimmeret that stretches the receptor.
6.4 Statocysts and visual input
The principal exteroceptive sensory system that affects the swimmeret system is the pair of statocyst organs at the base of the antennules. These organs detect gravitational force and angular acceleration. By comparing the output of the two statocysts, crustaceans can determine the orientation of their bodies in the pitch and roll planes, and monitor the rate of any angular rotation. Each statocyst has hundreds of sensory hairs, each innervated by a primary sensory afferent that projects through the statocyst nerve into the brain. Cohen (1955; 1960) described in elegant detail the anatomy of these organs and the response-properties of these sensory afferents.
In response to roll about the long axis of the body, macruran crustaceans beat their swimmerets asymmetrically to create a corrective torque that would return them to an upright position. High-speed cinematography of adult Homarus americanus revealed that these asymmetric movements are accomplished by rotating each swimmeret on the upward side outward and increasing the strength of power-stroke movement while reducing the force of movements made by swimmerets on the downward side (Davis, 1968a). Roll itself is enough to elicit swimmeret beating on the upward side if the system is not already active, and in these circumstances the swimmerets on the downward side sometimes remain immobile in their resting retracted positions. This righting response is unaffected by elimination of visual input from both compound eyes, but is abolished by destruction of both statocyst organs even when vision remains intact. Other parts of the animal's body also respond to roll and tilt, including the antennae, antennules, eyestalks, and uropods (Davis, 1971b).
Neil and Miyan (1986) described the biomechanics of these asymmetric movements in Nephrops. They showed that during a roll, muscles M9 and M10 (Davis, 1968c) of each swimmeret on the upward side are excited while the same muscles on the downward side are inhibited. From the mechanics of their insertions in the swimmeret, Neil and Miyan argue that M9 and M10 create the torque that twists the swimmeret during a righting response. They described the innervation of these two muscles, and recorded responses of the motor neurons that innervate them to roll about the long axis. In the same segment of an upright (not rolled) animal, left and right muscles received similar levels of activity. During sequences of left-right rolls about the long axis, motor activity increased on the elevated side but decreased on the lower side, and these differences reversed smoothly as the body rolled past the upright position. From Neil and Miyan's evidence, motor neurons that innervate M9 and M10 in Nephrops are members of the PS group of motor neurons (see 2.1.2), and fire during PSE bursts. Neil and Miyan also observed that the normal posterior-to-anterior progression of power-stroke movements (Fig. 2D) was maintained during roll even when power-stroke movements in each segment were highly asymmetric.
How is information from the statocysts integrated and conducted to the swimmeret systems? In a wonderful study, Takahata and Hisada (1982a) identified four pairs of descending axons that are sensitive to statocyst input and project from the brain through the interganglionic connectives posteriorly all the way to A6 (Fig. 2A). There appear to be two pathways from each statocyst to each interneuron in the brain, one short latency and one longer latency pathway that is probably polysynaptic. Each pair has a distinctive receptive field in the statocyst's population of primary sensory afferents (Takahata and Hisada, 1982b). Two of these neurons receive input from the statocyst contralateral to the side of the brain from which their axons project. The other two receive input from the ipsilateral statocyst. Together they encode information sufficient to distinguish pitch and roll about two body axes (Takahata and Hisada, 1982b). At least one pair of these neurons is multimodal, and responds also to visual and proprioceptive input from the walking legs. The direction and magnitude of body tilt is therefore encoded in the combined firing of these descending interneurons.
The best evidence that these four interneurons conduct information about pitch and roll to the swimmeret system comes from Davis's (1968a) behavioral description of the coordinated responses of swimmerets, abdomen, and uropods to imposed roll, and Takahata's group's analysis of the neural circuits in A6 through which these descending statocyst interneurons shape the responses of uropods to roll (Takahata and Murayama, 1992; Yoshino et al., 1980). These descending axons project through each swimmeret ganglion to reach A6, and we predict that they also will influence the activity of individual swimmeret modules in those ganglia. The circuitry through which they exercise this influence is undiscovered.
7. Interactions of swimmerets with other motor systems
In the intact animal, the swimmeret system's activity is integrated with the activities of other motor systems, particularly those whose movements affect or are affected by the movements of swimmerets. Abdominal posture (extended or flexed), escape tail-flips, and walking all affect swimmeret activity, and interactions between the neural systems controlling these behaviors have been explored from various perspectives (Cattaert and Le Ray, 2001). In a well-controlled series of experiments on command neurons that affected abdominal posture in semi-intact crayfish, Williams and Larimer (1981) demonstrated that stimulation of certain command axons that evoked postural extension usually excited swimmeret beating, too. The most effective unit they worked with is probably Acevedo's EC axon (see 3.1).
7.1 Interactions with abdominal posture
The posture of the abdomen of macruran crustaceans is determined by the relative activity of well-defined sets of motor neurons that innervate the segmentally-repeated slow-flexor muscles and slow-extensor muscles that work across each abdominal joint (Drummond and MacMillan, 1998; Kennedy et al., 1967; Leise et al., 1986; 1987; Wine et al., 1974). In its flexed posture, the curled abdomen effectively prevents swimmeret beating because it blocks power-strokes. As part of their study of neural control of abdominal posture, Kotak and Page (1986) demonstrated that local tactile stimulation of a swimmeret on abdominal segment two (innervated by A2) caused abdominal extension. All flexor excitor motor neurons were inhibited, while the flexor inhibitor and extensor excitor motor neurons were excited. This coordinated response to stimulating a swimmeret spread to serial homologues in neighboring ganglia.
In intact animals, changes in the orientation of the body relative to gravity (“pitch” and “roll”) are encoded by interneurons that project from the brain to A6, the full length of the ventral nerve cord (see 6). Beating swimmerets respond to roll with asymmetric movements that generate a rotational torque that serves to right the body (Davis, 1968a). Tatsumi et al. (1985) demonstrated that coordinated abdominal extension and swimmeret beating were elicited by forward pitch (head downward) of the body. Knox and Neil (1991) studied this coordinated response more quantitatively using video analysis of movements. They found that, in response to pitch, swimmeret movements remained symmetrical, but in response to roll, the upper swimmeret was directed strongly to the side, as Davis had observed. They also used semi-intact preparations to record extracellularly from an RS branch of A3's N1 and from the nerve innervating the slow flexor muscles of the same segment. They observed that sinusoidal rotation in the pitch plane modulated firing of a “tonic return stroke” unit and both excitors and inhibitors of the flexor muscles. Barthe et al. (1991) used an in vitro preparation of the complete thoracic and abdominal nerve cord (T1 to A6), isolated from all sensory input, to look more closely at central mechanisms coordinating swimmeret activity with walking and postural activity. Commonly in these preparations, units in the postural system fired tonically, but in one third of Barthe et al.'s experiments, flexor and extensor units were modulated simultaneously with swimmeret activity, and had the same period and a stable phase relative to PS bursts. In other preparations, they observed long-lasting (about ten second) bursts simultaneously in flexor and extensor recordings, and during these bursts the swimmeret output was inhibited.
