Abstract
Treadmill training is known to improve stepping in complete spinal cord injured animals. Few studies have examined whether treadmill training also enhances locomotor recovery in animals following incomplete spinal cord injuries. In the present study, we compared locomotor recovery in trained and untrained rats that received a severe mid-thoracic contusion of the spinal cord. A robotic device was used to train and to test bipedal hindlimb stepping on a treadmill. Training was imposed for 8 weeks. The robotic device supported the weight of the rats and recorded ankle movements in the hindlimbs for movement analyses. Both the trained and untrained rats generated partial weight bearing hindlimb steps after the spinal cord contusion. Dragging during swing was more prevalent in the untrained rats than the trained rats. In addition, only the trained rats performed step cycle trajectories that were similar to normal step cycle trajectories in terms of the trajectory shape and movement velocity characteristics. In contrast, untrained rats executed step cycles that consisted of fast, kick-like movements during forward swing. These findings indicate that spinal cord contused rats can generate partial weight bearing stepping in the absence of treadmill training. The findings also suggest that the effect of treadmill training is to restore normal patterns of hindlimb movements following severe incomplete spinal cord injury in rats.
Keywords: Bipedal, Locomotion, Robot, Kinematics, Velocity
Introduction
Treadmill training has been successfully used to improve the recovery of stepping in animal models of complete spinal cord injury (for review, see Edgerton et al., 2004). For example, spinally transected cats recover full weight bearing hindlimb stepping after undergoing several weeks of treadmill training (Lovely et al., 1986; Barbeau et al., 1987; Forssberg et al.,1980; Lovely et al.,1990; Belanger et al.,1996; de Leon et al., 1998a; Boyce et al., 2007). In contrast, untrained spinally transected cats stumble frequently and thus are unable to maintain weight bearing hindlimb stepping (Lovely et al., 1986; de Leon et al., 1998a; Boyce et al., 2007; de Leon et al., 1999a). Recent studies have reported that treadmill training also improves stepping in rats and mice that receive a complete spinal cord transection (Cha et al., 2007; Cai et al., 2006; de Leon and Acosta, 2006; Kubasak et al., 2005; Fong et al., 2005). Treadmill training improves the consistency of stepping in spinally transected rats (Kubasak et al., 2005) and spinally transected mice (Cai et al., 2006). The spatial and temporal characteristics of the step cycle in rats that are spinally transected as adults are improved by treadmill training (de Leon and Acosta, 2006). It also appears that treadmill training enhances the ability of the rats that are spinally transected as neonates to adjust stepping patterns in response to different weight bearing levels (Cha et al., 2007). Together, these findings indicate that the generation of stepping by neural networks in the lumbar spinal cord is enhanced by treadmill training.
In contrast to the number of studies performed in spinally transected animals, there have been fewer studies of treadmill training in animals following an incomplete spinal cord injury. Two previous studies reported a positive effect of treadmill training on the ability of spinal cord injured rats to perform open field locomotion (Multon et al., 2003; Stevens et al., 2006). Only two studies have used kinematic analyses to measure recovery in trained rats following an incomplete spinal cord injury. Thota et al. (2001) analyzed changes in the joint angles of the hindlimb following a moderate spinal cord contusion in rats. The authors reported that treadmill training improved the range of motion at the hip and ankle joints of the spinally contused rats (Thota et al., 2001). Untrained control rats, however, were not included in the study and so it is unclear if these changes occurred as a result of training or through spontaneous recovery. Fouad et al. (2000) reported that 5 weeks of daily treadmill training had little effect on hindlimb kinematics during treadmill locomotion in rats that received a dorsal transection of the spinal cord. Analyses of hindlimb extension at toe off and paw contact failed to reveal any significant differences between the trained and untrained dorsal spinal cord injured rats. The authors also reported no effect of training on BBB scores, grid walk, narrow beam walking and footprint scores (Fouad et al., 2000). A more recent study reported a positive effect of treadmill training in spinal cord hemisected mice (Goldshmit et al., 2008). Multiple measures of recovery were used, including open field locomotion, grid walking, foot print analyses and kinematic analyses of treadmill stepping. Based on the results of these few studies and the apparent discrepancy in some findings, the question of whether treadmill training enhances stepping recovery in animals with incomplete spinal injury requires further examination.
