Abstract
The temperature size rule (TSR) is the tendency for ectotherms to develop faster but mature at smaller body sizes at higher temperatures. It can be explained by a simple model in which the rate of growth or biomass accumulation and the rate of development have different temperature dependence. The model accounts for both TSR and the less frequently observed reverse-TSR, predicts the fraction of energy allocated to maintenance and synthesis over the course of development, and also predicts that less total energy is expended when developing at warmer temperatures for TSR and vice versa for reverse-TSR. It has important implications for effects of climate change on ectothermic animals.
Keywords: development rate, ectotherm development, energy budget, growth rate, temperature size rule
1. Introduction
Changes in environmental temperature regimes pose potentially severe problems for ectothermic organisms. Their body temperatures fluctuate with environmental temperatures and the rates of most biochemical reactions and biological processes increase approximately exponentially with temperature. So changing temperature literally changes the pace of life.
The rates of ontogenetic growth and development are not exceptions. Ectothermic animals develop faster at warmer temperatures [1], and they usually mature at smaller body sizes—as much as 20 per cent smaller for a 10°C temperature increase. This phenomenon has been called the ‘temperature size rule’ (TSR) [2]. Like most biological ‘rules’, however, there are exceptions, including well-documented cases of the reverse-TSR, where the mature body sizes are larger at higher temperatures. Different compilations give about 15 per cent (13–17%) of reverse-TSR cases [2,3].
Here, we develop a simple model for the effects of temperature on ontogenetic development of ectothermic animals. The model extends an earlier model for allocation of energy and biomass to growth on endotherms [4] by explicitly incorporating the temperature dependence of the rate of development and the rate of somatic growth. Any imbalance in these two rates results in either the TSR or reverse-TSR, depending on which process is more sensitive to temperature. The model predicts the fractions of energy allocated to maintenance and biomass synthesis at a given developmental stage, including the total quantity of energy expended during development. We model explicitly the case of post-hatching development, where an animal consumes food to fuel its metabolism. Later, we consider the case of embryonic development, where the organism fuels its metabolism from energy reserves stored in the egg.
2. The model
Growth and development are fuelled by metabolism. It is well known that within the normal temperature range, metabolic rate increases approximately exponentially with temperature [5]. This relationship can be described by the Boltzmann relation, 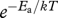 , where Ea is an ‘activation energy’ that reflects the kinetics of the underlying biochemical reactions and quantifies the temperature dependence, k is Boltzmann's constant and T is absolute temperature ([1,6], but see [7]). For processes governed by aerobic respiration, such as growth and development of most ectothermic animals, Ea is typically approximately 0.65 eV (corresponding to a Q10 of approx. 2.5 or a 2.5-fold increase in development rate for a 10°C increase in temperature) [1].
, where Ea is an ‘activation energy’ that reflects the kinetics of the underlying biochemical reactions and quantifies the temperature dependence, k is Boltzmann's constant and T is absolute temperature ([1,6], but see [7]). For processes governed by aerobic respiration, such as growth and development of most ectothermic animals, Ea is typically approximately 0.65 eV (corresponding to a Q10 of approx. 2.5 or a 2.5-fold increase in development rate for a 10°C increase in temperature) [1].
The effect of temperature on body size at maturity, however, depends on how energy and materials are allocated during ontogeny. The body mass m(t, T) at any time, t, during development depends on the magnitude of two different processes that can have different temperature dependence: (i) growth rate or rate of biomass accumulation, ∂m/∂t, with temperature dependence 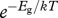 and (ii) development rate or rate of ontogenetic differentiation, ∂μ′/∂t, with temperature dependence
and (ii) development rate or rate of ontogenetic differentiation, ∂μ′/∂t, with temperature dependence 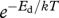 , where Eg and Ed are the respective ‘activation energies’ (figure 1). The relative developmental stage, μ′ ≡ t/tdev, is defined in terms of the time to the current developmental stage, t, relative to the total development time, tdev. Similarly, the relative body mass, μ ≡ m/M, is the body size at any time, t, relative to adult body size [4]. Since ∂μ′/∂t = 1/tdev, the total time of development
, where Eg and Ed are the respective ‘activation energies’ (figure 1). The relative developmental stage, μ′ ≡ t/tdev, is defined in terms of the time to the current developmental stage, t, relative to the total development time, tdev. Similarly, the relative body mass, μ ≡ m/M, is the body size at any time, t, relative to adult body size [4]. Since ∂μ′/∂t = 1/tdev, the total time of development 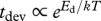 . The relative developmental stage is a simple way to standardize the overall ontogenetic trajectory on a 0–1 scale, so μ′ = 0.1 means 10 per cent of time to adulthood. The adult mass,
. The relative developmental stage is a simple way to standardize the overall ontogenetic trajectory on a 0–1 scale, so μ′ = 0.1 means 10 per cent of time to adulthood. The adult mass, 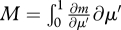 , is proportional to
, is proportional to 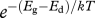 . It can also be calculated as
. It can also be calculated as 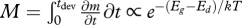 . Unless these two processes, growth and development, have exactly the same temperature dependence (Eg ≡ Ed), size at maturity will vary with temperature (figure 1).