Chrachri and Neil (1994; 1993) made an in vitro preparation of three thoracic ganglia and the abdominal nerve cord (T3-A6) and used microelectrode recordings to demonstrate several modes of interaction between swimmerets and the postural motor systems. At times in some preparations, units in both the flexor and extensor nerves fired bursts of spikes simultaneously with PS bursts from the same ganglion. Under these conditions, synchronous bursts in these postural neurons occurred bilaterally in each segment. When the postural system was producing coordinated alternation of bursts in flexor and extensor excitors, a pattern with a period of about twenty seconds elicited by bath-applied oxotremorine, PS motor neurons were entrained to fire protracted burst of spikes concurrently with the bursts in flexor excitors. They also found that stimulating the “Thoracic Second Root” (TSR) of one of the thoracic ganglia shortened the cycle period of swimmeret activity, and in different experiments excited either the slow flexor or slow extensor motor neurons. When the extensors were excited, so was the swimmeret system. When the flexors were excited, the swimmeret system was inhibited for several seconds. Chrachri et al. (1994) recorded from various interneurons in A1 that responded to stimulation of a TSR and affected the output of either the postural system or the swimmeret system, but did not succeed in identifying these neurons or in mapping synaptic connections between them.
In an extensive series of experiments, Murchison and Larimer (1990; 1992) made paired microelectrode recordings from “abdominal-positioning interneurons” in abdominal ganglia while recording motor activity from nerves innervating slow flexor, slow extensor, and swimmeret muscles. They classified neurons by their effects on these different motor systems, and sought to describe synaptic interactions between pairs of neurons. They described many individual interneurons that affected one or both motor systems, and observed polysynaptic interactions between some of them. Burdohan and Larimer (1995) extended this approach by looking for abdominal-positioning interneurons that affected motor output to the uropods, and found some that affected swimmerets and uropods simultaneously. However, because of uncertainties about identifications of neurons recorded in different preparations, this promising approach did not produce a cellular circuit model to account for coordination of swimmeret and postural systems. The neural circuits responsible for these coordinated responses of postural and swimmeret systems remain unknown.
7.2 Interactions with the walking system
Crayfish and lobsters can walk forward, backward, or sideways using the four pairs of walking legs on their thorax (Ayers and Davis, 1977; MacMillan, 1975). The innervation of these limbs closely resembles the innervation of swimmerets (Mulloney et al., 2003). In many behavioral situations, the walking system and swimmeret system are active simultaneously, and their coordination has been studied in several ways. Cattaert and Clarac (1983) recorded electromyograms simultaneously from PS and RS swimmeret muscles and from levator and depressor leg muscles of lobsters walking on a treadmill. They observed episodes of absolute coordination, where each step was accompanied by a swimmeret beat (1:1), and periods of relative coordination, where the periods of swimmeret movements were briefer (2:1 or 3:1) but there was still a phase-dependent effect of the step on the period of the swimmeret cycle during which the step occurred.
Working with their in vitro preparation (see 7.1) of the complete thoracic and abdominal nerve cord (T1 to A6), Barthe et al. (1991) observed clear evidence of inhibition of swimmeret activity during spontaneous bouts of walking. In their experiments, the swimmeret system was normally continuously active, but during the comparatively rare bouts of spontaneous slow backward walking (Chrachri and Clarac, 1990), swimmeret activity was effectively inhibited while depressor motor neurons of the fifth leg fired. Since these preparations had no sensory input, this inhibition is evidence of descending inhibition from central components of the thoracic walking system to the swimmeret system. Cattaert et al. (1992) modified this T1-A6 preparation by retaining a leg proprioceptor, the coxo-basal chordotonal organ (CBCO), that monitors movements of the fifth walking leg. Stretching this receptor sinusoidally could elicit expression of the swimmeret motor pattern when the system had previously been silent, and shorten the cycle periods if the system was already active. Brief electrical stimulation of the CBCO nerve caused a phase-dependent change in the period of the swimmeret cycle during which the stimulus occurred; stimulation during the PS burst shortened the period while stimulation during the RS burst lengthened it. The central projections of the different primary sensory afferent in the CBCO are restricted to the one LN that holds the dendrites of all that leg's motor neurons (El Manira et al., 1991a; 1991b; Mulloney et al., 2003), so these interactions between the walking and swimmeret systems cannot be due to posterior projections of proprioceptive axons.
7.3 Interactions with the tail-flip system
Macruran crustaceans escape using powerful tail-flips that propel them away from perceived threats (Edwards et al., 1999). During the flexion phase of a tail-flip, swimmeret motor output is actively inhibited. This inhibition is fast, and does not reset the phase of the swimmeret motor output (Tatsumi et al., 1985). How this inhibition is accomplished is unclear. Each LN of a swimmeret ganglion (A2 though A5) contains the dendrites of a large neuron, the Segmental Giant (SG), that relays spikes from the giant fibers to the Motor Giant neuron, and so causes rapid flexion of the tail (Wine, 1984). Although it has no function in the swimmeret system, the SG neuron does have an axon that projects from the LN into N1 toward the swimmeret (Heitler et al., 1985), and is weakly coupled to some swimmeret motor neurons through what appear to be electrical synapses (Heitler and Darrig, 1986). Each SG spike is accompanied by an EPSP in these swimmeret motor neurons, a connection that cannot account for the observed inhibition of motor output. What effects SG spikes might have on neurons of the local swimmeret pattern-generating circuit is unknown.
Following an initial escape tail-flip driven by a spike in either of the giant axons (Wine, 1984), crayfish and lobster often continue to swim away with an alternating series of powerful tail extensions and flexions. This continued backward swimming is termed “non-giant” swimming because the coordinated alternating extensor and flexor activity is not caused by firing of either of the giant-fiber systems. The Squat Lobster, Galathea strigosa, lacks giant fibers and escapes using just the non-giant system. Sillar and Heitler (1985) described the motor control of this non-giant swimming and observed that the unspecialized swimmerets of males make a rapid PS movement during each flexion of the tail. This movement is caused by a burst of spikes in one swimmeret motor unit that occurs simultaneously with each burst in the flexor motor units. Thus, in this specific behavior, movements of these swimmerets are commandeered to reinforce a different form of swimming.
8. Conclusions
The research efforts of the last fifty years have produced a thorough description of the behavioral and neural properties of the crustacean swimmeret system, and detailed descriptions of its neuronal components. Because the modules that control swimmerets are anatomically separated into left and right pairs in each ganglion and, in macruran crustaceans, the ganglia are separated by several millimeters, it is possible to examine individual modules and the circuit that coordinates them more easily than in other motor systems where segmental circuits are compressed together by developmental condensation of the CNS. The numbers of different neurons in the system are intermediate between small systems like the crustacean stomatogastric ganglion and the spinal cords of fish, amphibia, and reptiles.
Five factors make this system exceptionally suitable for computational and experimental investigation of neural mechanisms of coordination . 1. The system will express in vitro the periodic motor pattern that normally drives cycles of swimmeret movements during forward swimming. 2. The intersegmental neurons which encode information that is necessary and sufficient for normal coordination have been identified, and their activity can be recorded. Individual coordinating neurons affect the phase and strength of motor output from their target modules, and recordings from these neurons then gives access to this coded information. 3. The local commissural neurons that integrate this coordinating information and tune the phase of each swimmeret are known. 4. The complete set of synaptic connections between coordinating neurons and these commissural neurons have been described. 5. The synaptic connections onto each local pattern-generating circuit through which coordinating information tunes the circuit's phase have been discovered. The ensemble of these results form a complete map of this intersegmental circuit (Fig. 9).