In the present study, our approach to examining the effects of training following incomplete spinal cord injury was to use a robotic device to assist in both training and assessment of treadmill stepping in rats. The robotic device facilitates training by providing more precise control over hindlimb weight bearing relative to manual training techniques (Timoszyk et al., 2005). The robotic device also enhances assessment capabilities since kinematic analyses can be performed on a large number of limb movements (Cha et al., 2007; de Leon and Acosta, 2006). Previous studies have demonstrated that the robotic device is effective in training and testing stepping performed by spinally transected rats (Cha et al., 2007; de Leon and Acosta, 2006; Timoszyk et al., 2005). A recent study demonstrated that the robotic device can also be effective in assessing locomotor recovery in rats that have a moderate or severe spinal cord contusion injury (Nessler et al., 2006). Therefore, we used the robotic device in the present study to train rats that received a severe spinal cord contusion injury. We then performed an analyses of the hindlimb step cycle to compare stepping in the trained versus untrained spinally contused rats. We also used the robotic device to collect data from intact rats. We found that in the absence of treadmill training, the rats generated stepping movements in their hindlimbs but these movements did not resemble normal movement patterns. Only the rats that received treadmill training recovered movement patterns that were normal in trajectory shape and velocity.
Materials and methods
Experimental design
Twenty-six female Sprague–Dawley rats (8 weeks of age) received a severe spinal cord contusion injury at the mid-thoracic level (T9). Six weeks after the injury (Week 6), the ability of the rats to perform bipedal, hindlimb stepping on a treadmill was tested. A robotic device was used to support the weight of the body while the hindlimbs stepped on a treadmill belt. After baseline testing, 13 of the rats underwent robotic-assisted treadmill training for 5 days/week for 8 weeks and the other 13 rats served as untrained controls. Afterwards, hindlimb stepping performance was re-tested (Week 14). Robotic data were also collected from intact rats (n = 9).
Surgical procedures and animal care
The spinal contusion injury procedures were performed in the Roman Reed Core laboratory at the Reeve Irvine Research Center in the University of California in Irvine. The surgical procedures have been described in detail previously (Nessler et al., 2006). Briefly, the rats were anaesthetized with a ketamine/xylazine mixture (90:10 mg/kg, i.p.). The skin over the spine was shaved and cleaned with betadyne solution. The skin was incised and muscle and connective tissue were dissected to expose the T9–T11 vertebrae. A T9 laminectomy was performed and the animals received a 250 kdyn contusion with the Infinite Horizon Device (Precision Systems & Instrumentation, Lexington KY; Scheff et al., 2003). The behavioral and anatomical characteristics of the particular IH device used by the Reeve Irvine Research Center have been characterized in previous studies (Nessler et al., 2006; Radojicic et al., 2007; Keirstead et al., 2005; Totoiu and Keirstead, 2005). In short, the 250 kdyn impact force results in a severe contusion injury (i.e. large, centrally-located scar with white matter sparing at the lesion site) and typically, a long-term (i.e. >70 days post-injury) recovery of only an 8 BBB score during open field locomotor testing occurs. The connective tissue and muscle were closed with chromic gut and the skin was closed using staples. Carprofen (5 mg/kg) was given sub-cutaneously, post-operatively. Immediately following the surgery, rats were placed on a heating pad until they recovered from anesthesia, whereby they were returned to their cages. One week after the surgery, the rats were transferred to the animal facilities at California State University, Los Angeles for locomotor testing and training. The animals were monitored twice daily throughout the post-injury survival period for general health. Bladders were manually expressed twice daily for 10–14 days after which the animals developed an automatic bladder voidance reflex. The animals received Baytril (Enroflaxacin 2.5 mg/kg, sub-cutaneously) for 10–14 days after surgery and when necessary, were hydrated with lactated ringers (5 mg/100 g, sub-cutaneously). All procedures were approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
Locomotor testing and training
A robotic device was used (Rodent Robotic Motor Performance System, Robomedica Inc., Irvine, CA) to test and to train hindlimb stepping on a treadmill. The robotic device has been described previously (Timoszyk et al., 2005; Timoszyk et al., 2002) and consists of two light-weight robotic arms, a body weight support system and a variable-speed treadmill. The robotic arms attach to the ankles of the hindlimbs via small loops of neoprene that wrap around the ankle. The robotic arms move in the para-sagittal plane. A computer-controlled, spring-actuated body weight support system controlled the amount of weight on the hindlimbs. Rats were placed in a harness which was secured to the body weight support system using Velcro. All locomotor tests were performed at a treadmill speed of 8 cm s−1 while the robotic device supported 85% of the rat's body weight. During training, the amount of weight support was adjusted (80–95% body weight) to facilitate stepping in each rat. Most rats generated stepping in their hindlimbs independently during training. When stepping was not consistently performed, stepping was initiated by assisting hindlimb movements. The arms of the robotic device moved the ankle in a fixed semi-circular trajectory for several step cycles (de Leon and Acosta, 2006). If robotic assistance was not effective in facilitating ankle dorsiflexion during swing, the trainers manually moved the hindlimbs. Training was performed for 15–20 min/day, 5 days/week.
