Abstract
Global environmental changes, including ocean acidification, have been identified as a major threat to scleractinian corals. General predictions are that ocean acidification will be detrimental to reef growth and that 40 to more than 80 per cent of present-day reefs will decline during the next 50 years. Cold-water corals (CWCs) are thought to be strongly affected by changes in ocean acidification owing to their distribution in deep and/or cold waters, which naturally exhibit a CaCO3 saturation state lower than in shallow/warm waters. Calcification was measured in three species of Mediterranean cold-water scleractinian corals (Lophelia pertusa, Madrepora oculata and Desmophyllum dianthus) on-board research vessels and soon after collection. Incubations were performed in ambient sea water. The species M. oculata was additionally incubated in sea water reduced or enriched in CO2. At ambient conditions, calcification rates ranged between −0.01 and 0.23% d−1. Calcification rates of M. oculata under variable partial pressure of CO2 (pCO2) were the same for ambient and elevated pCO2 (404 and 867 µatm) with 0.06 ± 0.06% d−1, while calcification was 0.12 ± 0.06% d−1 when pCO2 was reduced to its pre-industrial level (285 µatm). This suggests that present-day CWC calcification in the Mediterranean Sea has already drastically declined (by 50%) as a consequence of anthropogenic-induced ocean acidification.
Keywords: cold-water coral, Lophelia pertusa, Madrepora oculata, Desmophyllum dianthus, calcification, ocean acidification
1. Introduction
Cold-water corals (CWCs) have recently come into the focus of research as biodiversity hotspots in the deep oceans [1]. Although, they have been described since the early eighteenth century, their more comprehensive study has been, and still is, restricted by the fact that they predominantly occur in deep depths or on steep cliffs, canyon walls or under overhangs. Technological developments with respect to shipboard logistics, as well as acoustic and video survey techniques, has led to the discovery of a large number of CWC sites during the last decades [2–9].
CWCs are ecosystem engineers which need to outgrow sedimentation and other destructive forces in order to maintain a three-dimensional structure that serves as the foundation for the ecosystem. Skeletal extension rates between 6 and 26 mm yr−1 for Lophelia pertusa were derived from indirect measurements of stable isotopes [10–13] and from corals growing on artificial substrate [14,15]. For corals maintained in aquaria, skeletal extension rates were 9.4 mm yr−1 and 15–17 mm yr−1 for L. pertusa from Norwegian fjords and the Mediterranean Sea, respectively [16,17], and 3–18 mm yr−1 for Madrepora oculata from the Mediterranean Sea [17]. One study reported average extension rates of 3.77 mm yr−1 and 2.44 mm yr−1 for branches of L. pertusa stained with Alizarin Red S and re-deployed in situ at coral and non-coral areas, respectively [18]. Studies of CWC growth usually refer to linear or radial extension which, without information on skeletal density, provides no information on calcification rates (mass of CaCO3 precipitated per unit time) [19]. So far, only two studies have reported rates of calcification in CWCs. Average calcification rates measured with the buoyant weighing technique ranged between 0.02 and 0.11% d−1 in four Mediterranean species [20]. Maier et al. [21] reported average rates of 0.02–0.07% d−1 obtained through labelling of colonies of L. pertusa with the radiotracer 45Ca.
It is well known that the distribution of CWCs is limited by temperature rather than depth and they are most commonly distributed at temperatures between 4°C and 12°C [1]. In the Mediterranean Sea, they occur at temperatures between 12.5°C, to almost 14°C, which might already be at the higher tolerance limit [2]. Furthermore, the uptake of anthropogenic CO2 by the ocean generates ocean acidification which has recently been proclaimed as one of the perhaps most harmful threats to CWCs [1]. That is because CWCs are mostly distributed at relatively high latitudes and/or deep water. Both environments will be the first to be affected by the lowering in ocean pH and a shallowing of the aragonite saturation (Ωa) horizon [22–24]. The Mediterranean Sea with its two Western and Eastern main basins, separated by the Straits of Sicily, and some smaller regional basins, can be regarded as a miniature ocean that is expected to react faster to global change than the open ocean [25]. Over the last decades, there appears to have been a warming trend attributed to global warming in deep [26] and surface waters [27,28]. A very recent study has shown that there is a high imprint of anthropogenic CO2, which has already affected Mediterranean pH by a decrease of 0.05–0.14 pH units since pre-industrial times [29]. Owing to the high alkalinity in the Mediterranean, more atmospheric CO2 is absorbed than in the open ocean [30]. It is consequently important to understand how anthropogenic partial pressure of CO2 (pCO2) has already affected and how it will affect Mediterranean Sea ecosystems and its key taxa.
Because of their restricted accessibility, it is a demanding task to study the biology and physiology of CWCs, making it important to develop reproducible methods for experimentation. One option is to maintain corals under controlled laboratory conditions, and attempt to mimic their natural environment. However, the maintenance of CWCs in the laboratory is very time consuming and prone to technical problems. We therefore conducted on-board experiments with freshly collected CWCs and ambient sea water. The aim of the study was to compare rates of calcification in three Mediterranean species at three study sites and to test the effects of ocean acidification on M. oculata calcification rates by subjecting colonies to three pCO2 levels: 280 µatm (pre-industrial), 400 µatm (present-day) and 800 µatm (as projected for the end of this century).
2. Material and methods
(a). Study sites and sampling
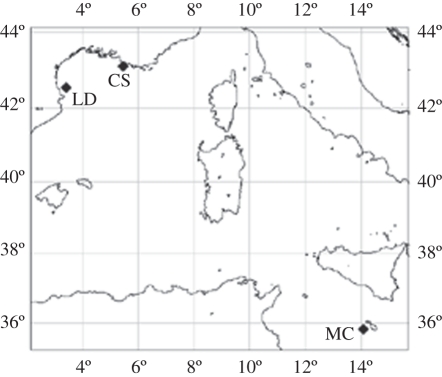
CWCs and ambient sea water were sampled during several cruises: June 2009 in the canyon of Lacaze-Duthiers (42°32.98′ N; 03°25.21′ E), in September 2009 in the Gulf of Cassidaigne (43°06.76′ N; 05°27.71′ N), both on board the R/V Minibex (COMEX, France), and in December 2009 in the Strait of Sicily at a site southwest off the island of Malta (35°49.77′ N; 14°04.90′ E) on board the R/V Urania (Italy; figure 1).
Figure 1.