Compared with our knowledge of the local pattern-generating circuits and the coordinating circuit, our knowledge of mechanisms of sensory modulation of swimmeret movements is quite sparse. During each cycle of movements, proprioceptive feedback from swimmerets to the modules controlling them clearly occurs, but how these sensory neurons connect with the system and how their information is gated and integrated during cycles of movements has only been sketched. Swimmerets also make drastic asymmetric righting movements in response to rolling the animal's body about its long axis, but how gravitational information encoded by the statocysts in the head reaches swimmeret modules is unknown. Studying the neural basis of these equilibrium reactions may contribute new, broadly-applicable insights into the cellular mechanisms of stability and balance.
The largest immediate gap in our understanding of this system is how the structure of the coordinating circuit produces the posterior-to-anterior phase progression characteristic of coordinated swimmeret movements. A new generation of conductance-based models of local pattern-generation and the coordinating circuit will be necessary before we can grasp the significance of the gradients of synaptic strength and the integration of information by ComInt 1 neurons to the system's performance. These new models will also be the framework within which new results about proprioceptive modulation and descending statocyst information can best be integrated into our current understanding of the system's properties.
Finally, how does the system maintain a constant intersegmental phase if period changes in response to changes in excitation? The evidence from non-uniform excitation experiments is that local levels of excitation determine the “intrinsic” period of the local pattern-generating circuit and the strengths of motor bursts, and adjust local encoding of coordinating information. But, what cellular and synaptic properties are affected by these changes in excitation, and how do these altered properties combine to stabilize phase? These questions arise in any multisegmental motor system, but further experimental and computational analysis of the swimmeret coordinating circuit may let us begin to answer them.
Highlights.
A modular nervous system with an intersegmental coordinating circuit that enables effective locomotion.
Neural substrates of encoding and decoding essential coordinating information
A comprehensive review of this motor system
Acknowledgments
We thank Cynthia Weller for reading the manuscript critically and assisting in preparing figures, and T. Michael Wright for critical reading and helping to resolve correct attribution of ideas. William Herrera, Jessie Schwarz, Angela Trinh, Mattias Karlsson, and Carolyn Sherff contributed unpublished data to this review. Our research has been supported by NIH grant NS04-8068, by NSF grant 0905063, and by DFG grant SM 206/3-1.
Abbreviations List
- A1, A2,…, A6
Abdominal ganglion 1, abdominal ganglion 2, through abdominal ganglion 6
- ASCE
Ascending coordinating neuron, early
- ASCL
Ascending coordinating neuron, late
- CBCO
Coxo-basal chordotonal organ
- CCAP
Crustacean cardioactive peptide
- ComInt 1
Commissural interneuron 1
- ComInt 2
Commissural interneurons 2
- CR
Cuticular-ridge receptor
- DA
Dopamine
- DC1
Dorsal commissure 1
- DF
Distal feathered hairs
- DSC
Descending coordinating neuron
- EA,EB,…,EE
Excitatory command axon A, excitatory command axon B, through excitatory command axon E
- Int 1A
Interneuron 1A
- Int 1B
Interneuron 1B
- Int 2A
Interneuron 2A
- IA, IC, IE
Inhibitory command axon A, inhibitory command axon C, inhibitory command axon E
- LG
Lateral Giant axon
- LN
Lateral Neuropil
- M9, M10
Muscle 9, muscle 10
- ML
Morris-Lecar model
- MnT
Minuscule tract
- N1
Nerve 1 of each abdominal ganglion; the swimmeret nerve
- N1a
Anterior branch of N1
- N1p
Posterior branch of N1
- NSSR
non-spiking stretch receptor
- NSSR-A, NSSR-P
NSSR anterior, NSSR posterior
- OA
Octopamine
- PF
Proximal feathered hairs
- PRC
Phase-response curve
- PS
Power-stroke
- PSE
Power-stroke excitor neuron
- PSI
Power-stroke inhibitor neuron
- RPCH
Red pigment concentrating hormone
- RS
Return-stroke
- RSE
Return-stroke excitor neuron
- RSI
Return stroke inhibitor neuron
- S1, S2
Strand 1, Strand 2
- SG
Segmental Giant neuron
- T1, T2,…, T5
Thoracic ganglion 1, thoracic ganglion 2, through thoracic ganglion 5
- WF
Wide-field receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo LD. Ph D Thesis. University of California; Davis: 1990. Control of the expression of a simple behavior: Activation and inhibition of the crayfish swimmeret rhythm by command neurons and their transmitters; p. 172. http://proquest.umi.com/ [Google Scholar]
- Acevedo LD, Hall WM, Mulloney B. Proctolin and excitation of the crayfish swimmeret system. J Comp Neurol. 1994;345:612–627. doi: 10.1002/cne.903450411. [DOI] [PubMed] [Google Scholar]
- Atwood HL. Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol. 1976;7:291–391. doi: 10.1016/0301-0082(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Wiersma CAG. Command interneurons in the crayfish central neurons system. J Exp Biol. 1967;46:249–261. doi: 10.1242/jeb.46.2.249. [DOI] [PubMed] [Google Scholar]
- Ayali A, Johnson BR, Harris-Warrick RM. Dopamine modulated graded and spike-evoked synaptic inhibition independently at single synapses in pyloric network of lobster. J Neurophysiol. 1998;79:2063–2069. doi: 10.1152/jn.1998.79.4.2063. [DOI] [PubMed] [Google Scholar]
- Ayers JL, Davis WJ. Neuronal control of locomotion in the lobster, Homarus americanus I. Motor programs for forward and backward walking. J Comp Physiol [A] 1977;115:1–28. [Google Scholar]
- Barker DL, Herbert E, Hildebrand JG, Kravitz EA. Acetylcholine and lobster sensory neurones. J Physiol (Lond) 1972a;226:205–229. doi: 10.1113/jphysiol.1972.sp009981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DL, Kushner PD, Hooper NK. Synthesis of dopamine and octopamine in the crustacean stomatogastric nervous system. Brain Res. 1979;161:99–113. doi: 10.1016/0006-8993(79)90198-7. [DOI] [PubMed] [Google Scholar]
- Barker DL, Molinoff PB, Kravitz EA. Octopamine in the Lobster Nervous System. Nature New Bio. 1972b;236:61–63. doi: 10.1038/newbio236061a0. [DOI] [PubMed] [Google Scholar]
- Barthe JY, Bévengut M, Clarac F. The swimmeret rhythm and its relationships with postural and locomotor activity in the isolated nervous system of the crayfish Procambarus clarkii. J Exp Biol. 1991;157:205–226. [Google Scholar]
- Barthe JY, Bevengut M, Clarac F. In vitro, proctolin and serotonin induced modulations of the abdominal motor system activities in crayfish. Brain Res. 1993;623:101–109. doi: 10.1016/0006-8993(93)90016-g. [DOI] [PubMed] [Google Scholar]
- Barthe JY, Mons N, Cattaert D, Geffard M, Clarac F. Dopamine and motor activity in the lobster Homarus gammarus. Brain Res. 1989;497:368–373. doi: 10.1016/0006-8993(89)90282-5. [DOI] [PubMed] [Google Scholar]