. Unless these two processes, growth and development, have exactly the same temperature dependence (Eg ≡ Ed), size at maturity will vary with temperature (figure 1).
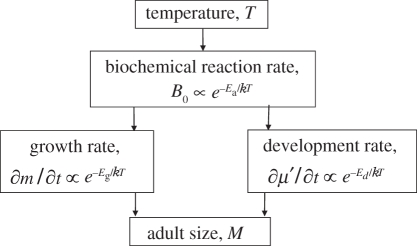
Figure 1.
Flow chart showing the effects of temperature on energy allocation during ontogenetic development. Metabolic rate, B, varies with body mass, m, and absolute temperature, T, as 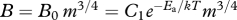 , where B0 is a temperature-dependent constant for a given species, Ea is the activation energy and k is Boltzmann's constant. Other variables: t is time, Eg and Ed are the activation energies for biomass accumulation rate and development rate, respectively.
, where B0 is a temperature-dependent constant for a given species, Ea is the activation energy and k is Boltzmann's constant. Other variables: t is time, Eg and Ed are the activation energies for biomass accumulation rate and development rate, respectively.
The animal consumes food to fuel growth and development from hatchling to adult. We use an extended ontogenetic growth model [4] to capture the energy allocation during growth. The rate of food assimilation, A, is the sum of the rates of energy consumption for maintenance and growth. So
| 2.1 |
and
| 2.2 |
where Bmaint is the rate of energy expended on maintenance, Bsyn is the rate of energy used to synthesize the new biomass, B is the total metabolic rate, S is the rate of energy allocation to storage in new biomass and Bsyn + S is the rate of energy expended on growth. Assuming that the energy content per unit of biomass, Ec, remains constant over ontogeny and is independent of temperature, these equations reflect energy and mass balance at any time, t. The integrated form over the entire development period is
| 2.3 |
where Qtot is the total quantity of energy expended, Qmaint is energy expended on maintenance, Qsyn is energy expended to synthesize new biomass and Qsto is energy stored in new biomass.
To incorporate effects of body size and temperature into equation (2.1), we make two assumptions:
— Throughout ontogeny, metabolic rate, B, scales with body mass as B = B0m3/4, where B0 is constant within and among individuals of the same species developing at the same temperature, but varies with temperature and species. The generality of m3/4 scaling of metabolic rate has been questioned (but see [4,8–10]). It is straightforward to substitute a generic scaling exponent, α, or a different numerical value when there is compelling evidence for deviation from three-quarter-power scaling.
— The temperature dependencies of the component processes can be characterized by Boltzmann relations as indicated above and below.
Rewriting equation (2.2) to explicitly incorporate body mass dependence gives
| 2.4 |
Additionally, Bmaint = mBm, where Bm = B0M−1/4 is the mass-specific rate of energy expenditure for maintenance, M is the body mass at maturity, Bsyn = Em(∂m/∂t), and Em is the quantity of energy expended to synthesize a unit of biomass, assumed here to be independent of temperature. Dividing both sides of equation (2.4) by m3/4 gives
| 2.5 |
Now incorporating the temperature dependencies in figure 1 and 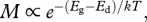 equation (2.5) gives
equation (2.5) gives
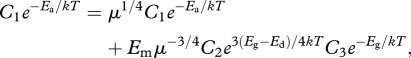 |
2.6 |
where μ ≡ m/M is the relative body mass at any time, t, which is temperature-independent; Ea, Eg and Ed are the activation energies for the rates of overall metabolism, growth and development, respectively; and C1, C2 and C3, are temperature-independent coefficients. Therefore, equation (2.6) gives the relationship among the temperature dependencies of rates of metabolism, growth and development
| 2.7 |
The model predicts the trajectories of biomass accumulation rate and development rate over ontogeny from hatching to maturity. Rewriting equation (2.4) by normalizing with respect to M, the body mass at maturity, gives a normalized biomass accumulation rate (see electronic supplementary material, appendix A)
| 2.8 |
where C1 is a temperature-independent coefficient. Normalizing with respect to the effect of temperature, 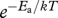 , in equation (2.8), predicts the biomass accumulation rate normalized to both temperature and body mass at maturity
, in equation (2.8), predicts the biomass accumulation rate normalized to both temperature and body mass at maturity
| 2.9 |
This model makes two additional predictions for the energy budgets of ectotherms during ontogeny. First, introducing effects of temperature into equation (2.2) gives (see electronic supplementary material, appendix B)
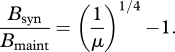 |
2.10 |
This predicts that the same fraction of metabolic energy is allocated to maintenance and synthesis at any given relative body mass, regardless of temperature and taxon. Second, integrating equation (2.1) with respect to time gives equation (2.3), and introducing effects of temperature gives
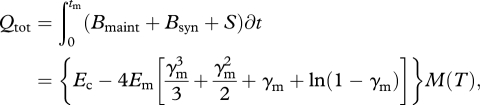 |
2.11 |
where tm is the time to reach some near-asymptotic adult size, madult = (1 − ɛ)M with ɛ ≪ 1 and γm = (madult/M)1/4 [11]. In equation (2.11), Ec, Em and γm are all independent of temperature, so the total quantity of energy consumed, Qtot, during ontogeny varies predictably with temperature as 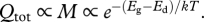 The model predicts that when developing at higher temperatures, TSR ectotherms should consume less energy and reverse-TSR ectotherms should consume more energy.