Sampling sites in the Mediterranean Sea: Lacaze-Duthiers (LD), Gulf of Cassidaigne (CS) and south of Malta (MC).
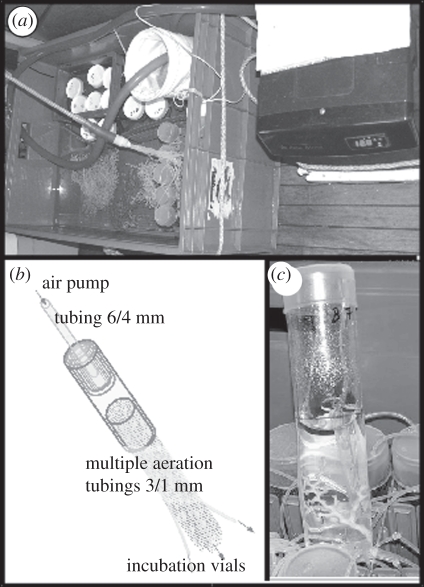
During the Minibex cruises, CWCs were sampled with a remotely operated vehicle (ROV), while during the R/V Urania cruise a mini-dredge had to be deployed for a hit-and-go sampling because of technical problems with the ROV. At all sites, sea water next to living CWC was sampled using 6 l or 10 l Niskin bottles. After collection, corals were directly transferred to a plastic container (1040 × 640 × 515 mm), containing about 120 l of sea water, which was continuously flowing through two layers of micron bags (5 and 1 µm) and a chilling unit at a rate of 1000 l h−1. Temperature was maintained at 12.5 ± 0.5°C (figure 2). Sea water was sub-sampled to determine the concentration of inorganic nutrients (phosphate and ammonium), total alkalinity (AT) and dissolved inorganic carbon (CT). Another part of the sea water collected from CWC sites was used for on-board incubation to determine calcification rates of corals by the alkalinity anomaly technique.
Figure 2.
(a) Experimental set-up showing a large container with open and closed system incubation vials and some coral samples. In the right corner of the container is a double layer of micron bags (5 and 1 µm) to filter the recycled and chilled sea water. Next to the container is the chiller unit that is connected to a pump (1000 l h−1), which exchanges sea water between the container and chiller. (b) Aeration system to supply a multiple set of incubation vials. Tubing is joined by several silicon tubes with various diameters to fit to a standard aeration (4/6 mm inner/outer diameter) tube and an air pump. The space between tubing is sealed air-tight using silicone glue, and an (c) open system incubation vial containing a small branch of M. oculata. Inside the vial is another small tube of ca 1 cm in diameter in which the small 1 mm air tubing ends. The upward moving air bubbles inside the 1 cm tube generate an upward flow of sea water and thus water mixing.
(b). On-board incubation to determine calcification rates of CWCs in ambient sea water
Small fragments were broken off from CWCs placed in either open or closed vials containing 150 ml or 180 ml sea water, respectively, and directly incubated after fragmentation (table 2 and figure 2). Sea water for incubation was sampled close to CWC sites to provide similar conditions with respect to the carbonate chemistry and nutrients. Open vials contained a small tube (Ø 1 cm) in which the outlet of a thin air tubing (Ø 3 mm) ended (figure 2b,c) to aerate the sea water with ambient air and to equilibrate the pCO2 during incubation. In the open system set-up, the vials were aerated with ambient air, which served two purposes: (i) to keep pCO2 stable and (ii) generate an air lift and thus a vertical flow and mixing of the incubation water. For closed incubation, vials were filled almost to the rim (180 ml) with sea water, and closed with a screw-on lid after corals were placed inside. Closed vials had a couple of millilitre of air space to avoid large changes in dissolved oxygen and to allow for some water mixing via ship movement. As controls, at least three vials contained no corals (blank) and also sea water was sub-sampled prior to starting incubation (T0) to determine initial carbonate chemistry and inorganic nutrient concentration. Corals were incubated for 24 h, and then sea water was sub-sampled for determination of inorganic nutrients, CT and AT.
Table 2.
Results from on-board incubation of CWC in ambient sea water at three Mediterranean CWC sites: Lacaze-Duthiers (LD), Gulf of Cassidaigne (CS) and south of Malta (MC), numbers no._no. indicate station number and depth, respectively. (The three CWC species were L. pertusa (LP), M. oculata (MO) and D. dianthus (DE). Corals were either incubated in a closed or open system, DW is skeletal dry weight, average pCO2 and Ωa are average between beginning and end of incubation, G is calcification rate (corrected for inorganic nutrient excretion). CS5_500*: initially, seven corals were incubated, but only three could be evaluated with respect to carbonate chemistry and G in the open system. Nevertheless, DW and no. of polyps are given for all seven fragments because they were later used along with the eight fragments of the closed system of CS5_500 for repeated measurements of G under variable pCO2 (figure 4).)