Data analyses
Ankle movements during all tests were recorded by the robotic arms at 500 Hz and stored on a computer for subsequent analyses as previously described (Timoszyk et al., 2005; Timoszyk et al., 2002). Briefly, toe off and paw contact events were detected based on changes in the horizontal and vertical velocities of the robotic arm end points. The execution of both toe off and paw contact was used to count the number of successful steps and drag steps. Successful steps were defined as step cycles in which the hindpaw was lifted during swing (see “s” in Fig. 1A). Steps cycles with paw dragging, i.e. drag steps, occurred when there was little or no vertical movement in the ankle (see “d” in Fig. 1A). Movement analyses was performed only on successful step cycles. The step cycle trajectory was divided into forward and backward phases (see black and grey lines respectively in Fig. 1B) and the points in the trajectory at which the ankle was highest (maximum height) and lowest (minimum height) were identified (see 2 and 4 in Fig. 1B). The following trajectory characteristics were measured: 1) lift during swing, which was the difference between the height of the ankle at the start of forward movement and the height of the ankle at the maximum height (see 1 to 2 in Fig. 1B); 2) ankle lowering during forward movement, which was the difference in the ankle height from the maximum height to the end of forward movement (see 2 to 3 in Fig. 1B); 3) ankle elevation during stance which was the difference in ankle height from the minimum height to the end of backward movement (see 4 to 5 in Fig. 1B); and 4) peak horizontal and peak vertical velocities during forward and backward movements.
Fig. 1.
Examples of stepping movements recorded by the robotic device in an untrained rat 14 weeks after a spinal contusion. (A) The horizontal (thin lines) and vertical (thick lines) displacement of the ankle is shown. The “s” indicate successful partial weight bearing steps with lift of the hindpaw whereas the “d” indicates steps with paw dragging. (B) The trajectory of the ankle during a single step cycle from one spinally contused rat. For movement analyses, the step cycle was divided into forward movement (black line with black squares) and backward movement (grey line with grey squares). Arrows indicate the direction of movement. At 1, forward movement begins. 2 denotes the point in the trajectory that the ankle reached maximum height. At 3, forward movement ends and backward movement begins. 4 denotes the point in the trajectory that the ankle reached minimum height. At 5, backward movement ends.
A repeated measures ANOVA was used to determine if the average number of step cycles on Week 6 was significantly different than average number of steps on Week 14. Significant differences in the number of steps, trajectory characteristics and velocity characteristics between the trained spinally contused rats, untrained spinally contused rats and intact rats were determined using one-way ANOVA. Bonferroni post-hoc tests were used to identify pairs of groups that were significantly different. Differences between groups were considered statistically significant at p values <0.05. Since not all spinally contused rats performed consistent stepping, only rats that performed at least 15 weight bearing steps were included in the analyses of movement trajectory and velocity (n = 8/13 trained rats; n = 9/13 untrained rats). All statistical analyses were performed using SPSS 13.0 for Windows Software.
Results
Trained and untrained spinally contused rats recovered the ability to perform weight bearing stepping on a treadmill
We used a robotic device to test locomotor ability in trained and untrained spinally contused rats following a severe spinal cord contusion. The baseline test was performed 6 weeks after the contusion. We also used the robotic device to collect data from intact rats (n = 9). During all tests, the robotic device supported 85% of the body weight so that the ability to perform partial weight bearing steps was assessed.
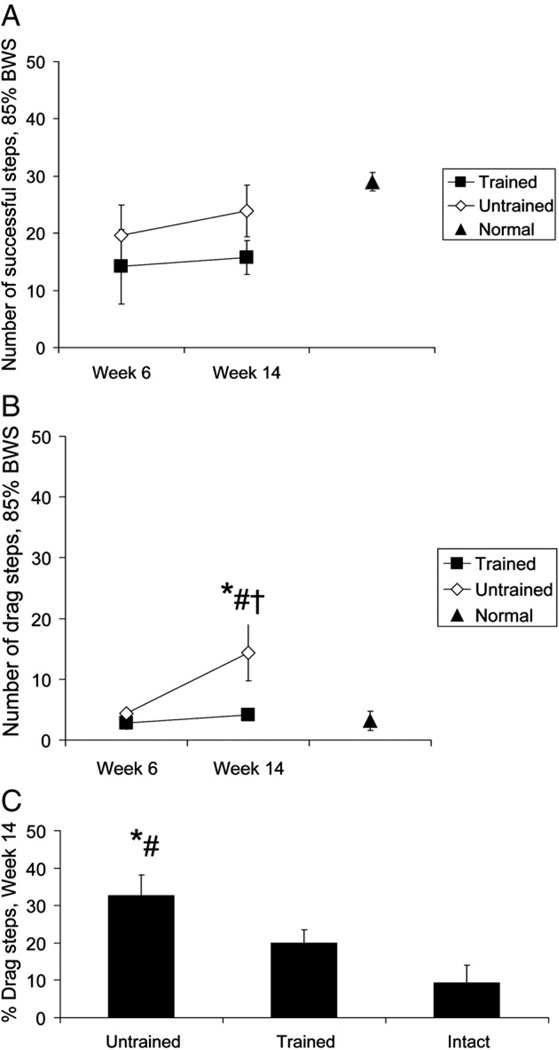
The robotic device was used to count the number of successful steps defined as a step in which the hindpaw was lifted during swing (see “s” in Fig. 1A). The average number of successful steps performed by the trained and untrained rats was not significantly different during baseline tests (see Week 6 in Fig. 2A). When stepping was re-tested 8 weeks after the baseline tests the average number of successful steps increased in the contused rats from Week 6 to Week 14, but this change was not significant (compare Week 6 and Week 14 in Fig. 2A). There were no significant differences in the number of successful steps between the contused rats and intact rats during testing at Week 6 or 14 (compare Week 6 and 14 with triangle in Fig. 2A).