The model predicts that when developing at higher temperatures, TSR ectotherms should consume less energy and reverse-TSR ectotherms should consume more energy.
3. Empirical evaluation
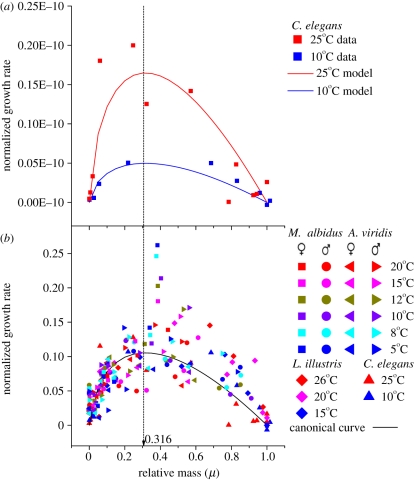
Experimental data for a variety of ectotherm taxa, such as nematodes, molluscs, crustaceans, insects and amphibians (table 1) support the model. The activation energy for metabolic rate, Ea, for each organism has been calculated based on equation (2.7). The average calculated Ea is 0.62 ± 0.03 eV. This average and most of the individual values are close to the predicted 0.65 eV, but a few outliers in table 1 and earlier studies [1,12] encompass a total range from 0.15 to 1.2 eV. Equation (2.9) predicts that all organisms should exhibit identical ‘canonical’ curves for normalized rates of biomass accumulation over ontogeny. Data for several organisms generally support this prediction. As predicted, the absolute rates, normalized only with respect to mass, increase with increasing temperature (figure 2a), but when normalized with respect to both mass and temperature, these curves all converge on the same shape with a peak at μ ≈ 0.3 (figure 2b). So, the highest rate of growth or biomass accumulation occurs at about 1/3 of adult mass, independent of temperature. This peak occurs at the same fraction of adult mass in ectotherms as it does in endotherms [4].
Table 1.
Effects of temperature on rate of development, biomass accumulation and metabolism and on body size at maturity. Experimental studies conducted at different temperatures provide data on development rate (Ed in 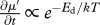 ) and body size (Eg − Ed in
) and body size (Eg − Ed in 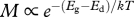 ) of different species. Effects of temperature on growth rate (Eg in
) of different species. Effects of temperature on growth rate (Eg in 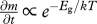 ) and metabolic rate (Ea in
) and metabolic rate (Ea in 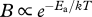 ) are calculated by Ea − (1/4)Eg − (3/4)Ed = 0 from the model. (Original data are in electronic supplementary material, appendix D.)
) are calculated by Ea − (1/4)Eg − (3/4)Ed = 0 from the model. (Original data are in electronic supplementary material, appendix D.)