| station | species | open/closed | n | DW (G) | no. of polyps | initial pCO2 | average pCO2 | Ωa | G (% d−1) |
|---|---|---|---|---|---|---|---|---|---|
| LD9_500 | LP | open | 3 | 0.49 ± 0.09 | 3.3 ± 1.5 | 354 | 378 ± 4 | 2.6 ± 0 | 0.090 ± 0.020 |
| LD10_267 | LP | open | 3 | 2.03 ± 0.72 | 8.3 ± 1.5 | 354 | 389 ± 10 | 2.5 ± 0.1 | 0.033 ± 0.011 |
| LD9_500 | LP | closed | 3 | 3.36 ± 2.63 | 7.0 ± 1.7 | 347 | 441 ± 56 | 2.1 ± 0.4 | 0.034 ± 0.013 |
| LD9_500 | MO | closed | 3 | 2.21 ± 1.38 | n.a. ± n.a. | 347 | 387 ± 20 | 2.5 ± 0.2 | 0.018 ± 0.018 |
| CS5_500 | MO | open | 7 | 1.42 ± 0.15 | 47.1 ± 10.6 | n.a. | n.a. | n.a. | n.a. |
| CS5_500* | MO | open | 3 | 1.33 ± 0.10 | 40.3 ± 5.0 | 367 | 444 ± 127 | 2.4 ± 0.8 | 0.021 ± 0.017 |
| CS5_500 | MO | closed | 8 | 1.03 ± 0.42 | 43.3 ± 12.5 | 404 | 863 ± 588 | 1.5 ± 0.8 | 0.068 ± 0.068 |
| MC25_690 | LP | open | 1 | 3.53 ± 0 | 7.0 ± n.a. | 433 | 686 ± n.a. | 1.3 ± 0 | 0.028 ± 0 |
| MC25_690 | MO | open | 6 | 2.79 ± 2.38 | 23.5 ± 8.3 | 433 | 614 ± 72 | 1.6 ± 0.3 | 0.037 ± 0.036 |
| MC25_690 | DE | open | 5 | 4.49 ± 2.50 | 3.4 ± 1.1 | 433 | 563 ± 57 | 1.8 ± 0.2 | 0.011 ± 0.007 |
| all groups | 40 | 2.15 ± 1.85 | 25.4 ± 20.1 | 390 | 585 ± 340 | 1.9 ± 0.6 | 0.042 ± 0.040 |
(c). Repeated incubation of CWCs under variable pCO2
First, seven fragments were used in open system incubation and eight in closed system incubation to assess calcification rates using ambient sea water and thus ambient pCO2 (table 2, CS5_500) directly on board. This on-board incubation served as a control to assess if calcification rates change over time (in a couple of days) and as a function of handling and transport. The same 15 coral fragments (seven open and eight closed system) were then used for repeated incubation at variable pCO2 using the closed system set-up for all 15 coral fragments. About 2 h after transport back to the laboratory the first incubation was carried out at elevated pCO2 (ca 867 µatm), then at ambient pCO2 (404 µatm) and reduced pCO2 (285 µatm) for 24 h each without feeding the corals. The timespan between the first on-board incubation and the last incubation at reduced pCO2 was 5 days. Over this time period, we can also exclude that starvation affects calcification rates of CWCs [31]. For each treatment, at least three additional controls containing only sea water at respective pCO2 (blank) were incubated along with coral samples. Except for ambient pCO2, the pCO2 level of bulk sea water was regulated before incubation using mass flow controllers (AnalytMTC, GFC 171) using a mix of CO2-free air and pure CO2. CO2-free air was produced by stripping CO2 from ambient using soda lime. pCO2 was measured using an infrared gas analyser (LI-COR-6262).
(d). Analyses of inorganic nutrients
Approximately 30 ml of sea water from Niskin bottles or experimental incubation vials were sub-sampled for inorganic nutrient analysis. Most of the sea water was used for rinsing syringe, filter and vials before a final volume of ca 5 ml of sea water was filtered over a 0.2 µm Acrodisc into 6 ml Pony Vials (Perkin Elmer). The samples were frozen and stored at −20°C pending analysis at the Royal Netherlands Institute for Sea Research. Ammonium and dissolved inorganic phosphorus concentrations were determined using an AxFlow Bran & Luebbe Traacs800 autoanalyser [32]. Precision (±1 s.d.) of an internal standard was 0.009 µmol kg−1 and 0.023 µmol kg−1 for phosphate and ammonium (n = 17 each), respectively.
(e). Analysis of AT and CT and determination of sea water carbonate chemistry
Ambient sea water was sampled to analyse the dissolved inorganic carbon (CT) and total alkalinity (AT) and to calculate other parameters of the carbonate system. Triplicate 500 ml samples were collected from a Niskin bottle. Sea water was poisoned with 100 µl saturated mercuric chloride, the stoppers of the bottles was greased (Apiezon) to seal bottles air-tight, and samples were stored at 10°C pending analysis. Analyses of CT and AT were performed by the Service National d'Analyse des Paramètres Océaniques du CO2 (http://soon.ipsl.jussieu.fr/SNAPOCO2/) following the method of Edmond [33] and the software described by DOE [34]. Reproducibility of the Dickson standard (batch 99, http://www.andrew.uc.edu/co2qc/batches.html) was 3 µmol kg−1 for AT and 2.8 µmol kg−1 for CT.
Initial and final CT and AT of the incubations were measured on 125 ml samples at the Laboratoire d'Océanographie de Villefranche. They were processed and stored as described earlier for the in situ samples. Bottles were carefully filled as described for samples from Niskin bottles, poisoned with 25 µl mercuric chloride, sealed air-tight and stored at 10°C.
CT was determined immediately after opening the bottle using an inorganic carbon analyser (AIRICA, Marianda, Kiel, Germany) coupled to an infrared gas analyser (LI-COR 6262). This system was calibrated prior to sample analysis against a certified standard [34].
The sample bottle was kept in a water bath at a constant temperature (25°C). The coefficient of variation of at least three replicate measurements was 0.08 per cent and 2 µmol kg−1. Also, an internal laboratory standard was measured at regular intervals and revealed a precision of ±2.54 µmol kg−1 (mean = 2281.87, n = 17).
AT was determined on samples filtered on 0.2 µm membranes by a potentiometric titration (Metrohm Titrando 80 titrator) coupled to a glass electrode (Metrohm, electrode plus) and thermometer. The pH electrode was calibrated on the total scale using Tris/HCl and 2-aminopyridine/HCl buffer solutions with a salinity of 38 (Dickson and co-workers [35]). Measurements were carried out in triplicate at 25°C with pre-weighed samples of ca 25 g and 0.1 N HCl (TitriPAC, Merck) was used for titration. AT was calculated as described by Dickson and co-workers [35]. Standards provided by Dickson (batch 99 and 102) were run regularly. Accuracy was on average 2.38 µmol kg−1 and 0.94 µmol kg−1 below the nominal values of batches 99 and 102, respectively. Precision (±1 s.d.) of repeated measurements was 3.53 and 4.63 µmol kg−1 for the same batches (n = 34 and n = 70). Other parameters (pCO2, Ωa, pH) of the carbonate chemistry were determined from CT, AT, temperature, depth, and salinity using the software package Seacarb [36].
(f). Determination of calcification rates (G) by the alkalinity anomaly technique
The alkalinity anomaly technique was used to estimate rates of calcification using the 2 : 1 stoichiometric relationship between the decrease of AT and the precipitation of CaCO3 [37]. However, other biogeochemical processes, such as changes in inorganic nutrient concentration can also affect AT [35]. Previous work has shown that the CWC L. pertusa can release significant amounts of inorganic nutrient during incubation [38]. Changes of AT during the incubations were therefore corrected for the release of phosphate and ammonium as follows:
Calcification rates were normalized to initial skeletal weight of the corals and are given in per cent per day (% d−1).