Fig. 2.
Number of steps executed by trained, untrained spinally contused rats and intact rats. The mean number of successful steps (A) and drag steps (B) performed by trained (black squares) and untrained (white diamonds) rats 6 weeks and 14 weeks after spinal contusion are shown. Data from intact animals (black triangle) are also shown. Successful steps are defined as steps in which the paw was lifted during swing (see “s” in Fig. 1A). Drag steps are steps in which the paw dragged during swing (see “d” in Fig. 1A). In (C), the percentage of drag steps relative to the total number of stepping movements is shown. All of the data in Fig. 2 are partial weight bearing steps with 85% of the body weight supported by the robotic device. The average ± standard error is shown (n = 13 trained rats; n = 13 untrained rat; n = 9 intact rats). * indicates significant difference between untrained and trained (p ≤ 0.01), # indicates significant difference between untrained and normal (p ≤ 0.01) and † indicates significant difference between Week 14 and week 6.
We next examined the number of steps in which the hindpaw dragged during swing (see “d” in Fig. 1A). These are abnormal steps and indicate an inability to flex the ipsilateral hindlimb during swing and/or extend the contralateral hindlimb during stance. The average number of drag steps was not significantly different between the trained and untrained spinally contused rats during baseline testing (see Week 6, Fig. 2B). However, the number of drag steps significantly increased in the untrained spinally contused rats from Week 6 to Week 14 (compare diamond Week 6 and Week 14, Fig. 2B; p<0.05). During testing at Week 14, the untrained spinally contused rats performed significantly more drag steps than the trained spinally contused rats (p<0.01) and normal rats (p<0.01; compare Week 14 diamond with Week 14 square and triangle, Fig. 2B). The result was that the percentage of drag steps during Week 14 was significantly greater in the untrained rats relative to the trained and normal rats (Fig. 2C; p<0.01). No other significant differences were found.
Comparison of ankle movements during stepping
We performed an analyses of the movement of the ankle as the rats stepped on the treadmill. Because less than half of the spinally contused rats were capable of consistent stepping during Week 6, we focused our analyses on the data collected during Week 14. We divided the step cycle into two phases based on the forward and backward movements of the ankle (see Fig. 1B). When we examined movement patterns in this manner, it was apparent that the untrained rats moved their ankles in a different pattern than the trained and intact rats. We will first describe differences during forward movements then describe the differences during backward movements.
Forward movements
In the untrained rats, the ankle was lifted as it moved forward (see black line, Fig. 3A). On average, the ankle was lifted 11 ± 1.9 mm from the beginning of forward movement to maximum height (see 1–2, Fig. 3A). After reaching peak height, the ankle moved down only 1.4 ± 0.4 mm lower than maximum height (see 2–3, Fig. 3A). A different movement pattern was observed in the trained and intact rats. First, the ankles in the trained and intact rats moved forward with little upward movement (see black lines, Figs. 3B and C). The ankle was lifted only 3.2 ± 0.9 mm and 2.4 ± 0.9 mm to maximum height in the trained and intact rats respectively (see 1–2 in Figs. 3B and C). Second, after reaching maximum height, the ankle in the trained and untrained rats was lowered 4.6 ± 1.5 mm and 6.6 ± 1.2 mm respectively (see 2–3 in Figs. 3B and C). One-way ANOVA showed significant differences in the amount of lift and the amount of lowering during forward movement between the groups (p < 0.001 for amount of lift and lowering). Post hoc comparisons showed the amount of ankle lift and lowering was significantly different in the untrained rats relative to the trained and intact rats (p < 0.01 for lift and lowering comparisons between untrained vs trained, and untrained vs intact).
Fig. 3.