- Bässler U. On the definition of central pattern generator and its sensory control. Biol Cybern. 1986;54:65–69. [Google Scholar]
- Bässler U. Functional principles of pattern generation for walking movements of stick insect forelegs: the role of femoral chordotonal organ afferences. J Exp Biol. 1988;136:125–147. [Google Scholar]
- Bent SA, Chapple WD. Peripheral and central asymmetry in the swimmeret system of the hermit crab, Pagurus pollicarus. J Comp Physiol [A] 1977a;118:75–92. [Google Scholar]
- Bent SA, Chapple WD. Simplification of swimmeret musculature and innervation in the hermit crab, Pagurus pollicarus, in comparison to macrurans. J Comp Physiol [A] 1977b;118:61–74. [Google Scholar]
- Blitz DM, Nusbaum MP. Neural flexibility in a small sensorimotor system. Cur Opin Neurobiol. 2011;21:1–9. doi: 10.1016/j.conb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun G, Mulloney B. Cholinergic modulation of the swimmeret system in crayfish. J Neurophysiol. 1993;70:2391–2398. doi: 10.1152/jn.1993.70.6.2391. [DOI] [PubMed] [Google Scholar]
- Braun G, Mulloney B. Acetylcholinesterase activity in neurons of crayfish abdominal ganglia. J Comp Neurol. 1994;350:272–280. doi: 10.1002/cne.903500210. [DOI] [PubMed] [Google Scholar]
- Braun G, Mulloney B. Coordination in the crayfish swimmeret system: Differential excitation causes changes in intersegmental phase. J Neurophysiol. 1995;73:880–885. doi: 10.1152/jn.1995.73.2.880. [DOI] [PubMed] [Google Scholar]
- Burdohan JA, Larimer JL. Interneurons involved in the control of multiple motor centers in crayfish. J Exp Zool. 1995;273:204–215. doi: 10.1002/jez.1402730305. [DOI] [PubMed] [Google Scholar]
- Büschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Araque A, Buño W, Clarac F. Nicotinic and muscarinic activation of motoneurons in the crayfish locomotor network. J Neurophysiol. 1994;72:1622–1633. doi: 10.1152/jn.1994.72.4.1622. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Barthe JY, Neil DM, Clarac F. Remote control of the swimmeret central pattern generator in crayfish (Procambarus clarkii and Pacifastacus leniusculus): effect of a walking leg proprioceptor. J Exp Biol. 1992;169:181–206. [Google Scholar]
- Cattaert D, Clarac F. Influence of walking on swimmeret beating in the lobster Homarus gammarus. J Neurobiol. 1983;14:421–439. doi: 10.1002/neu.480140603. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Clarac F. Rami motor neurons and motor control of the swimmeret system of Homarus gammarus. J Comp Physiol [A] 1987a;160:55–68. [Google Scholar]
- Cattaert D, Clarac F. Rami motor neurons and motor control of the swimmeret system of Homarus gammarus. J Comp Physiol [A] 1987b;160:55–68. [Google Scholar]
- Cattaert D, Clarac F, Neil DM. Anatomical and physiological organization of the swimmeret system of the spiny lobster Jasus lalandii as adaptive components of the tail flick. J Comp Physiol [A] 1988;162:187–200. [Google Scholar]
- Cattaert D, Le Ray D. Adaptive motor control in crayfish. Prog Neurobiol. 2001;63:199–240. doi: 10.1016/s0301-0082(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Chrachri A. Ionic currents in identified swimmeret motor neurones of the crayfish Pacifastacus leniusculus. J Exp Biol. 1995;198:1483–1492. doi: 10.1242/jeb.198.7.1483. [DOI] [PubMed] [Google Scholar]
- Chrachri A, Clarac F. Fictive locomotion in the fourth thoracic ganglion of the crayfish. J Neurosci. 1990;10:707–719. doi: 10.1523/JNEUROSCI.10-03-00707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrachri A, Neil DM. Interaction and synchronization between two abdominal motor systems in crayfish. J Neurophysiol. 1993;69:1373–1383. doi: 10.1152/jn.1993.69.5.1373. [DOI] [PubMed] [Google Scholar]
- Chrachri A, Neil DM, Mulloney B. State-dependent responses of two motor systems in the crayfish, Pacifastacus leniusculus. J Comp Physiol [A] 1994;175:371–380. doi: 10.1007/BF00192996. [DOI] [PubMed] [Google Scholar]
- Clarac F. Some historical reflections on the neural control of locomotion. Brain Res Revs. 2008;57:13–21. doi: 10.1016/j.brainresrev.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Wallén P. The neuronal correlate of locomotion in fish: “Fictive swimming” induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. The function of receptors in the statocyst of the lobster Homarus americanus. J Physiol (Lond) 1955;130:9–34. doi: 10.1113/jphysiol.1955.sp005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ. The response patterns of single receptors in the crustacean statocyst. Proc Roy Soc Lond B. 1960;152:30–49. doi: 10.1098/rspb.1960.0020. [DOI] [PubMed] [Google Scholar]
- Connor JA. Neural repetitive firing: A comparative study of membrane propertie s of crustacean walking leg axons. J Neurophysiol. 1975;38:922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- Connor JA, Walter D, McKown R. Neural repetitive firing: Modifications of the Hodgkin-Huxley axon suggested by experimental results from crustacean axons. Biophys J. 1977;18:81–102. doi: 10.1016/S0006-3495(77)85598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournil I, Casasnovas B, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus .2 Dopamine- immunoreactive neurons and development of the nervous system. J Comp Neurol. 1995;362:1–16. doi: 10.1002/cne.903620102. [DOI] [PubMed] [Google Scholar]
- Cournil I, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus. I Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the nervous system of the juvenile. J Comp Neurol. 1994;344:455–469. doi: 10.1002/cne.903440308. [DOI] [PubMed] [Google Scholar]
- Crapse T, Sommer M. Corollary discharge across the animal kingdom. Nature Reviews Neuroscience. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WJ. Lobster righting responses and their neural control. Proc Roy Soc Lond B. 1968a;70:435–456. doi: 10.1098/rspb.1968.0049. [DOI] [PubMed] [Google Scholar]
- Davis WJ. Quantitative analysis of swimmeret beating in the lobster. J Exp Biol. 1968b;48:643–662. [Google Scholar]
- Davis WJ. The neuromuscular basis of lobster swimmeret beating. J Exp Zool. 1968c;168:363–378. [Google Scholar]
- Davis WJ. Neural control of swimmeret beating in the lobster. J Exp Biol. 1969a;50:99–117. doi: 10.1242/jeb.50.1.99. [DOI] [PubMed] [Google Scholar]
- Davis WJ. Reflex organization in the swimmeret system of the lobster. I. Intrasegmental reflexes. J Exp Biol. 1969b;51:547–564. [Google Scholar]
- Davis WJ. Reflex organization in the swimmeret system of the lobster. II. Reflex dynamics. J Exp Biol. 1969c;51:565–574. [Google Scholar]
- Davis WJ. Motoneuron morphology and synaptic contacts: Determination by intracellular dye injection. Science. 1970;168:1358–1360. doi: 10.1126/science.168.3937.1358. [DOI] [PubMed] [Google Scholar]
- Davis WJ. Functional significance of motoneuron size and soma position in swimmeret system of the lobster. J Neurophysiol. 1971a;34:274–288. doi: 10.1152/jn.1971.34.2.274. [DOI] [PubMed] [Google Scholar]