(g). Statistical analyses
Statistical analyses were conducted using the software package Statistica v. 7.0. The tests used are mentioned in the results subsections. Values are reported as mean ± standard deviation.
For parameters of the carbonate chemistry and inorganic nutrients at the start and end of the experiments, see tables in the electronic supplementary material.
3. Results
(a). In situ sea water carbonate chemistry and inorganic nutrients
AT and CT of ambient sea water ranged from 2577 to 2623 and 2316 to 2333, respectively (table 1). The calculated pCO2, pHT and Ωa values ranged between 349 µatm and 395 µatm, 8.07 and 8.11, and 2.58 and 2.95, respectively. Average phosphate and ammonium concentrations ranged between 0.20 µmol kg−1 and 0.41 µmol kg−1 and 0.03–0.29 µmol kg−1, respectively.
Table 1.
In situ sea water chemistry of CWC sampling sites. (Total alkalinity (AT), concentration of dissolved inorganic carbon (CT), bicarbonate (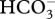 ), carbonate (
), carbonate (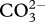 ), phosphate (PO4) and ammonium (NH4) (all in µmol kg−1). Also shown are pCO2 (µatm), pH on total scale (pHT), and the saturation state of aragonite (Ωa). Numbers given in italics were calculated from TA and CT, at respective depth (metre), salinity and temperature using the software package Seacarb, under the program R. For the stations of Lacaze-Duthiers (LD_9, LD_10) and the Gulf of Cassidaigne (CS_5) salinity and temperature were 38°C, and 13°C, and at the site south of Malta (MC_30) they were 38.75°C, and 12.75°C, respectively.)
), phosphate (PO4) and ammonium (NH4) (all in µmol kg−1). Also shown are pCO2 (µatm), pH on total scale (pHT), and the saturation state of aragonite (Ωa). Numbers given in italics were calculated from TA and CT, at respective depth (metre), salinity and temperature using the software package Seacarb, under the program R. For the stations of Lacaze-Duthiers (LD_9, LD_10) and the Gulf of Cassidaigne (CS_5) salinity and temperature were 38°C, and 13°C, and at the site south of Malta (MC_30) they were 38.75°C, and 12.75°C, respectively.)
| station | depth | AT | CT | HCO3− | CO3 | CO2 | pCO2 | Ωa | pHT | PO4 | NH4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LD_9 | 500 | 2577 | 2317 | 2114 | 187 | 15.4 | 395 | 2.6 | 8.07 | 0.41 | 0.29 |
| LD_10 | 267 | 2577 | 2316 | 2112 | 189 | 15.4 | 394 | 2.7 | 8.08 | 0.22 | 0.16 |
| CS_5 | 200 | 2615 | 2333 | 2115 | 204 | 14.4 | 368 | 3.0 | 8.11 | 0.28 | 0.20 |
| MC_30 | 690 | 2623 | 2328 | 2104 | 210 | 13.7 | 349 | 2.8 | 8.11 | 0.20 | 0 |
(b). Changes of inorganic nutrient concentrations during the incubations
The average phosphate and ammonium release during the incubations was 0.06 ± 0.07 µmol g−1 and 1.12 ± 1.19 µmol g−1 skeleton per day, respectively. The lowest release of phosphate was found for Desmophyllum dianthus with 0.002 ± 0.002 µmol g−1 skeleton per day (n = 2), while in M. oculata and L. pertusa incubations, phosphate release was 0.06 ± 0.07 µmol g−1 skeleton per day (n = 27) and 0.08 ± 0.06 µmol g−1 skeleton per day (n = 10), respectively. For ammonium, the highest release rates were found for M. oculata with 1.32 ± 1.37 µmol g−1 skeleton per day, while L. pertusa released an average of 0.77 ± 0.53 µmol g−1 skeleton per day and D. dianthus 0.92 ± 0.53 µmol g−1 skeleton per day. Between species effects were tested for L. pertusa and M. oculata, while inorganic nutrient release was only determined for two D. dianthus specimens and was thus not considered for statistical testing. There was no species-specific effect on the release of phosphate or ammonium (t-test, t = 0.65, p = 0.52 for phosphate and t = −1.22, p = 0.23 for ammonium, n = 35 for both nutrients). Also, phosphate and ammonium excretion exhibit a positive and significant correlation when normalized to skeletal weight (R = 0.79, p ≪ 0.001, n = 39).
(c). Contribution of changes in nutrient concentration to changes in AT
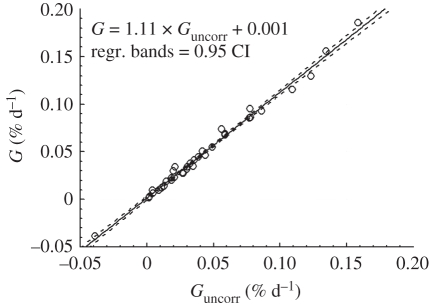
There is a significant correlation between the release of nutrients and the rate of calcification (PO4: R = 0.72, NH4; R = 0.79, p ≪ 0.001 for both, n = 39). The calcification rates estimated without (Guncorr) and with (G) correction for changes in the concentration of nutrients are significantly correlated (figure 3; G = 1.11 × Guncorr + 0.001, r = 0.995, p ≪ 0.001; n = 37). Therefore, the rate of calcification is underestimated by ca 10 per cent if nutrient excretion is not accounted for. As not all incubation vials were sub-sampled for inorganic nutrients, this linear regression function was used to correct for nutrient excretion where respective data were missing.
Figure 3.
Correlation between G corrected for inorganic nutrient increase during incubation and uncorrected (r2 = 0.991, p ≪ 0.001; n = 37).
(d). Calcification rates in ambient sea water
The overall mean calcification rates of corals incubated in ambient sea water was 0.04 ± 0.04% d−1 and ranged between −0.01 and 0.23% d−1 (n = 36). Species-specific rates for L. pertusa, M. oculata and D. dianthus were 0.05 ± 0.03% d−1 (n = 10), 0.05 ± 0.05% d−1 (n = 21) and 0.01 ± 0.01% d−1 (n = 5). Rates of calcification of L. pertusa were on average higher in the open incubation system with 0.05 ± 0.04% d−1 (n = 7) versus 0.03 ± 0.01% d−1 (n = 3) in the closed system, while the opposite was true for M. oculata with 0.03 ± 0.03% d−1 (n = 9) and 0.06 ± 0.06% d−1 (n = 12) in open and closed systems, respectively (table 2). There was no significant species effect on calcification rates between M. oculata and L. pertusa, between open and closed system incubation nor a combined effect of species and type of incubation (two-way ANOVA, p = 0.98 for the species effect, p = 0.93 for the incubation effect and p = 0.23 for the interaction term).