Average step cycle trajectories in (A) untrained and (B) trained spinally contused rats and (C) intact rats. Forward and backward movements are indicated by the black and white lines respectively. Arrows and the numbers (1–5) indicate the direction and progression of movement (see Fig. 1 for details). The trajectories shown are group averages. Grey areas around the mean trajectory indicate standard error. Only the trajectories from rats that performed at least 15 steps were included in these averages (n = 9 untrained; n = 8 trained; n = 9 intact). The data from the contused rats are from tests performed 14 weeks after the contusion.
Based on velocity measurements, the untrained rats moved their ankles faster during forward movement than the trained and intact rats. For example, the peak horizontal velocity in the untrained rats (268 ± 72 mm s−1) was twice as fast as the peak horizontal velocities in the trained and intact rats (111 ± 20 and 122 ± 68 mm s−1 respectively). The difference in peak vertical velocities was even greater. Untrained rats reached a peak vertical velocity (181 ± 43 mm s−1) that was six times faster than in trained rats (31 ± 12 mm s−1) and nine times faster than in intact rats (20 ± 16 mm s−1). Significant group differences in peak horizontal and vertical velocities were found between the groups with one-way ANOVA (p < 0.01 for peak horizontal and vertical velocities). Post hoc comparisons showed peak horizontal and vertical velocities were significantly faster in the untrained rats relative to the trained and intact rats (p < 0.01 for comparisons of vertical velocities between untrained vs trained and untrained vs intact; p < 0.01 for horizontal velocities between untrained and intact; p < 0.05 for horizontal velocities between untrained and trained).
Differences were also apparent in how velocity changed during forward movement. In all groups, the horizontal velocity increased then decreased as the ankle moved forward (Fig. 4A). However, the untrained rats reached peak horizontal velocity earlier in the step cycle than the trained and intact rats (see circles in Fig. 4A). Furthermore, whereas vertical velocity in the untrained rats rapidly increased then decreased during the first 10% of the step cycle, little change in vertical velocity occurred in the trained and intact rats (Fig. 4B). One-way ANOVA showed significant differences in the onset of peak horizontal velocity between the groups (p < 0.01). Post hoc comparisons showed the onset of peak horizontal velocity was significantly earlier in the untrained rats relative to the trained (p < 0.05) and intact rats (p < 0.01).
Fig. 4.
Changes in the velocity during forward movement in untrained (thin black line), trained (thick grey line) and intact (thick black line) rats. Horizontal velocity is shown in (A) and vertical velocity is shown in (B). The changes in velocity are shown relative to the step cycle (the start of forward movement marks the beginning of the step cycle, 0%). Circles and vertical lines indicate the onset of the peak velocity during the step cycle. The data shown are group averages. Only the trajectories from rats that performed at least 15 steps were included in these averages (n = 9 untrained; n = 8 trained; n = 9 intact). The data from the contused rats are from tests performed 14 weeks after the contusion. *† indicates that the onset of the peak velocity is significantly earlier than the onset of the peak velocity in the trained (p < 0.05) and normal rats respectively (p ≤ 0.01).
Changes in the horizontal and vertical velocities in the untrained rats were coupled. As the ankle moved forward, the horizontal and vertical velocities initially increased, reached a peak and afterwards decreased together (Fig. 5A). A different relationship was found in the trained and intact rats. As horizontal velocity increased, there was little change in vertical velocity (Figs. 5B,C). After peak horizontal velocity was reached, both the horizontal and vertical velocities decreased (Figs. 5B,C).
Fig. 5.
Plot of horizontal versus vertical velocity of the ankle during forward movement in (A) untrained, (B) trained and (C) intact rats. Arrows indicate the direction of movement. The velocity data shown are group averages. Only the trajectories from rats that performed at least 15 steps were included in these averages (n = 9 untrained; n = 8 trained; n = 9 intact). The data from the contused rats are from tests performed 14 weeks after the contusion.
In summary, the untrained rats moved their ankles rapidly upward during forward movement giving the appearance of a kicking motion. In contrast, the paws in the trained and intact rats moved forward at a steady vertical position and at a slower speed.
Backward movements
Backward movement in the ankle began while the hindpaw was still in the air. In each group, the ankle was moving downward when backward movement began (see 3 in Fig. 3). In the untrained rats, the ankle traveled 12.6 ± 2.0 mm downward to the minimum (see 3 to 4 in Fig. 3) whereas the ankle in the trained and intact rats traveled only 6.0 ± 2.1 mm and 5.6 ± 1.9 mm, respectively. One-way ANOVA showed a significant difference in the amount of downward movement between the groups (p < 0.05).