- Davis WJ. The Integrative Action of the Nervous System in Crustacean Equilibrium Reaction. In: Gordon SA, Cohen MJ, editors. Gravity and the Organism. Chicago Press; 1971b. pp. 237–250. [Google Scholar]
- Davis WJ, Kennedy D. Command interneurons controlling swimmeret movements in the lobster I. Types of effects on motorneurons. J Neurophysiol. 1972a;35:1–12. doi: 10.1152/jn.1972.35.1.1. [DOI] [PubMed] [Google Scholar]
- Davis WJ, Kennedy D. Command interneurons controlling swimmeret movements in the lobster. II. Interaction of effects on motor neuron. J Neurophysiol. 1972b;35:13–19. doi: 10.1152/jn.1972.35.1.13. [DOI] [PubMed] [Google Scholar]
- Davis WJ, Kennedy D. Command interneurons controlling swimmeret movements in the lobster. III. Temporal relationships among bursts in different motorneurons. J Neurophysiol. 1972c;35:20–29. doi: 10.1152/jn.1972.35.1.20. [DOI] [PubMed] [Google Scholar]
- Davis WJ, Murphey RK. Discharge patterns of swimmeret motoneurons in the lobster, simulated with a digital computer. J Exp Biol. 1969;50:119–128. doi: 10.1242/jeb.50.1.119. [DOI] [PubMed] [Google Scholar]
- Deller SRT, MacMillan DL. Entrainment of the swimmeret rhythm of the crayfish to controlled movements of some of the appendages. J Exp Biol. 1989;144:257–278. [Google Scholar]
- Dickinson PS, Marder E. Peptidergic modulation of a multioscillator system in the lobster. I. Activation of the cardiac sac motor pattern by the neuropeptides proctolin and red pigment-concentrating hormone. J Neurophysiol. 1989;61(4):833–844. doi: 10.1152/jn.1989.61.4.833. [DOI] [PubMed] [Google Scholar]
- Drummond JM, Jackson D, MacMillan DL. The result of evolutionary limb loss on the complement of motor neurons in a crayfish limb nerve revealed by serial homology. Mar Fresh Behav Physiol. 1998;31:123–132. [Google Scholar]
- Drummond JM, MacMillan DL. The abdominal motor system of the crayfish, Cherax destructor. I. Morphology and physiology of the superficial extensor motor neurons. J Comp Physiol [A] 1998;183:583–601. [Google Scholar]
- Dudel J, Kuffler SW. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol (Lond) 1961;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M, Rapus J, Nürnberger A, Penzlin H. A new specific antibody reveals octopamine-like immunoreactivity in cockroach ventral nerve cord. J Comp Neurol. 1992;322:1–15. doi: 10.1002/cne.903220102. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–161. [Google Scholar]
- El Manira A, Cattaert D, Clarac F. Monosynaptic connections mediate resistance reflex in crayfish (Procambarus clarkii) walking legs. J Comp Physiol [A] 1991a;168:337–349. [Google Scholar]
- El Manira A, DiCaprio RA, Cattaert D, Clarac F. Monosynaptic interjoint reflexes and their central modulation during fictive locomotion in crayfish. Eur J Neurosci. 1991b;3:1219–1231. doi: 10.1111/j.1460-9568.1991.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Florey E. Acetylcholine as sensory transmitter in crustacea. New evidence from experiments demonstrating release of ACh during sensory stimulation. J Comp Physiol [A] 1973;83:1–16. [Google Scholar]
- Freud S. Uber den Bau der Nervenfasern und Nervenzellen beim Flusskrebs. 3. Vol. 85. Sitzungsber d k Akad der Wissensch; Wien: 1882. pp. 9–46. [Google Scholar]
- Friesen WO, Stent GS. Neural circuits for generating rhythmic movements. Annu Rev Biophys Biophys Chem. 1978;7:37–61. doi: 10.1146/annurev.bb.07.060178.000345. [DOI] [PubMed] [Google Scholar]
- Heitler WJ. Coupled motoneurones are part of the crayfish swimmeret central oscillator. Nature. 1978;275:231–234. doi: 10.1038/275231a0. [DOI] [PubMed] [Google Scholar]
- Heitler WJ. Neural mechanisms of central pattern generation in the crayfish swimmeret system. Adv Physiol Sci. 1981;23:369–383. [Google Scholar]
- Heitler WJ. Non-spiking stretch receptors in the crayfish swimmeret system. J Exp Biol. 1982;96:355–366. doi: 10.1242/jeb.114.1.521. [DOI] [PubMed] [Google Scholar]
- Heitler WJ. The control of rhythmic limb movements in crustacea. Symp Soc Exp Biol. 1983;37:351–382. [PubMed] [Google Scholar]
- Heitler WJ. Motor programme switching in the crayfish swimmeret system. J Exp Biol. 1985;114:521–549. doi: 10.1242/jeb.114.1.521. [DOI] [PubMed] [Google Scholar]
- Heitler WJ. Aspects of sensory integration in the crayfish swimmeret system. J Exp Biol. 1986;120:387–402. [Google Scholar]
- Heitler WJ, Cobb JLS, Fraser K. Ultrastructure of the segmental giant neuron of crayfish. J Neurocytol. 1985;14:921–941. doi: 10.1007/BF01224805. [DOI] [PubMed] [Google Scholar]
- Heitler WJ, Darrig S. The segmental giant neurone of the signal crayfish, Pacifastacus leniusculus, and its interactions with abdominal fast flexor and swimmeret motoneurones. J Exp Biol. 1986;121:55–75. [Google Scholar]
- Heitler WJ, Pearson KG. Non-spiking interactions and local interneurones in the central pattern generator of the crayfish swimmeret system. Brain Res. 1980;187:206–211. doi: 10.1016/0006-8993(80)90506-5. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Barker DL, Herbert E, Kravitz EA. Screening for neurotransmitters: A rapid radiochemical procedure. J Neurobiol. 1971;2:231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- Hill AAV, Masino MA, Calabrese RL. Intersegmental coordination of rhythmic motor patterns. J Neurophysiol. 2003;90:531–538. doi: 10.1152/jn.00338.2003. [DOI] [PubMed] [Google Scholar]
- Hughes GM, Wiersma CAG. The co-ordination of swimmeret movements in the crayfish, Procambarus clarkii. J Exp Biol. 1960;37:657–670. [Google Scholar]
- Huxley TH. The Crayfish: An Introduction to the Study of Zoology. MIT Press; Cambridge, MA: 1880. [Google Scholar]
- Ikeda K, Wiersma CAG. Autogenic rhythmicity in the abdominal ganglion of the crayfish: The control of swimmeret movements. Comp Biochem Physiol. 1964;12:107–115. doi: 10.1016/0010-406x(64)90053-2. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kopell N. Local network parameters can affect inter-network phase lags in central pattern generators. J Math Biol. 2006;52:115–140. doi: 10.1007/s00285-005-0348-0. [DOI] [PubMed] [Google Scholar]
- Jones SR, Mulloney B, Kaper TJ, Kopell N. Coordination of cellular pattern-generating circuits that control limb movements: The sources of stable differences in intersegmental phase. J Neurosci. 2003;23:3457–3468. doi: 10.1523/JNEUROSCI.23-08-03457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim W. Das Nervensystem von Astacus fluviatus. Ein Beitrag zur Morphologie der Dekapoden. Z wiss Zool. 1915;113:485–545. [Google Scholar]
- Kennedy D, Evoy WH, Dane B, Hanawalt JT. The central nervous organization underlying control of antagonistic muscles in the crayfish. II. Coding of position by command fibers. J Exp Zool. 1967;165:239–248. [Google Scholar]
- Killian KA, Page CH. Mechanosensory afferents innervating the swimmerets of the lobster. I. Afferents activated by cuticular deformation. J Comp Physiol [A] 1992a;170:491–500. doi: 10.1007/BF00191464. [DOI] [PubMed] [Google Scholar]