(e). Repeated incubations of Madrepora oculata under various pCO2 levels
During the on-board incubation, there was a problem with the open system and only three of the seven corals were sub-sampled for AT. Calcification of the three corals was 0.02 ± 0.02% d−1 and 0.03 ± 0.03% d−1 for on-board and laboratory (closed system) incubation, respectively. Calcification rates for the corals incubated in the closed incubation system were 0.07 ± 0.07% d−1 for both on-board and laboratory incubation. Under ambient pCO2, paired t-tests revealed no significant difference between on-board and laboratory incubation (open system: p = 0.74, n = 3; closed system: p = 0.93, n = 8). Pooling the calcification data for the 11 corals (open and closed system), average calcification rates were 0.06 ± 0.06 and 0.06 ± 0.07% d−1 and were not significantly different from each other (paired t-test: p = 0.84, n = 11).
The initial pCO2 levels for the three subsequent experiments were 867, 445 and 285 µatm, corresponding to Ωa values of 1.9, 2.6 and 3.7, respectively. pCO2 increased during the closed system incubations owing to respiration and calcification. The average final pCO2 levels were 1186 ± 461, 629 ± 248 and 331 ± 57 µatm, which decreased Ωa to 1.3 ± 0.6, 1.8 ± 0.6 and 2.9 ± 0.6, respectively.
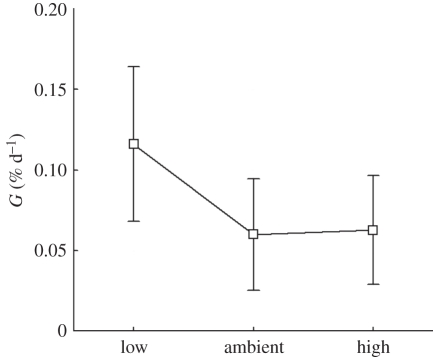
Average calcification rates were 0.06 ± 0.06, 0.06 ± 0.06 and 0.12 ± 0.06% d−1 for high, ambient and low pCO2 levels, respectively (figure 4). Repeated-measures ANOVA revealed that pCO2 had a significant effect on calcification rates (F2,28 = 11.3, p < 0.001). Post hoc comparison showed, that G of high and ambient pCO2 levels were significantly different from the low pCO2 (Tukey honest significant difference test (HSD): p = 0.001 and p < 0.001, respectively), while G at ambient and high pCO2 were not significantly different (Tukey HSD: p = 0.98).
Figure 4.
Average calcification rates of M. oculata of repeated closed system incubation at high (867 µatm), ambient (445 µatm) and low (285 µatm) pCO2. Data are from repeated incubations in the laboratory shortly after return from the cruise using the same coral fragments (n = 15). A repeated-measures ANOVA revealed a significant effect of pCO2 on calcification. Vertical bars denote 0.95 confidence intervals.
4. Discussion
(a). Methodological constraints
(i). On-board experiments
Measuring calcification of CWCs on board allows investigation of corals in ambient sea water over a broad biogeographic range. It requires a method that is not logistically demanding in terms of laboratory facilities. The set-up used for maintaining and incubating corals served that purpose well. It uses small incubation chambers that enable measurement of changes in total alkalinity large enough to estimate rates of calcification with satisfactory replication. We also tested the use of a closed incubation system. In closed systems, the CO2 released to the incubation water by coral respiration and calcification changes the sea water carbonate chemistry during incubation. This is likely to affect calcification rates as pCO2 increases and Ωa and pH decrease. However, systematic comparison revealed no significant difference in calcification between open and closed system incubation. Working with relatively simple and small incubation systems makes replication easier. This is an important experimental prerequisite given the high natural growth variability of CWCs that is largely owing to the age composition of polyps of a branch [16,21] and sporadic skeletal growth [16,18].
(ii). Pitfalls of alkalinity anomaly technique
The three most common methods to determine calcification rates of corals are 45Ca-labelling [39], the buoyant weight determination [40] and the AT anomaly technique [37]. The alkalinity anomaly technique used in this study has the advantage that it is sensitive enough to use for short-term incubations and that corals can be kept alive. However, it has been shown that L. pertusa and M. oculata excrete inorganic nutrients and that bacterial growth is stimulated during incubations owing to the release of organic matter [38,41,42]. This may also alter the TA during incubation. Negatively charged surface groups of phytoplankton and bacteria can react during titration with hydrochloric acid and the acid-base functional groups of dissolved organic carbon (DOC) in sea water can also have a small influence on TA [43]. It is therefore critical to pre-filter sea water over a 0.2 µm Acrodisc. Changes in the DOC pool during incubation was not accounted for in the present study, but Kim et al. [43] suggested that the influence of DOC on AT might be rather small. The release of inorganic nutrients induces a 10 per cent underestimation of calcification which was accounted for by an empirical relationship (this study). Also, a 10 per cent bias is relatively small when compared with the large natural variability of CWC calcification, with a coefficient of variation of up to 100 per cent.
(b). Calcification under ambient conditions
Calcification data are now available on L. pertusa determined by the three methods mentioned earlier. Calcification ranges between 0.02 and 0.07% d−1 under initial ambient conditions for corals collected in Mingulay and Skagerrak and investigated with 45Ca [21]. For Mediterranean samples, Orejas et al. [20] estimated calcification rates of 0.02% d−1 using the buoyant weight technique, and rates ranging between 0.03 and 0.09% d−1 were found in the present study with the alkalinity anomaly technique. The rate of calcification of the Mediterranean species D. dianthus and M. oculata determined by buoyant weight were 0.04% d−1 and 0.11% d−1, respectively [20], and 0.01 and 0.02–0.07% d−1 determined by the alkalinity anomaly technique (this study). Interestingly, there seems to be no systematic bias to either higher or lower calcification rates between the methods used, which provides some confidence that all three methods provide similar estimates. The method used by Orejas et al. [20] was based on long-term aquarium maintenance (256 days), while the 45Ca and AT approaches were based on short-term on-board incubations (1 day) with freshly collected colonies. Our results suggest that calcification of L. pertusa and M. oculata is similar. By contrast, Orejas et al. [20] reported a calcification rate fivefold higher in M. oculata than in L. pertusa and they hypothesize that this might be owing to different allocation of energy into tissue and skeletal growth. However, there is no evidence yet that M. oculata allocates more energy into skeletal growth than L. pertusa or D. dianthus.