After reaching the minimum, the ankle in the untrained rats moved backward with little vertical movement (see 4–5, Fig. 3A). In contrast, the ankle in the trained and intact rats rose during most of the backward movement indicating that the ankle joint extended during stance (see 4–5 in Fig. 3B and C). The ankle traveled upward a significantly greater distance in the trained and intact rats than in the untrained rats. On average, the ankle rose 7.4 ± 1.8 mm and 10.1 ± 1.3 mm in the trained and intact rats respectively versus only 1.4 ± 0.5 mm in the untrained rats. One-way ANOVA showed significant differences in the amount of ankle rise during backward movement between the groups (p < 0.01). Post hoc comparisons showed the amount of upward movement was significantly less in the untrained rats relative to the trained (p < 0.01) and intact rats (p < 0.01).
Interestingly, as the ankle of the untrained rats moved backward, it reached a faster peak horizontal velocity than in the trained and intact rats (untrained, 58 ± 6.6 mm s−1; trained, 40 ± 6.6 mm s−1; intact, 27 ± 6.6 mm s−1). The peak horizontal velocity in the untrained rats occurred later than the peak horizontal velocity in the trained and intact rats (see white squares in Fig. 6). One-way ANOVA showed significant differences in peak horizontal velocity and onset of the peak horizontal velocity between the groups (p < 0.01). Post hoc comparisons showed the peak horizontal velocity was significantly different in the untrained rats relative to the intact rats (p < 0.01) and onset of peak horizontal velocity was significantly different in the untrained rats relative to the trained (p < 0.01) and intact rats (p< 0.01). No significant differences in peak vertical velocity were found between the groups.
Fig. 6.
Changes in the velocity during backward movement in (A) untrained, (B) trained and (C) intact rats. Horizontal velocity (grey line) and vertical velocity is shown (black line). The changes in velocity are shown relative to the step cycle (backward movements began after 10% of the step cycle had been completed). The white squares indicate the mean onset of the peak horizontal velocity during the step cycle. The bars around the white squares indicate the standard error of the mean onset. The data shown are group averages. Only the trajectories from rats that performed at least 15 steps were included in these averages (n = 9 untrained; n = 8 trained; n = 9 intact). The data from the contused rats are from tests performed 14 weeks after the contusion. *† indicates that the onset of the peak velocity is significantly earlier than the onset of the peak velocity in the trained and normal rats respectively (p ≤ 0.01).
Discussion
In the present study, we found that spinally contused rats that received daily treadmill training recovered stepping that had similar step cycle trajectory shape and velocity characteristics as stepping in intact rats. Untrained rats did recover the ability to perform stepping on the treadmill. However, they moved their ankles with a different trajectory shape and at a faster speed relative to the trained and intact rats. Hindpaw dragging during swing was also more prevalent in the untrained rats. Together, these findings are consistent with the hypothesis that treadmill training restored normal ankle movements during stepping in rats following a severe contusion spinal cord injury.
Effect of treadmill training on the performance of the swing and stance phases of the step cycle
Previous studies have reported a beneficial effect of treadmill training on the swing phase of stepping in spinal cord injured animals. For example, spinally transected cats that undergo several weeks of treadmill training perform greater lift of the hindpaws during swing than untrained spinal cats (de Leon et al., 1998a; Boyce et al., 2007; de Leon et al., 1999b). Recordings from flexor hindlimb muscles during stepping indicate that the trained spinally transected cats are better able to recruit flexor motor pools than the untrained spinal cats (de Leon et al., 1998a). Similarly, treadmill training resulted in an improved ability of spinal cord contused rats to flex the hip thereby reducing the amount of hindpaw dragging during swing (Thota et al., 2001). In the present study, one effect of treadmill training was to reduce the amount of paw dragging during swing. A second effect was to restore normal ankle trajectory shape during swing. Together, these findings suggest that the effect of treadmill training on swing trajectory depends on the specific movement errors that exist. Spinal cord injured animals learn to make the necessary adjustments through training. This conclusion is consistent with previous studies that showed that when stepping in spinally transected rats is perturbed, the animals learn to correct errors in swing trajectory in order to avoid stumbling and to maintain stepping (Timoszyk et al., 2002; Heng and de Leon, 2007; Edgerton et al., 2001).
We also found that training had a significant effect on the stance phase of the step cycle. Training increased the amount of rise in the ankle indicating ankle extension improved. Training has been shown to increase the duration of the stance phase of stepping in spinally transected rats (Cha et al., 2007) and to increase stance length in spinally transected cats (Boyce et al., 2007). In addition, stand training has been shown to improve the duration of hindlimb standing in spinally transected cats (Pratt et al., 1994; de Leon et al., 1998b). These findings indicate that hindlimb motor training improves the ability of spinal cord injured animals to maintain weight bearing in the hindlimbs. One previous study reported that weight bearing (estimated by measuring the height of the hip relative to the ground during stance) in rats that received a dorsal lesion of the spinal cord was not improved by 5weeks of treadmill training (Fouad et al., 2000). However, there is evidence that treadmill training improved weight bearing during open field locomotion in rats that received a partial compression injury of the spinal cord (Multon et al., 2003). Different injury models may account for the apparent disparity in these results. Further studies that combine detailed kinematic analyses of the limb movements and recordings of muscular activity will be necessary to more closely examine the effect of treadmill training on weight bearing in the incomplete injured animal.