| taxon | species | sex | development rate, 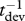
|
adult size, M | biomass accumulation rate, ∂m/∂t | metabolic rate, B |
|---|---|---|---|---|---|---|
| Ed (eV) (empirical) | Eg − Ed (eV) (empirical) | Eg (eV) (calculated) | Ea (eV) (calculated) | |||
| nematode | C. elegans | n.a. | 0.61 | −0.14 | 0.47 | 0.58 |
| mollusc | Crepidula plana | n.a. | 0.45 | −0.16 | 0.29 | 0.41 |
| crustacean | Acanthocyclops viridis | F | 0.66 | −0.13 | 0.53 | 0.63 |
| M | 0.73 | −0.14 | 0.60 | 0.69 | ||
| Macrocyclops albidus | F | 0.78 | −0.20 | 0.57 | 0.73 | |
| M | 0.77 | −0.13 | 0.64 | 0.74 | ||
| Acanthocyclops vernalis | F | 0.83 | −0.14 | 0.69 | 0.80 | |
| M | 0.86 | −0.24 | 0.62 | 0.80 | ||
| insect | Drosophila willistoni | M | 0.63 | −0.26 | 0.37 | 0.57 |
| F | 0.63 | −0.15 | 0.48 | 0.59 | ||
| Drosophila equinoxialis | M | 0.70 | −0.12 | 0.58 | 0.67 | |
| F | 0.70 | −0.16 | 0.54 | 0.66 | ||
| Drosophila pseudoobscura | M | 0.62 | −0.3 | 0.32 | 0.55 | |
| F | 0.62 | −0.3 | 0.32 | 0.55 | ||
| Drosophila persimilis | M | 0.59 | −0.19 | 0.4 | 0.54 | |
| F | 0.59 | −0.17 | 0.42 | 0.55 | ||
| Drosophila melanogaster | n.a. | 0.81 | −0.17 | 0.64 | 0.77 | |
| Chaoborus flavicans | n.a. | 0.53 | −0.46 | 0.07 | 0.42 | |
| Lucilia illustris | n.a. | 0.63 | 0.05 | 0.68 | 0.64 | |
| Chorthippus brunneus | F | 0.73 | 0.41 | 1.15 | 0.84 | |
| Omocestus viridulus | F | 0.11 | 0.18 | 0.29 | 0.15 | |
| Myrmeleotettix maculatus | F | 0.43 | 0.31 | 0.75 | 0.51 | |
| Stenobothrus lineatus | F | 0.57 | 0.33 | 0.90 | 0.66 | |
| amphibian | Rana sylvatica | n.a. | 1 | −0.86 | 0.14 | 0.79 |
Figure 2.
Different species of ectotherms developing at different temperatures follow the same canonical curve of biomass accumulation rate. (a) Normalized for differences in adult mass, rates of biomass accumulation are higher at higher temperatures, but the trajectories differ only in absolute rates (heights). (b) So, when normalized with respect to temperature as well as mass, the rates for different species and temperatures all cluster closely around the theoretically predicted curve, which peaks at 31.6% of adult body mass. Species are Macrocyclops albidus, Acanthocyclops viridis, Lucilia illustris and Caenorhabditis elegans. Macrocyclops albidus, A. viridis and C. elegans are temperature size rule (TSR) species; L. illustris is a reverse-TSR species. (Original data and calculations are in electronic supplementary material, appendix C.)
4. Discussion
It is well documented that, in ectotherms, rates of both somatic growth and ontogenetic development increase with increasing temperature, so time to maturity is shorter at higher temperatures. Whether body size at maturity is smaller or larger, however, depends on the difference in the temperature dependence between these two rates: the TSR occurs when development rate is more temperature-sensitive, the reverse-TSR when biomass accumulation rate is more sensitive.
The TSR and reverse-TSR are necessary consequences of differences in the temperature dependence of somatic growth rate and development rate. Growth is the trajectory of increase in somatic mass owing to the uptake, transformation and allocation of materials, so it must obey mass balance. Development is the trajectory of differentiation from fertilized egg or some later stage to adult, regulated by gene-by-environment interactions and physiological and biochemical signals. Growth and development usually occur together during ontogeny, but do not necessarily proceed at the same rates. For example, in arthropods, the pace of post-embryonic development is marked by moulting through a series of instars, and in holometabolous insects, the final stage, pupa, undergoes differentiation (development) but no growth (since the pupa metabolizes but does not feed, mass actually decreases) [13].
Many investigators have studied the temperature dependence of these two processes [2,14–17]. It is now well documented that differences in size at maturity after developing at different temperatures can be due to differences in cell size, number of cells or some combination of these. For example, Caenorhabditis elegans, other nematodes, rotifers and some arthropods, have a determinate fixed number of cells at maturity, so variation in adult body size is due entirely to variation in cell size. In Drosophila, however, differences in adult body size after developing at different temperatures may be due primarily to differences in either cell size or cell number [18,19].
The current study appears to offer four important advances over previous theoretical and empirical treatments of the TSR. First, we present an analytical model that is both very simple and very general. It incorporates a minimum number of assumptions, parameters and functions required to characterize the primary effect of temperature on the two critical processes: rate of biomass accumulation and rate of development. These can all potentially be measured to evaluate the model, its assumptions, and its predictions empirically. Model predictions provide a quantitative baseline against which to compare data for different kinds of animals developing under different physiological and environmental conditions. The assumptions can be relaxed to generate more complicated models for animals where they may not apply. This level of simplicity and generality stands in contrast to studies using a Sharpe–Schoolfield model [17,20], which incorporates multiple parameters of enzyme kinetics that may be only indirectly relevant and are difficult to measure directly, and to studies on other aspects of development, such as hormonal regulation [15,21,22].
Second, our model easily accommodates cases where differences in adult body size after developing at different temperatures are due to any combination of variation in cell size or cell number. In organisms with a fixed number of cells at maturity, such as C. elegans, variation in adult body size depends on the amount of somatic growth and hence the increase in cell size. This case can be analysed quantitatively by modifying the model to define the relative developmental stage by relative number of cells rather than relative time.