(i). Size matters
It has been demonstrated in L. pertusa that calcification is four orders of magnitude lower in older than in younger polyps [38]. Hence, calcification rates normalized to skeletal weight diminish relative to increasing size and age of a colony. There is no information available on the weight of coral fragments used in the study of Orejas et al. [20], but the average polyp numbers were 2.9 and 6.2 polyps per fragment for L. pertusa and M. oculata, respectively. This means these fragments were very small with half and one-fifth of the polyp number used in the present study, respectively. One exception was the size of L. pertusa used in the first open system incubation (table 2) of the present study, as these specimens were as small as the ones used by Orejas et al. [20]. The calcification rate of these small fragments was 0.09% d−1 while a rate of 0.02% d−1 was found by Orejas et al. [20]. This indicates that the long-term aquarium conditions were less favourable for calcification of L. pertusa of the same size class than were the short-term on-board incubations, while the calcification rates of M. oculata reported by the two studies are in good agreement.
(ii). Site-specific calcification rates
The present study also reports calcification measured in three distinct sites, providing the opportunity to assess geographical differences. Although, the Mediterranean exhibits an east–west trend in nutrient concentration [44] and in anthropogenic CO2 uptake [29], the in situ carbonate chemistry was very similar between the sites (table 1) and there is no significant difference in calcification rate between sites. However, on a broader biogeographic and depth scale where parameters of the carbonate chemistry are more distinct from each other, patterns in calcification rates might exist. In situ studies would be the ideal method to investigate the response of CWCs to changing environmental conditions, but are logistically too demanding for empirical comparisons on a broad geographical and depth scale. However, it is important to gather comparable and empirical datasets as close to in situ as possible to derive a baseline understanding, and moreover, to better predict the potential response to environmental change scenarios.
(c). Response to reduced and elevated pCO2
Our results clearly show that there is no negative response on calcification rates at the higher pCO2 level (867 µatm). However, calcification almost doubled at the pre-industrial pCO2 level (285 µatm) relative to calcification under the control at ambient pCO2. So far, only one study has tested the effect of ocean acidification on the calcification of CWCs. It revealed a negative response with decreasing pH (higher pCO2) in L. pertusa from the Skagerrak [21]. However, in contrast to the study on L. pertusa, Ωa did not drop below 1 for M. oculata in the higher pCO2 treatment, which might explain why M. oculata did not reveal a similar negative response at higher pCO2. While for most reef-building zooxanthellate corals a negative and often linear response to increasing pCO2 levels and lower Ωa had been reported [45–47], it had already been shown for temperate corals that calcification rates can remain unaffected by pCO2 levels as high as that projected for the end of the century [48,49]. In the study of Ries et al. [48], a nonlinear response was shown with calcification rates of Oculina arbuscula remaining constant at pCO2 levels between 400 and 900 µatm and decreasing at a pCO2 of 2850 µatm. It is therefore possible that M. oculata exhibits a similar nonlinear response and additional studies would be necessary to determine at which pCO2 level (or Ωa) calcification rates start to decrease. Few studies are actually available where calcification rates were tested for lower pCO2 levels. For the tropical coral Porites lutea, a linear response to Ωa, and increasing calcification rates with decreasing pCO2 were revealed [50]. For the Mediterranean Sea, it has been shown, that pH has decreased by 0.1 pH units owing to anthropogenic CO2 [29] and our results thus indicate that ocean acidification already has had a detrimental effect on calcification rates of M. oculata with a reduction by 50 per cent since pre-industrial time. Desmophyllum dianthus, M. oculata and L. pertusa have distinct sensitivities to increased temperature, with L. pertusa being the least tolerant to higher temperatures (C. Maier 2009, personal observation). This species is probably already at its highest temperature limit in the Mediterranean Sea. If the pCO2 increase since pre-industrial time had a similarly negative effect on L. pertusa calcification than it had on M. oculata, it could mean that L. pertusa might already be more negatively affected by climate change in the Mediterranean, where both temperature rise [26] and anthropogenic CO2 [29] are well documented. This might also partly explain, why M. oculata is, at the present-day, more widespread in the Mediterranean Sea than L. pertusa despite the fact that in the North Atlantic L. pertusa is the dominant CWC species. However, more studies including other parameters that may influence coral growth are needed to draw a final conclusion on a species-specific response to climate change scenarios.
Acknowledgements
We thank captains, crew and scientific shipboard staff of R/V Minibex, and R/V Urania, respectively. Cruise MEDCOR was partially funded by CNR and the EU HERMES programme, cruises on RV Minibex were part of a project on canyons of the Mediterranean Sea (MedSeaCan) lead by the Agences des aires marines protegées. Special thanks to L. Angeletti and A. Ceregato for on-board assistance, to S. Comeau for his assistance with the measurement of total alkalinity and J. Ries and an anonymous reviewer for their helpful comments on the manuscript. Financial support was provided by the European Commission through a Marie-Curie Fellowship (MECCA, project no 220299 and the project COMP via the Prince Albert II of Monaco Foundation. This work is a contribution to the ‘European Project on Ocean Acidification’ (EPOCA) which received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 211384, and also contributes to EU HERMIONE project (contract no. 226354) and is ISMAR-CNR Bologna scientific contribution 1733.