A unique movement parameter that one is able to measure with the robotic device is the velocity of the ankle during stepping. These data proved to be valuable for finding several key differences between the groups. For example, the untrained rats moved their ankles significantly faster during forward swing than the trained and intact rats. The values for peak velocity that we found in the untrained rats were similar to what has been reported in a previous study that also used a robotic device to assess treadmill stepping in untrained contused rats (Nessler et al., 2006). Given the speed and the trajectory that the ankle traveled, one possibility is that forward swing in the untrained rats was influenced by hindlimb reflexes. Mechanical stimuli applied to the paw in spinal animals triggers flexion reflexes that enhance the swing phase of stepping (Forssberg et al., 1977). Similar flexion reflexes could have been stimulated by contact with the treadmill and/or the interface of the ankle with the robotic device. With training, the rats learn to adapt their movement patterns to these various stimuli and consequently, normal stepping velocities and trajectories were restored. An unexpected finding was that the peak horizontal velocity during stance was faster in the untrained rats. No differences between the groups were expected for horizontal velocity during stance since treadmill speed was constant. This finding, however, is not unique and a similar result was reported in a previous study that used a robotic device to assess stepping spinally transected rats (Timoszyk et al., 2002). Ankle flexion movements during stance likely accounted for these velocity changes, however, detailed kinematic analyses of the hindlimb are necessary.
Factors influencing the effects of training on functional recovery after spinal cord injury
One of the interesting findings in the present study was that treadmill training failed to increase the amount of successful, partial weight bearing steps (see Fig. 2A). Factors that could have accounted for the lack of effect on successful steps are the onset of training following injury and injury severity. There is evidence that beginning motor training immediately after spinal cord injury (i.e. 3–4 days) is more beneficial than if training is delayed (Norrie et al., 2005). Treadmill training began 1 month after the contusion injuries in the present study and it is possible that this long delay could have reduced the effectiveness of training on stepping recovery. Another possibility is that the effects of treadmill training following a severe spinal cord contusion injury may be limited. Locomotor performance is improved by treadmill training in rats with a moderate spinal cord contusion injury, but whether training increased the number of step cycles executed by the rats is unknown.
One could also argue that training consisting of bipedal hindlimb stepping is not a natural form of locomotion for rats. Other activities may provide longer periods of stepping under more natural conditions. Increasing spontaneous exercise via an enriched environment or wheel running has been shown to produce gains in locomotor function in spinal cord inured rats (Lankhorst et al., 2001; Van Meeteren et al., 2003) and spinal cord injured mice (Engesser-Cesar et al., 2005). Other studies have shown that swimming exercise improved hindlimb function in spinally contused rats (Hutchinson et al., 2004; Smith et al., 2006). Whether these forms of exercise are more effective than treadmill training in improving locomotor deficits, however, remains to be determined.
Mechanism of training
Based on the present findings, it is impossible to determine how treadmill training improved stepping in the spinally contused rats. Recent findings suggest that treadmill training may improve muscle function (Stevens et al., 2006) and sensory function (Hutchinson et al., 2004) in the hindlimbs of spinally contused rats. There is also a growing amount of evidence from studies of spinally transected animals that neurons within the lumbar spinal cord are modified by treadmill training (Tillakaratne et al., 2002; Petruska et al., 2007; Cote and Gossard, 2004; Cote et al., 2003). It is possible that treadmill training has a similar effect on the lumbar spinal circuitry following an incomplete spinal cord injury. In support of this hypothesis, Ying et al. (2005) recently showed that wheel running exercise enhances BDNF levels and synaptic plasticity in the lumbar spinal cord of rats following a spinal cord hemisection injury. Treadmill training may also improve locomotor function by inducing plasticity in the brain and in the pathways that connect the brain with the lumbar spinal circuitry. For example, the findings from recent studies performed in humans with spinal cord injuries suggest that treadmill training may enhance activity in the corticospinal tract and (Norton and Gorassini, 2006; Winchester et al., 2005; Thomas and Gorassini, 2005) and in the sensorimotor cortex and cerebellum (Winchester et al., 2005). Together, these findings indicate that treadmill training influences plasticity in multiple areas of the neuromuscular system.