Third, our model not only accounts for how temperature gives rise to both the TSR and reverse-TSR, it also predicts the effect of developmental temperature on three other important aspects of development. The first is the trajectory of biomass accumulation rate over ontogeny (equations (2.8) and (2.9); figure 2). The model predicts that after normalizing for body mass at maturity, all ectotherms should exhibit quantitatively similar trajectories of ontogenetic growth, with temperature affecting only the absolute growth rate. The second prediction is that the proportion of energy allocated to maintenance as opposed to biosynthesis at a given relative size is independent of temperature (equation 2.10). So the net growth efficiency [23] at any given relative mass, μ, is independent of temperature, although it decreases over ontogeny (see electronic supplementary material, appendix E). The third novel prediction is how temperature affects the total quantity of energy used at each stage of development (equation 2.11). We know of no other model that predicts these important unifying features of ontogeny. They are relevant to understanding effects of environmental temperature on the life history, ecology and evolution of ectotherms. And again, when deviations from model predictions are observed empirically, these cases call attention to the importance of other factors left out of our deliberately very simple model.
Fourth, although developed above explicitly for the case of post-embryonic development, the model can easily be modified for the case of an embryonic development. An embryo developing within an egg is fuelled by energy reserves stored in yolk, so the total quantity of energy available is fixed by egg size. The model predicts that when eggs of the same size are incubated at higher temperatures, a TSR ectotherm consumes less energy during incubation and may hatch with unused yolk, whereas a reverse-TSR ectotherm uses more energy, consumes more yolk and may hatch at a less-developed stage. Actually, some TSR ectotherms appear to compensate by producing smaller eggs at higher temperatures [24–29], supporting the prediction that they consume less total energy during development [30]. More complicated treatments may be required to incorporate other features of embryonic development, such as: (i) cell size usually decreases over ontogeny with the multiple of cycles of cell division as a single-celled zygote develops into a multi-cellular hatchling and (ii) relative water content of the embryo may decrease and energy density of accumulated biomass may increase over ontogeny [31,32].
It remains to be explained why the majority of ectotherms follow the TSR, whereas only a minority exhibit the reverse-TSR. Several authors have proposed adaptive explanations for the preponderance of the TSR [23,33–42], and many other studies in the context of geographical variation in ectotherm body size in gradients of environmental temperature, especially in Drosophila. Usually selection can be expected to minimize the time and the total energy consumed during ontogeny. Time can be minimized by behavioural temperature regulation, selecting higher temperatures for both incubation and post-hatching development. This tendency for ‘warmer to be better’ [43] should translate into a TSR. Selection to incorporate a margin of safety so that embryos do not run out of yolk should also favour TSR. A reverse-TSR should be expected only in rare cases when it is advantageous to develop at colder temperatures, such as to behaviourally select cold microclimates to avoid predators or to prolong development owing to constraints of environmental seasonality [42]. The theory developed here should also apply to special cases, such as when temperature dependence of solubility and diffusion of oxygen in aqueous media limit egg size and development of aquatic organisms [44,45].
The phenomenon of ‘Bergman's Rule’ in ectotherms is consistent with the TSR. In latitudinal and elevational gradients of increasing temperature, both aquatic and terrestrial arthropods and other ectotherms are often smaller in warmer environments [46]. So this pattern could potentially be just a direct phenotypic response to environmental temperature. Alternatively, if the TSR is most often adaptive for any of the above reasons, then the Bergmann's rule phenomenon may reflect selection for decreased developmental time and energy consumption in warmer environments. Application of our model should allow predictions for effects of global warming on ectotherm development.
Our model is generally consistent with the above adaptive hypotheses, but offers additional insights. Both growth rate and development rate vary approximately exponentially with temperature, and the magnitude of the temperature dependence of each rate is subject to natural selection. In general, natural selection should tend to keep the temperature dependence of these rates very nearly equal, so that the developmental programme buffers size at maturity against perturbations owing to differences in temperature. However, equation (2.7) shows that Eg − Ed is very sensitive to Ea and Ed (e.g. Δ(Eg − Ed) = 4(ΔEa −ΔEd)), and consequently body mass at maturity, M, is extremely sensitive to these temperature dependencies.
One other interesting application of the theory is to organisms that have temperature-dependent sex determination, which occurs in many reptiles and amphibians, some fish, and at least one bird [47–50]. Small differences in temperature during development should potentially affect not only the gender of the hatchling, but also the time of development and body size at hatching. It is known that warmer temperatures during development can produce either males or females, depending on taxon: for example, generally males in crocodilians and females in turtles. In the Australian brush turkey (Alectura lathami), higher temperatures during incubation of eggs result in proportionately more females with larger body mass at hatching [48], consistent with reverse-TSR. Temperature-induced sex-related differences in development time and body size at hatching should have potentially important consequences for subsequent life history and ecology.