References
- 1.Roberts J. M., Wheeler A. J., Freiwald A. 2006. Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547 10.1126/science.1119861 (doi:10.1126/science.1119861) [DOI] [PubMed] [Google Scholar]
- 2.Freiwald A., Beuck L., Rüggeberg A., Taviani M., Hebbeln D. 2009. The white coral community in the central Mediterranean Sea revealed by ROV surveys. Oceanography 22, 36–52 10.5670/oceanog.2009.06 (doi:10.5670/oceanog.2009.06) [DOI] [Google Scholar]
- 3.Heifetz J. 2002. Coral in Alaska: distribution, abundance, and species associations. Hydrobiologia 471, 19–28 10.1023/A:1016528631593 (doi:10.1023/A:1016528631593) [DOI] [Google Scholar]
- 4.Hovland M., Vasshus S., Indreeide A., Austdal L., Nilsen Ø. 2002. Mapping and imaging deep-sea coral reefs off Norway, 1982–2000. Hydrobiologia 471, 13–17 10.1023/A:1016576514754 (doi:10.1023/A:1016576514754) [DOI] [Google Scholar]
- 5.Orejas C., Gori A., Iacono C. L., Puig P., Gili J.-M., Dale M. R. T. 2009. Cold-water corals in the Cap de Creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar. Ecol. Prog. Ser. 397, 37–51 10.3354/meps08314 (doi:10.3354/meps08314) [DOI] [Google Scholar]
- 6.Roberts J. M., Brown C. J., Long D., Bates C. R. 2005. Acoustic mapping using a multibeam echosounder reveals cold-water coral reefs and surrounding habitats. Coral Reefs 24, 654–669 10.1007/s00338-005-0049-6 (doi:10.1007/s00338-005-0049-6) [DOI] [Google Scholar]
- 7.Taviani M., Freiwald A., Zibrowius H. 2005. Deep coral growth in the Mediterranean Sea: an overview. In Cold-water corals and ecosystems (eds Freiwald A., Roberts J. M.), pp. 137–156 Heidelberg, Germany: Springer [Google Scholar]
- 8.Taviani M., et al. 2010. Pleistocene to Recent scleractinian deep-water corals and coral facies in the eastern Mediterranean. Facies 57, 579–603 10.1007/s10347-010-0247-8 (doi:10.1007/s10347-010-0247-8) [DOI] [Google Scholar]
- 9.White M., Mohn C., Stigter H., Mottram G. 2005. Deep-water coral development as a function of hydrodynamics and surface productivity around the submarine banks of the Rockall Trough, NE Atlantic. In Cold-water corals and ecosystems (eds Freiwald A., Roberts J. M.), pp. 503–514 Heidelberg, Germany: Springer [Google Scholar]
- 10.Freiwald A., Henrich R., Pätzold J. 1997. Anatomy of a deep-water coral reef mound from Stjernsund, West-Finnmark, Northern Norway. SEPM Spec. Vol. 56, 141–162 [Google Scholar]
- 11.Mikkelsen N., Erlenkauser H., Killingley J. S., Berger W. H. 1982. Norwegian corals: radiocarbon and stable isotopes in Lophelia pertusa. Boreas 5, 163–171 [Google Scholar]
- 12.Mortensen P. B., Rapp H. T. 1998. Oxygen and carbon isotope ratios related to growth line patterns in skeletons of Lophelia pertusa (L) (Anthozoa, Scleractinia): implications for determination of linear extension rates. Sarsia 83, 443–446 [Google Scholar]
- 13.Rogers A. D. 1999. The biology of Lophelia pertusa (Linnaeus 1758) and other deep-water reef-forming corals and impacts from human activities. Internat. Rev. Hydrobiol. 84, 315–406 [Google Scholar]
- 14.Bell N., Smith J. 1999. Coral growing on North Sea oil rigs. Nature 402, 601. 10.1038/45127 (doi:10.1038/45127)10604464 [DOI] [Google Scholar]
- 15.Gass S. E., Roberts J. M. 2006. The occurrence of the cold-water coral Lophelia pertusa (Scleractinia) on oil and gas platforms in the North Sea: colony growth, recruitment and environmental controls on distribution. Mar. Poll. Bull. 52, 549–559 10.1016/j.marpolbul.2005.10.002 (doi:10.1016/j.marpolbul.2005.10.002) [DOI] [PubMed] [Google Scholar]
- 16.Mortensen P. B. 2001. Aquarium observations on the deep-water coral Lophelia pertusa (L., 1758) (Scleractinia) and selected associated invertebrates. Ophelia 54, 83–104 [Google Scholar]
- 17.Orejas C., Gori A., Gili J. M. 2008. Growth rates of live Lophelia pertusa and Madrepora oculata from the Mediterranean Sea maintained in aquaria. Coral Reefs 27, 255. 10.1007/s00338-007-0350-7 (doi:10.1007/s00338-007-0350-7) [DOI] [Google Scholar]
- 18.Brooke S., Young C. M. 2009. In situ measurement of survival and growth of Lophelia pertusa in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 397, 153–161 10.3354/meps08344 (doi:10.3354/meps08344) [DOI] [Google Scholar]
- 19.Buddemeier R. W. 1978. Coral growth: retrospective analysis. In Coral reefs: research methods (eds Stoddart D. R., Johannes R. E.), pp. 551–571 Paris, France: Unesco [Google Scholar]
- 20.Orejas C., Ferrier-Pagès C., Reynaud S., Gori A., Beraud E., Tsounis G., Allemand D., Gili J. M. 2011. Long-term growth rates of four Mediterranean cold-water coral species maintained in aquaria. Mar. Ecol. Prog. Ser. 429, 57–65 10.3354/meps09104 (doi:10.3354/meps09104) [DOI] [Google Scholar]
- 21.Maier C., Hegeman J., Weinbauer M. G., Gattuso P. 2009. Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences 6, 1671–1680 10.5194/bg-6-1671-2009 (doi:10.5194/bg-6-1671-2009) [DOI] [Google Scholar]
- 22.Guinotte J. M., Orr J., Cairns S., Freiwald A., Morgan L., George R. 2006. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front Ecol. Environ. 4, 141–146 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2 (doi:10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2) [DOI] [Google Scholar]
- 23.Orr J. C., et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 10.1038/nature04095 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 24.Turley C. M., Roberts J. M., Guinotte J. M. 2007. Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448 10.1007/s00338-007-0247-5 (doi:10.1007/s00338-007-0247-5) [DOI] [Google Scholar]
- 25.Bethoux J. P., Gentili B., Morin P., Nicolas E., Pierre C., Ruiz-Pino D. 1999. The Mediterranean Sea: a miniature ocean for climatic and environmental studies and a key for the climatic functioning of the North Atlantic. Prog. Oceanogr. 44, 131–146 10.1016/S0079-6611(99)00023-3 (doi:10.1016/S0079-6611(99)00023-3) [DOI] [Google Scholar]