- Killian KA, Page CH. Mechanosensory afferents innervating the swimmerets of the lobster. II. Afferents activated by hair deflection. J Comp Physiol [A] 1992b;170:501–508. doi: 10.1007/BF00191465. [DOI] [PubMed] [Google Scholar]
- Kirk MD, Govind CK. Early innervation of abdominal swimmeret muscles in developing lobsters. J Exp Zool. 1992;261:298–309. doi: 10.1002/jez.1402610309. [DOI] [PubMed] [Google Scholar]
- Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E. Invertebrate synapsins: A single gene codes for several isoforms in Drosophila. J Neurosci. 1996;10:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol. 1999;81:29–38. doi: 10.1152/jn.1999.81.1.29. [DOI] [PubMed] [Google Scholar]
- Knox PC, Neil DM. The coordinated action of abdominal postural and swimmeret motor systems in relation to body tilt in the pitch plane in the norway lobster Nephrops norvegicus. J Exp Biol. 1991;155:605–627. [Google Scholar]
- Kopell N, Ermentrout GB. Symmetry and phaselocking in chains of weekly coupled oscillators. Comm Pure Appl Math. 1986;39:623–660. [Google Scholar]
- Kopell N, Ermentrout GB. Coupled oscillators and the design of central pattern generators. Math Biosci. 1988;90:87–109. [Google Scholar]
- Kotak VC, Page CH. Tactile stimulation of the swimmeret alters motor programs for abdominal posture in the lobster Homarus americanus. J Comp Physiol [A] 1986;158:225–233. doi: 10.1007/BF01338565. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Evans PD, Talamo BR, Wallace BG, Battelle BA. Octopamine neurons in lobsters: Location, morphology, release of octopamine and possible physiological role. Cold Spring Harbor Symp Quant Biol. 1976;40:127–133. doi: 10.1101/sqb.1976.040.01.014. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Glusman S, Harris-Warrick RM, Livingstone MS, Schwarz T. Amines and a peptide as neurohormones in lobsters: Actions on neuro muscular preparations and preliminary behavioral studies. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Weiss KR. The command neuron concept. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- Laverack MS, MacMillan DL, Neil DM. A comparison of beating parameters in larval and post-larval locomotor systems of the lobster Homarus gammarus (L.) Phil Trans Roy Soc Lond B. 1976;274:87–99. doi: 10.1098/rstb.1976.0040. [DOI] [PubMed] [Google Scholar]
- Leise EM, Hall WM, Mulloney B. Functional organization of crayfish abdominal ganglia: I. The flexor systems. J Comp Neurol. 1986;253:25–45. doi: 10.1002/cne.902530104. [DOI] [PubMed] [Google Scholar]
- Leise EM, Hall WM, Mulloney B. Functional organization of crayfish abdominal ganglia: II. Sensory afferents and extensor motor neurons. J Comp Neurol. 1987;266:495–518. doi: 10.1002/cne.902660405. [DOI] [PubMed] [Google Scholar]
- MacMillan DL. A physiological analysis of walking in the American lobster (Homarus americanus) Phil Trans Roy Soc Lond B. 1975;270:1–59. doi: 10.1098/rstb.1975.0003. [DOI] [PubMed] [Google Scholar]
- MacMillan DL, Deller SRT. Sensory systems of the swimmerets of the crayfish Cherax destructor and their effectiveness in entraining the swimmeret rhythm. J Exp Biol. 1989;144:279–301. [Google Scholar]
- Marder E. Acetylcholine as an excitory neuromuscular transmitter in the stomatogastric system of the lobster. Nature. 1974;251:730–731. doi: 10.1038/251730a0. [DOI] [PubMed] [Google Scholar]
- Marder E. Cholinergic motor neurons in the stomatogastric system of the lobster. J Physiol (Lond) 1976;257:63–86. doi: 10.1113/jphysiol.1976.sp011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino MA, Calabrese RL. Period differences between segmental oscillators produce intersegmental phase differences in the leech heartbeat timing network. J Neurophysiol. 2002;87:1603–1615. doi: 10.1152/jn.00338.2001. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Grillner S. Neural mechanisms of intersegmental coordination in lamprey: Local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol. 1992;67:373–388. doi: 10.1152/jn.1992.67.2.373. [DOI] [PubMed] [Google Scholar]
- Miyan JA, Neil DM. Swimmeret proprioceptors in the lobsters Nephrops norvegicus L. and Homarus gammarus L. J Exp Biol. 1986;126:181–204. [Google Scholar]
- Mulloney B. A test of the excitability-gradient hypothesis in the swimmeret system of crayfish. J Neurosci. 1997;17:1860–1868. doi: 10.1523/JNEUROSCI.17-05-01860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B. During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J Neurosci. 2003;23:5953–5962. doi: 10.1523/JNEUROSCI.23-13-05953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Acevedo LD, Bradbury AG. Modulation of the crayfish swimmeret rhythm by octopamine and the neuropeptide proctolin. J Neurophysiol. 1987;58:584–597. doi: 10.1152/jn.1987.58.3.584. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. Local and intersegmental interactions of coordinating neurons and local circuits in the swimmeret system. J Neurophysiol. 2007a;98:405–413. doi: 10.1152/jn.00345.2007. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. Not by spikes alone: Responses of coordinating neurons and the swimmeret system to local differences in excitation. J Neurophysiol. 2007b;97:436–450. doi: 10.1152/jn.00580.2006. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. GABAergic neurons in the crayfish nervous system: An immunocytochemical census of the segmental ganglia and stomatogastric system. J Comp Neurol. 1990;291:383–394. doi: 10.1002/cne.902910306. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. Functional organization of crayfish abdominal ganglia: III. Swimmeret motor neurons. J Comp Neurol. 2000;419:233–243. doi: 10.1002/(sici)1096-9861(20000403)419:2<233::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. Local commissural interneurons integrate information from intersegmental coordinating interneurons. J Comp Neurol. 2003;466:366–376. doi: 10.1002/cne.10885. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Harness PI, Hall WM. Bursts of information: coordinating interneurons encode multiple parameters of a periodic motor pattern. J Neurophysiol. 2006;95:850–861. doi: 10.1152/jn.00939.2005. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Murchison D, Chrachri A. Modular organization of pattern-generating circuits in a segmental motor system: the swimmerets of crayfish. Semin Neurosci. 1993a;5:49–57. [Google Scholar]
- Mulloney B, Namba H, Agricola HJ, Hall WM. Modulation of force during locomotion: differential action of Crustacean Cardioactive Peptide on power-stroke and return-stroke motor neurons. J Neurosci. 1997;17:6872–6883. doi: 10.1523/JNEUROSCI.17-18-06872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Rapus J, Eckert M, Hall WM. Projections of octopaminergic neurons in the CNS of crayfish. Pacifastacus leniusculus. 1993b:127.2. [Google Scholar]