The quantitative model developed here, and more complicated analytical mathematical or computer simulation versions that could be developed for cases that do not meet the simplifying assumptions, provide a theoretical basis for assessing responses of ectothermic organisms to changes in environmental temperature regimes [51]. The magnitude of recent anthropogenic global warming is already substantial and likely to increase for decades and perhaps centuries [52]. The impacts on ectothermic animals and their ecology will undoubtedly be profound. Many of these impacts can be understood in a general theoretical context that is based on the fundamental effect of temperature on metabolism, and the effects of metabolism on many aspects of organism structure and function, population and ecosystem ecology, and biological evolution [53–55]. It will be impossible to conduct the detailed studies, on one species at a time, to predict effects of climate change on the abundance, distribution and diversity of species. A practical alternative will be to start by developing general theory, like the model presented here, which is based on fundamental biological principles and can make testable quantitative predictions.
Acknowledgements
We thank HHMI-NIBIB Interfaces grant (to W.Z. and J.H.B.), National Institutes of Health grant P20RR-018754 (to M.E.M.), National Science Foundation grants DEB-0083422 and CCF0621900 (to J.H.B.) and PHY0706174 and PHY0202180 (to G.B.W.), and the Thaw Charitable Trust and the Bryan and June Zwan Foundation (to G.B.W.) for support. Numerous colleagues contributed helpful discussions and Hans Heesterbeek, Daniel Costa and several anonymous reviewers made helpful comments on earlier drafts of the manuscript.
References
- 1.Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 2.Atkinson D. 1994. Temperature and organism size—a biological law for ectotherms? Adv. Ecol. Res. 25, 1–58 10.1016/S0065-2504(08)60212-3 (doi:10.1016/S0065-2504(08)60212-3) [DOI] [Google Scholar]
- 3.Willott S. J., Hassall M. 1998. Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct. Ecol. 12, 232–241 10.1046/j.1365-2435.1998.00180.x (doi:10.1046/j.1365-2435.1998.00180.x) [DOI] [Google Scholar]
- 4.Hou C., Zuo W., Moses M. E., Woodruff W. H., Brown J. H., West G. B. 2008. Energy uptake and allocation during ontogeny. Science 323, 736–739 10.1126/science.1162302 (doi:10.1126/science.1162302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochachka P. W., Somero G. N. 2002. Biochemical adaptation: mechanism and process in physiological evolution. New York, NY: Oxford University Press [Google Scholar]
- 6.Arrhenius S. 1889. Ueber die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Saeuren [On the rate of reaction of the inversion of sucrose by acids]. Z. Phys. Chem. 4, 226–248 [Google Scholar]
- 7.Knies J. L., Kingsolver J. G. 2010. Erroneous Arrhenius: modified Arrhenius model best explains the temperature dependence of ectotherm fitness. Am. Nat. 176, 227–233 10.1086/653662 (doi:10.1086/653662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Savage V. M., Gillooly J. F., Woodruff W. H., West G. B., Allen A. P., Enquist B. J., Brown J. H. 2004. The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282 10.1111/j.0269-8463.2004.00856.x (doi:10.1111/j.0269-8463.2004.00856.x) [DOI] [Google Scholar]
- 10.Moses M. E., Hou C., Woodruff W. H., West G. B., Nekola J. C., Zuo W., Brown J. H. 2008. Revisiting a model of ontogenetic growth: estimating model parameters from theory and data. Am. Nat. 171, 632–645 10.1086/587073 (doi:10.1086/587073) [DOI] [PubMed] [Google Scholar]
- 11.West G. B., Brown J. H., Enquist B. J. 2001. A general model for ontogenetic growth. Nature 413, 628–631 10.1038/35098076 (doi:10.1038/35098076) [DOI] [PubMed] [Google Scholar]
- 12.Irlich U. M., Terblanche J. S., Blackburn T. M., Chown S. L. 2009. Insect rate–temperature relationships: environmental variation and the metabolic theory of ecology. Am. Nat. 174, 819–835 10.1086/647904 (doi:10.1086/647904) [DOI] [PubMed] [Google Scholar]
- 13.Cressa C., Lewis W. M., Jr 1984. Growth and development patterns in a tropical Chaoborus species and their ecological significance. Arch. Hydrobiol. 100, 21–28 [Google Scholar]
- 14.Van Voorhies W. A. 1996. Bergmann size clines: a simple explanation for their occurrence in ectotherms. Evolution 50, 1259–1264 10.2307/2410666 (doi:10.2307/2410666) [DOI] [PubMed] [Google Scholar]