- 26.Bethoux J. P., Gentili B., Raunet J., Tailliez D. 1990. Warming trend in the western Mediterranean deep water. Nature 347, 660–662 10.1038/347660a0 (doi:10.1038/347660a0) [DOI] [Google Scholar]
- 27.Lelieveld J., Berresheim H., Borrmann S., Crutzen P. J. 2002. Global air pollution crossroads over the Mediterranean. Science 298, 794–799 10.1126/science.1075457 (doi:10.1126/science.1075457) [DOI] [PubMed] [Google Scholar]
- 28.Nykjaer L. 2009. Mediterranean Sea surface warming 1985–2006. Clim. Res. 39, 11–17 10.3354/cr00794 (doi:10.3354/cr00794) [DOI] [Google Scholar]
- 29.Touratier F., Goyet C. 2011. Impact of the eastern Mediterranean transient on the distribution of anthropogenic CO2 and first estimate of acidification for the Mediterranean Sea. Deep Sea Res. I 58, 1–15 [Google Scholar]
- 30.CIESM 2008. Impacts of ocean acidification on biological, chemical and physical systems in the Mediterranean and Black Seas. In CIESM Workshop Monographs, vol. 36 (ed. Briand F.), pp. 124 Monaco [Google Scholar]
- 31.Schubert A. 2010. The impact of ocean acidification on calcification of Mediterranean cold-water corals. Diplomarbeit, IFM-GEOMAR, pp. 68 Germany: Christian Albrechts-Universität zu Kiel [Google Scholar]
- 32.Herfort L., Schouten S., Abbas B., Veldhuis M. J. W., Coolen M. J. L., Wuchter C., Boon J. P., Herndl G. J., Damsté J. S. S. 2007. Variations in spatial and temporal distribution of Archaea in the North Sea in relation to environmental variables. FEMS Microbiol. Ecol. 62, 242–257 10.1111/j.1574-6941.2007.00397.x (doi:10.1111/j.1574-6941.2007.00397.x) [DOI] [PubMed] [Google Scholar]
- 33.Edmond J. M. 1970. High precision determination of titration alkalinity and total carbon dioxide content of seawater by potentiometric titration. Deep Sea Res. 17, 737–750 [Google Scholar]
- 34.DOE 1994. Recommended standard operation procedures. In Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water, version 2 (eds A. G. Dickson & C. Goyet), pp. 1–187. (ORNL/CDIAC-74). Online version. [Google Scholar]
- 35.Wolf-Gladrow D. A., Zeebe R. E., Klaas C., Körtzinger A., Dickson A. G. 2007. Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Deep Sea Res. I 106, 287–300 [Google Scholar]
- 36.Lavigne H., Gattuso P. 2011. Seacarb: seawater carbonate chemistry with R. R package version 2.4. See http://www.CRAN.R-project.org/package=seacarb
- 37.Chisholm J. R. M., Gattuso P. 1991. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 36, 1232–1239 10.4319/lo.1991.36.6.1232 (doi:10.4319/lo.1991.36.6.1232) [DOI] [Google Scholar]
- 38.Maier C., de Kluijver A., Agis M., Brussaard C. P. D., Van Duyl F. C., Weinbauer M. G. 2011. Dynamics of nutrients, total organic carbon, prokaryotes and viruses in onboard incubations of cold-water corals. Biogeosci. Discuss. 8, 3829–3861 10.5194/bgd-8-3829-2011 (doi:10.5194/bgd-8-3829-2011) [DOI] [Google Scholar]
- 39.Tambutté É., Allemand D., Mueller E., Jaubert J. 1996. A compartmental approach to the mechanism of calcification in hermatypic corals. J. Exp. Biol. 199, 1029–1041 [DOI] [PubMed] [Google Scholar]
- 40.Davies P. S. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395 10.1007/BF00428135 (doi:10.1007/BF00428135) [DOI] [Google Scholar]
- 41.Wild C., Mayr C., Wehrmann L., Schöttner S., Naumann M., Hoffmann F., Rapp H. T. 2008. Organic matter release by cold-water corals and its implication for fauna-microbe interaction. Mar. Ecol. Prog. Ser. 372, 67–75 10.3354/meps07724 (doi:10.3354/meps07724) [DOI] [Google Scholar]
- 42.Wild C., Wehrmann L. M., Mayr C., Schöttner S. I., Allers E., Lundälv T. 2009. Microbial degradation of cold-water coral-derived organic matter: potential implication for organic C cycling in the water column above Tisler Reef. Aquat. Biol. 7, 71–80 10.3354/ab00185 (doi:10.3354/ab00185) [DOI] [Google Scholar]
- 43.Kim H.-C., Lee K., Choi W. 2006. Contribution of phytoplankton and bacterial cells to the measured alkalinity of seawater. Limnol. Oceanogr. 51, 331–338 10.4319/lo.2006.51.1.0331 (doi:10.4319/lo.2006.51.1.0331) [DOI] [Google Scholar]
- 44.Bethoux J. P., Morin P., Chamuery C., Connan O., Gentili B., Ruiz-Pino D. 1998. Nutrients in the Mediterranean Sea, mass balance and statistical analysis of concentrations with respect to environmental change. Mar. Chem. 63, 155–169 10.1016/S0304-4203(98)00059-0 (doi:10.1016/S0304-4203(98)00059-0) [DOI] [Google Scholar]
- 45.Langdon C., Atkinson M. J. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07. 10.1029/2004JC002576 (doi:10.1029/2004JC002576) [DOI] [Google Scholar]
- 46.Marubini F., Ferrier-Pagès C., Furla P., Allemand D. 2008. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs 27, 491–499 10.1007/s00338-008-0375-6 (doi:10.1007/s00338-008-0375-6) [DOI] [Google Scholar]
- 47.Schneider K., Erez J. 2006. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293 10.4319/lo.2006.51.3.1284 (doi:10.4319/lo.2006.51.3.1284) [DOI] [Google Scholar]
- 48.Ries J. B., Cohen A. L., McCorkle D. C. 2010. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 29, 661–674 10.1007/s00338-010-0632-3 (doi:10.1007/s00338-010-0632-3) [DOI] [Google Scholar]
- 49.Rodolfo-Metalpa R., Martin S., Ferrier-Pagès C., Gattuso P. 2010. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7, 289–300 10.5194/bg-7-289-2010 (doi:10.5194/bg-7-289-2010) [DOI] [Google Scholar]
- 50.Ohde S., Hossain M. M. M. 2004. Effect of CaCO3 (aragonite) saturation state of seawater on calcification of Porites coral. Geochem. J. 38, 613–621 10.2343/geochemj.38.613 (doi:10.2343/geochemj.38.613) [DOI] [Google Scholar]