- Mulloney B, Skinner FK, Namba H, Hall WM. Intersegmental coordination of swimmeret movements: Mathematical models and neural circuits. In: Kiehn O, Hultborn H, Kudo N, Jordan LM, Harris-Warrick RM, editors. Neuronal mechanisms for generating locomotor activity. 1998. pp. 266–280. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Smarandache CR. Fifty years of CPGs: Two neuroethological papers that shaped the course of neuroscience. Frontiers in Behavioral Neuroscience. 2010;4(45):1–8. doi: 10.3389/fnbeh.2010.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Tschuluun N, Hall WM. Architectonics of crayfish ganglia. Microscopy Res and Tech. 2003;60:253–265. doi: 10.1002/jemt.10265. [DOI] [PubMed] [Google Scholar]
- Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. J Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- Murchison D, Larimer JL. Dual motor output interneurons in the abdominal ganglia of the crayfish Procambarus clarkii: Synaptic activation of motor outputs in both the swimmeret and abdominal positioning systems by single interneurons. J Exp Biol. 1990;150:269–293. doi: 10.1242/jeb.150.1.269. [DOI] [PubMed] [Google Scholar]
- Murchison D, Larimer JL. Synaptic interactions among neurons that coordinate swimmeret and abdominal movements in the crayfish. J Comp Physiol [A] 1992;170:739–747. doi: 10.1007/BF00198985. [DOI] [PubMed] [Google Scholar]
- Nagayama T. GABAergic and glutamatergic inhibition of nonspiking local interneurons in the terminal abdominal ganglion of the crayfish. 2005. pp. 66–75. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Mulloney B. A presynaptic basis for gradients in strength of synapses between different abdominal stretch-receptor axons and their common target neurons. J Neurosci. 2001;21:1645–1655. doi: 10.1523/JNEUROSCI.21-05-01645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba H, Mulloney B. Coordination of limb movements: Three types of intersegmental interneurons in the swimmeret system, and their responses to changes in excitation. J Neurophysiol. 1999;81:2437–2450. doi: 10.1152/jn.1999.81.5.2437. [DOI] [PubMed] [Google Scholar]
- Neil DM, Miyan JA. Phase-dependent modulation of auxiliary swimmeret muscle activity in the equilibrium reactions of the Norway Lobster, Nephrops norvegicus L. J Exp Biol. 1986;126:157–179. [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Adams ME. Pentapeptide (proctolin) associated with an identified neuron. Science. 1981;213:567–569. doi: 10.1126/science.6113690. [DOI] [PubMed] [Google Scholar]
- Olsen ØH, Murray-Smith D. Quantifying periodic activity in central pattern generators: The crayfish swimmeret. J Neurosci Meth. 1993;50:25–35. doi: 10.1016/0165-0270(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Kravitz EA, Potter DD. Physiological and chemical architecture of a lobster ganglion with particular reference to GABA and glutamate. J Neurophysiol. 1967;30:725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- Pabst H, Kennedy D. Cutaneous mechanoreceptors influencing motor output in the crayfish abdomen. Z vergleich Physiol. 1967;57:190–208. [Google Scholar]
- Page CH. Sexual dimorphism of pleopod motor neurons in lobster. Brain Res. 1985;339:154–157. doi: 10.1016/0006-8993(85)90636-5. [DOI] [PubMed] [Google Scholar]
- Paul DH. Decremental conduction over “giant” afferent processes in an arthropod. Science. 1972;176:680–683. doi: 10.1126/science.176.4035.680. [DOI] [PubMed] [Google Scholar]
- Paul DH. Nonspiking stretch receptors of the crayfish swimmeret receive an efference copy of the central motor pattern for the swimmeret. J Exp Biol. 1989;141:257–264. [Google Scholar]
- Paul DH, Mulloney B. Local interneurons in the swimmeret system of the crayfish. J Comp Physiol [A] 1985a;156:489–502. [Google Scholar]
- Paul DH, Mulloney B. Nonspiking local interneuron in the motor pattern generator for the crayfish swimmeret. J Neurophysiol. 1985b;54:28–39. doi: 10.1152/jn.1985.54.1.28. [DOI] [PubMed] [Google Scholar]
- Paul DH, Mulloney B. Intersegmental coordination of swimmeret rhythms in isolated nerve cords of crayfish. J Comp Physiol [A] 1986;158:215–224. [Google Scholar]
- Poon MLT. Induction of swimming in lamprey by L-DOPA and amino acids. J Comp Physiol [A] 1980;136:337–344. [Google Scholar]
- Retzius G. Zur Kenntniss des Nervensystems der Crustaceen. Samson & Wallin; Stockholm: 1890. [Google Scholar]
- Rinzel J, Ermentrout GB. Analysis of neuronal excitability and oscillations. In: Koch C, Segev I, editors. Methods in Neuronal Modeling. MIT Press; Cambridge MA: 1998. pp. 251–291. [Google Scholar]
- Ripley SH, Bush BMH, Roberts A. Crab muscle receptor which responds without impulses. Nature. 1968;218:1170–1171. doi: 10.1038/2181170a0. [DOI] [PubMed] [Google Scholar]
- Schmidt W. Die Muskulatur von Astacus fluviatilis (Potamobius astacus L.). Ein Beitrag zur Morphologie der Decapoden. Z wiss Zool. 1915;113:165–251. [Google Scholar]
- Schneider H, Trimmer BA, Rapus J, Eckert M, Valentine DE, Kravitz EA. Mapping of octopamine-immunoreactive neurons in the central nervous system of the lobster. J Comp Neurol. 1993;329:129–142. doi: 10.1002/cne.903290109. [DOI] [PubMed] [Google Scholar]
- Schwarz TL, Lee GMH, Siwicki KK, Standaert DG, Kravitz EA. Proctolin in the lobster: The distribution, release, and chemical characterization of a likely neurohormone. J Neurosci. 1984;4:1300–1311. doi: 10.1523/JNEUROSCI.04-05-01300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmer MA, Lewis TJ. The Theory of Weakly Coupled Oscillators. In: Schultheiss N, Prinz AA, Butera RJ, editors. PRCs in Neuroscience: Theory, Experiment and Analysis. Springer Verlag; Heidleberg: 2012. in press. [Google Scholar]
- Sherff CM, Mulloney B. Red Pigment Concentrating Hormone is a modulator of the crayfish swimmeret system. J Exp Biol. 1991;155:21–35. doi: 10.1242/jeb.155.1.21. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Mulloney B. Tests of the motor neuron model of the local pattern-generating circuits in the swimmeret system. J Neurosci. 1996;16:2839–2859. doi: 10.1523/JNEUROSCI.16-08-02839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherff CM, Mulloney B. Passive properties of swimmeret motor neurons. J Neurophysiol. 1997;78:92–102. doi: 10.1152/jn.1997.78.1.92. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Heitler WJ. The neural basis of escape swimming behavior in the squat lobster Galathea strigosa. II. The motor programme and sensory feedback interactions. J Exp Biol. 1985;117:271–289. [Google Scholar]
- Siwicki KK, Bishop CA. Mapping of proctolin-like immunoreactivity in the nervous systems of lobster and crayfish. J Comp Neurol. 1986;243:435–453. doi: 10.1002/cne.902430402. [DOI] [PubMed] [Google Scholar]
- Skinner FK, Kopell N, Mulloney B. How does the crayfish swimmeret system work? Insights from nearest neighbor coupled oscillator models. J Comput Neurosci. 1997;4:151–160. doi: 10.1023/a:1008891328882. [DOI] [PubMed] [Google Scholar]