- 15.Nijhout H. F., Davidowitz G., Roff D. A. 2006. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 5, 16. 10.1186/jbiol43 (doi:10.1186/jbiol43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong G., van der Have T. M. 2009. Temperature dependence of development rate, growth rate and size: from biophysics to adaptation. In Phenotypic plasticity of insects: mechanisms and consequence (eds Whitman D. W., Ananthakrishnan T. N.), pp. 461–526 Plymouth, UK: Science Publishers, Inc [Google Scholar]
- 17.de Jong G. 2010. A biophysical interpretation of temperature-dependent body size in Drosophila aldrichi and D. buzzatii. J. Thermal Biol. 35, 85–99 10.1016/j.jtherbio.2009.12.001 (doi:10.1016/j.jtherbio.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 18.Partridge L., Barrie B., Fowler K., French V. 1994. Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48, 1269–1276 10.2307/2410384 (doi:10.2307/2410384) [DOI] [PubMed] [Google Scholar]
- 19.French V., Feast M., Partridge L. 1998. Body size and cell size in Drosophila: the developmental response to temperature. J. Insect Physiol. 44, 1081–1089 10.1016/S0022-1910(98)00061-4 (doi:10.1016/S0022-1910(98)00061-4) [DOI] [PubMed] [Google Scholar]
- 20.van der Have T. M., de Jong G. 1996. Adult size in ectotherms: temperature effects on growth and differentiation. J. Theor. Biol. 138, 329–340 10.1006/jtbi.1996.0224 (doi:10.1006/jtbi.1996.0224) [DOI] [Google Scholar]
- 21.Davidowitz G., Nijhout H. F. 2004. The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integr. Comp. Biol. 44, 443–449 10.1093/icb/44.6.443 (doi:10.1093/icb/44.6.443) [DOI] [PubMed] [Google Scholar]
- 22.Davidowitz G., D'Amico L. J., Nijhout H. F. 2004. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 6, 49–62 [Google Scholar]
- 23.Angilletta M. J., Dunham A. E. 2003. The temperature-size rule in ectotherms: simple evolutionary explanations may not be general. Am. Nat. 162, 332–342 10.1086/377187 (doi:10.1086/377187) [DOI] [PubMed] [Google Scholar]
- 24.Azevedo R. B. R., French V., Partridge L. 1996. Thermal evolution of egg size in Drosophila melanogaster. Evolution 50, 2338–2345 10.2307/2410702 (doi:10.2307/2410702) [DOI] [PubMed] [Google Scholar]
- 25.Atkinson D., Morley S. A., Weetman D., Hughes R. N. 2001. Offspring size responses to maternal environment in ectotherms. In Environment and animal development: genes, life histories and plasticity (eds Atkinson D., Thorndyke M.), pp. 269–286 Oxford, UK: BIOS Scientific [Google Scholar]
- 26.Fischer K., Fiedler K. 2001. Egg weight variation in the butterfly Lycaena hippothoe: more small or fewer large eggs? Popul. Ecol. 43, 105–109 10.1007/PL00012009 (doi:10.1007/PL00012009) [DOI] [Google Scholar]
- 27.Fischer K., Bot A. N. M., Brakefield P. M., Zwaan B. J. 2003. Fitness consequences of temperature-mediated egg size plasticity in a butterfly. Funct. Ecol. 17, 803–810 10.1111/j.1365-2435.2003.00798.x (doi:10.1111/j.1365-2435.2003.00798.x) [DOI] [Google Scholar]
- 28.Steigenga M. J., Zwaan B. J., Brakefield P. M., Fischer K. 2005. The evolutionary genetics of egg size plasticity in a butterfly. J. Evol. Biol. 18, 281–289 10.1111/j.1420-9101.2004.00855.x (doi:10.1111/j.1420-9101.2004.00855.x) [DOI] [PubMed] [Google Scholar]
- 29.Steigenga M. J., Fischer K. 2007. Ovarian dynamics, egg size, and egg number in relation to temperature and mating status in a butterfly. Entomol. Exp. Appl. 125, 195–203 10.1111/j.1570-7458.2007.00610.x (doi:10.1111/j.1570-7458.2007.00610.x) [DOI] [Google Scholar]
- 30.Booth D. T., Kiddell K. 2007. Temperature and the energetics of development in the house cricket (Acheta domesticus). J. Insect Physiol. 53, 950–953 10.1016/j.jinsphys.2007.03.009 (doi:10.1016/j.jinsphys.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 31.Carey C., Rahn H., Parisi P. 1980. Calories, water, lipid and yolk in avian eggs. Condor 82, 335–343 10.2307/1367405 (doi:10.2307/1367405) [DOI] [Google Scholar]
- 32.Ar A., Rahn H. 1980. Water in the avian egg overall budget of incubation. Am. Zool. 20, 373–384 [Google Scholar]
- 33.Perrin N. 1988. Why are offspring born larger when it is colder? Phenotypic plasticity for offspring size in the cladoceran Simocephalus vetulus (Müller). Funct. Ecol. 2, 283–288 10.2307/2389399 (doi:10.2307/2389399) [DOI] [Google Scholar]