Other possibilities are that treadmill training increased sprouting and/or increased the amount of spared white matter thereby improving functional recovery. Locomotor recovery is known to be correlated with the amount of spared white matter following contusion injury in rats (Basso et al., 1996). However, previous studies of activity-based therapies in incomplete injured rats have failed to find any beneficial effect of exercise on white matter sparing (Multon et al., 2003; Fouad et al., 2000; Lankhorst et al., 2001). There is recent evidence that collateral sprouting was significantly enhanced by treadmill training in spinally hemisected mice (Goldshmit et al., 2008). We did not perform a histological or anatomical examination of the spinal cord injury site and thus, we do not know if there were any differences in sprouting or in spared white matter between the trained and untrained rats. The lack of histological data in the present study means that we also cannot rule out the possibility that the trained rats performed better than the untrained rats because the trained rats were less injured. However, it is noteworthy to point out that treadmill stepping performance was not significantly different between the two groups during baseline testing (see Week 6, Fig. 2) which indicates the two groups were similar at least in terms of locomotor recovery.
Implications
Weight supported treadmill training has been successfully used to improve walking function in humans with incomplete spinal cord injury (Wirz et al., 2005; Hicks et al., 2005; Behrman et al., 2005). An important, new technological development in body weight supported treadmill training has been the use of robotic devices (Wirz et al., 2005; Hornby et al., 2005). Recent studies have begun to test the effectiveness of different robotic assistance algorithms in animal models of complete spinal cord injuries (Cai et al., 2006; de Leon and Acosta, 2006). The present findings indicate that recovery in spinally contused rats is also responsive to robotic-assisted training. Thus, it appears feasible to develop and test robotic assistance algorithms in the spinally contused rats to begin to understand how robotic assistance can be used to enhance body weight supported treadmill training following incomplete spinal cord injury.
Acknowledgments
The authors would like to thank Kelli Sharp and Jacqueline Leung for their excellent technical assistance.
Footnotes
Grants: This work was supported by the Roman Reed Spinal Cord Injury Research Fund of California (RR03-079) and NIH award NS055911.
References
- Barbeau H, Julien C, Rossignol S. The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res. 1987;437:83–96. doi: 10.1016/0006-8993(87)91529-0. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Behrman AK, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys. Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- Belanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J. Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Tumolo MA, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J. Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 2006;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, de Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma. 2007;24:1000–1012. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J. Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J. Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Acosta CA. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrauma. 2006;23:1147–1163. doi: 10.1089/neu.2006.23.1147. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 1998a;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998b;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon RD, London NJ, Roy RR, Edgerton VR. Failure analysis of stepping in adult spinal cats. Prog. Brain Res. 1999a;123:341–348. doi: 10.1016/s0079-6123(08)62869-1. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 1999b;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J. Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol. Scand. 1980;108:269–281. doi: 10.1111/j.1748-1716.1980.tb06533.x. [DOI] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. J. Neurosci. 2007;27:8558–8562. doi: 10.1523/JNEUROSCI.1635-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys. Ther. 2005;85:52–66. [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasak MD, Hedlund E, Roy RR, Carpenter EM, Edgerton VR, Phelps PE. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp. Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Lankhorst AJ, ter Laak MP, van Laar TJ, van Meeteren NL, de Groot JC, Schrama LH, Hamers FP, Gispen WH. Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J. Neurotrauma. 2001;18:203–215. doi: 10.1089/08977150150502622. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J. Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- Nessler JA, De Leon RD, Sharp K, Kwak E, Minakata K, Reinkensmeyer DJ. Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J. Neurotrauma. 2006;23:882–896. doi: 10.1089/neu.2006.23.882. [DOI] [PubMed] [Google Scholar]
- Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J. Neurophysiol. 2005;94:255–264. doi: 10.1152/jn.00970.2004. [DOI] [PubMed] [Google Scholar]
- Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J. Neurophysiol. 2006;95:2580–2589. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt CA, Fung J, Macpherson JM. Stance control in the chronic spinal cat. J. Neurophysiol. 1994;71:1981–1985. doi: 10.1152/jn.1994.71.5.1981. [DOI] [PubMed] [Google Scholar]
- Radojicic M, Nistor G, Keirstead HS. Ascending central canal dilation and progressive ependymal disruption in a contusion model of rodent chronic spinal cord injury. BMC Neurol. 2007;7:30. doi: 10.1186/1471-2377-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Smith RR, Shum-Siu A, Baltzley R, Bunger M, Baldini A, Burke DA, Magnuson DS. Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J. Neurotrauma. 2006;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JE, Liu M, Bose P, O'Steen WA, Thompson FJ, Anderson DK, Vandenborne K. Changes in soleus muscle function and fiber morphology with one week of locomotor training in spinal cord contusion injured rats. J. Neurotrauma. 2006;23:1671–1681. doi: 10.1089/neu.2006.23.1671. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J. Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- Thota A, Carlson S, Jung R. Recovery of locomotor function after treadmill training of incomplete spinal cord injured rats. Biomed. Sci. Instrum. 2001;37:63–67. [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J. Neurophysiol. 2002;88:3108–3117. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J. Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, Williamson J. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil. Neural Repair. 2005;19:313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch. Phys. Med. Rehabil. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]