- Skinner FK, Mulloney B. Intersegmental coordination of limb movements during locomotion: mathematical models predict circuits that drive swimmeret beating. J Neurosci. 1998;18:3831–3842. doi: 10.1523/JNEUROSCI.18-10-03831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner K. The structure of the fourth abdominal ganglion of the crayfish, Procambarus clarkii I. Tracts in the ganglionic core. J Comp Neurol. 1985a;234:168–181. doi: 10.1002/cne.902340204. [DOI] [PubMed] [Google Scholar]
- Skinner K. The structure of the fourth abdominal ganglion of the crayfish, Procambarus clarkii II. Synaptic neuropils. J Comp Neurol. 1985b;234:182–191. doi: 10.1002/cne.902340205. [DOI] [PubMed] [Google Scholar]
- Smarandache CR, Hall WM, Mulloney B. Coordination of rhythmic motor activity by gradients of synaptic strength in a neural circuit that couples modular neural oscillators. J Neurosci. 2009;29:9351–9360. doi: 10.1523/JNEUROSCI.1744-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers ME, Nunnemacher RF. Microanatomy of the ganglionic roots of the abdominal cord of the crayfish, Oronectes virilis. J Comp Neurol. 1970;138:209–218. doi: 10.1002/cne.901380207. [DOI] [PubMed] [Google Scholar]
- Stein PSG. Intersegmental coordination of swimmeret motor neuron activity in crayfish. J Neurophysiol. 1971;34:310–318. doi: 10.1152/jn.1971.34.2.310. [DOI] [PubMed] [Google Scholar]
- Stein PSG. The relationship of interlimb phase to oscillator activity gradients in crayfish. In: Stein RB, Pearson KG, Smith RS, Redford JB, editors. Control of Posture and Locomotion. Plenum; New York: 1973. p. 621. [Google Scholar]
- Stein PSG. Neural control of interappendage phase during locomotion. Am Zool. 1974;14:1003–1016. [Google Scholar]
- Stein PSG. Mechanisms of interlimb phase control. In: Herman RM, editor. Neural Control of Locomotion. Plenum; New York: 1976. pp. 465–487. [Google Scholar]
- Stein PSG. Application of the mathematics of coupled oscillator systems to the analysis of the neural control of locomotion. Fed Proc. 1977;36:2056–2059. [PubMed] [Google Scholar]
- Stein PSG. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Revs. 2008;57:118–124. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RM, Nunnemacher RF. Microanatomy of crayfish thoracic cord and roots. J Comp Neurol. 1968;132:499–518. doi: 10.1002/cne.901320402. [DOI] [PubMed] [Google Scholar]
- Takahata M, Hisada M. Statocyst interneurons in the crayfish, Procambarus clarkii. I. Identification and response characteristics. J Comp Physiol [A] 1982a;149:301–306. [Google Scholar]
- Takahata M, Hisada M. Statocyst interneurons in the crayfish, Procambarus clarkii. II Directional sensitivity and its mechanism. J Comp Physiol [A] 1982b;149:287–300. [Google Scholar]
- Takahata M, Murayama M. Multiple gate control of the descending statocyst-motor pathway in the crayfish Procambarus clarkii Girard. J Comp Physiol [A] 1992;170:463–477. doi: 10.1007/BF00191462. [DOI] [PubMed] [Google Scholar]
- Tatsumi H, Haragashira M, Suzuki R. Interrelations between posture and locomotion in response to body rotation in crayfish. J Comp Physiol [A] 1985;157:509–517. [Google Scholar]
- Tatsumi H, Suzuki R. Phase plane description of crayfish swimmeret oscillator. Biol Cybern. 1983;47:59–68. [Google Scholar]
- Thomas JB, Bastiani MJ, Bate M, Goodman CS. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984;310:203–207. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: disribution in the central nervous system and physiological functions. Microscopy Res and Tech. 2003;60:325–335. doi: 10.1002/jemt.10271. [DOI] [PubMed] [Google Scholar]
- Trube A, Audehm U, Dircksen H. Crustacean cardioactive peptide-immunoreactive neurons in the ventral nervous system of crayfish. J Comp Neurol. 1994;348:80–93. doi: 10.1002/cne.903480104. [DOI] [PubMed] [Google Scholar]
- Tschuluun N, Hall WM, Mulloney B. State-changes in the swimmeret system: a neural circuit that drives locomotion. J Exp Biol. 2009;212:3605–3611. doi: 10.1242/jeb.033621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschuluun N, Hall WM, Mulloney B. Limb movements during locomotion: Tests of a model of an intersegmental coordinating circuit. J Neurosci. 2001;21:7859–7869. doi: 10.1523/JNEUROSCI.21-19-07859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Roberts A. A longitudinal gradient of synaptic drive in the spinal cord of Xenopus embryos and its role in co-ordination of swimming. J Physiol (Lond) 1994;474:393–405. doi: 10.1113/jphysiol.1994.sp020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A, Wiersma CAG. The triple innervation of crayfish muscle and its function in contraction and inhibition. J Exp Biol. 1937;14:448–459. [Google Scholar]
- Vogt G. Functional Anatomy. In: Holdich DM, editor. Biology of Freshwater Crayfish. Blackwell Science; Oxford: 2002. pp. 53–151. [Google Scholar]
- Von Holst E, Mittelstädt H. Das Reafferenzprinzip (Wechselwirkungen zwischen Zentralnervensystem und Peripherie) Naturwissenschaften. 1950;25:464–476. [Google Scholar]
- West L, Jacobs G, Mulloney B. Intrasegmental proprioceptive influences on the period of the swimmeret rhythm in crayfish. J Exp Biol. 1979;82:289–301. doi: 10.1242/jeb.82.1.281. [DOI] [PubMed] [Google Scholar]
- Wiens TJ, Wolf H. The inhibitory motoneurons of crayfish thoracic limbs: identification, structures, and homology with insect common inhibitors. J Comp Neurol. 1993;336:261–278. doi: 10.1002/cne.903360208. [DOI] [PubMed] [Google Scholar]
- Wiersma CAG. On the number of nerve cells in a crustacean nervous system. Acta Physiol Pharmacol Neederlandica. 1957;6:135–142. [PubMed] [Google Scholar]
- Wiersma CAG. The original definition of command neuron. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- Wiersma CAG, Hughes GM. On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii. J Comp Neurol. 1961;116:209–228. doi: 10.1002/cne.901160209. [DOI] [PubMed] [Google Scholar]
- Wiersma CAG, Ikeda K. Interneurons commanding swimmeret movements in the crayfish, Procambarus clarkii. Comp Biochem Physiol. 1964;12:509–525. doi: 10.1016/0010-406x(64)90153-7. [DOI] [PubMed] [Google Scholar]
- Willard AL. Effects of serotonin on the generation of the motor program for swimming in the medicinal leech. J Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Larimer JL. Neural pathways of reflex-evoked behaviors and command systems in the abdomen of the crayfish. J Comp Physiol [A] 1981;143:27–42. [Google Scholar]
- Wilson DM. Inherent asymmetry and reflex modulation of the locust flight motor pattern. J Exp Biol. 1968;48:631–641. doi: 10.1242/jeb.48.3.631. [DOI] [PubMed] [Google Scholar]
- Wine JJ. The structural basis of an innate behavior. J Exp Biol. 1984;112:283–319. [Google Scholar]
- Wine JJ, Mittenthal JE, Kennedy D. The structure of tonic flexor motoneurones in crayfish abdominal ganglia. J Comp Physiol [A] 1974;93:315–335. [Google Scholar]
- Winfree AT. The geometry of biological time. Springer Verlag; Heidelberg: 2001. [Google Scholar]
- Yoshino M, Takahata M, Hisada M. Statocyst control of the uropod movement in response to body rolling in crayfish. J Comp Physiol [A] 1980;139:243–250. [Google Scholar]