- 34.Sibly R. M., Atkinson D. 1994. How rearing temperature affects optimal adult size in ectotherms. Func. Ecol. 8, 486–493 10.2307/2390073 (doi:10.2307/2390073) [DOI] [Google Scholar]
- 35.Nunney L. 1996. The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution 50, 1193–1204 10.2307/2410660 (doi:10.2307/2410660) [DOI] [PubMed] [Google Scholar]
- 36.Partridge L., French V. 1996. Thermal evolution of ectotherm body size: why get big in the cold? In Animals and temperature: phenotypic and evolutionary adaptation (eds Johnston I. A., Bennett A. F.), pp. 265–292 Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Yampolsky L. Y., Scheiner S. M. 1996. Why larger offspring at lower temperatures? A demographic approach. Am. Nat. 147, 86–100 10.1086/285841 (doi:10.1086/285841) [DOI] [Google Scholar]
- 38.Atkinson D., Sibly R. M. 1997. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 12, 235–239 10.1016/S0169-5347(97)01058-6 (doi:10.1016/S0169-5347(97)01058-6) [DOI] [PubMed] [Google Scholar]
- 39.Nunney L., Cheung W. 1997. The effect of temperature on body size and fecundity in female Drosophila melanogaster: evidence for adaptive plasticity. Evolution 51, 1529–1535 10.2307/2411205 (doi:10.2307/2411205) [DOI] [PubMed] [Google Scholar]
- 40.Angilletta M. J., Steury T. D., Sears M. W. 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509 10.1093/icb/44.6.498 (doi:10.1093/icb/44.6.498) [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist G. W., Huey R. B. 2004. Plastic and genetic variation in wing loading as a function of temperature within and among parallel clines in Drosophila subobscura. Integr. Comp. Biol. 44, 461–470 10.1093/icb/44.6.461 (doi:10.1093/icb/44.6.461) [DOI] [PubMed] [Google Scholar]
- 42.Walters R. J., Hassall M. 2006. The temperature-size rule in ectotherms: may a general explanation exist after all? Am. Nat. 167, 510–523 10.1086/501029 (doi:10.1086/501029) [DOI] [PubMed] [Google Scholar]
- 43.Frazier M., Huey R. B., Berrigan D. 2006. Thermodynamics constrains the evolution of insect population growth rates: ‘warmer is better’. Am. Nat. 168, 512–520 10.1086/506977 (doi:10.1086/506977) [DOI] [PubMed] [Google Scholar]
- 44.Atkinson D. 1995. Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. J. Thermal Biol. 20, 61–74 10.1016/0306-4565(94)00028-H (doi:10.1016/0306-4565(94)00028-H) [DOI] [Google Scholar]
- 45.Gillooly J. F., Dodson S. I. 2000. Latitudinal patterns in the size distribution and seasonal dynamics of new world, freshwater cladocerans. Limnol. Oceanogr. 45, 22–30 10.4319/lo.2000.45.1.0022 (doi:10.4319/lo.2000.45.1.0022) [DOI] [Google Scholar]
- 46.Ray C. 1960. The application of Bergmann's and Allen's rules to the poikilotherms. J. Morphol. 106, 85–108 10.1002/jmor.1051060104 (doi:10.1002/jmor.1051060104) [DOI] [PubMed] [Google Scholar]
- 47.Bull J. J. 1980. Sex determination in reptiles. Quart. Rev. Biol. 55, 3–21 10.1086/411613 (doi:10.1086/411613) [DOI] [Google Scholar]
- 48.Göth A., Booth D. T. 2005. Temperature-dependent sex ratio in a bird. Biol. Lett. 1, 31–33 10.1098/rsbl.2004.0247 (doi:10.1098/rsbl.2004.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crews D., Bull J. J. 2008. Sex determination: some like it hot (and some don't). Nature 451, 527–528 10.1038/451527a (doi:10.1038/451527a) [DOI] [PubMed] [Google Scholar]
- 50.Ospina-Álvarez N., Piferrer F. 2008. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 3, e2837. 10.1371/journal.pone.0002837 (doi:10.1371/journal.pone.0002837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo W. 2011. Coda: the role of mechanistical models. From growth to extinction: explored by life history and metabolic theory. PhD thesis, pp. 80–83 See http://hdl.handle.net/1928/12854. [Google Scholar]
- 52.IPCC (Intergovernmental Panel on Climate Change) 2007. Climate change 2007: The physical science basis. Working Group I contribution to the Fourth Assessment Report of the IPCC. Cambridge, UK: Cambridge University Press [Google Scholar]
- 53.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 54.Dillon M. E., Wang G., Huey R. B. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 10.1038/nature09407 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 55.Sibly R. M., Brown J. H., Kodric-Brown A. 2012. Metabolic ecology, a scaling approach. Oxford, UK: Wiley-Blackwell [Google Scholar]