Abstract
The Ediacaran Doushantuo biota has yielded fossils interpreted as eukaryotic organisms, either animal embryos or eukaryotes basal or distantly related to Metazoa. However, the fossils have been interpreted alternatively as giant sulphur bacteria similar to the extant Thiomargarita. To test this hypothesis, living and decayed Thiomargarita were compared with Doushantuo fossils and experimental taphonomic pathways were compared with modern embryos. In the fossils, as in eukaryotic cells, subcellular structures are distributed throughout cell volume; in Thiomargarita, a central vacuole encompasses approximately 98 per cent cell volume. Key features of the fossils, including putative lipid vesicles and nuclei, complex envelope ornament, and ornate outer vesicles are incompatible with living and decay morphologies observed in Thiomargarita. Microbial taphonomy of Thiomargarita also differed from that of embryos. Embryo tissues can be consumed and replaced by bacteria, forming a replica composed of a three-dimensional biofilm, a stable fabric for potential fossilization. Vacuolated Thiomargarita cells collapse easily and do not provide an internal substrate for bacteria. The findings do not support the hypothesis that giant sulphur bacteria are an appropriate interpretative model for the embryo-like Doushantuo fossils. However, sulphur bacteria may have mediated fossil mineralization and may provide a potential bacterial analogue for other macroscopic Precambrian remains.
Keywords: Doushantuo, Ediacaran, Thiomargarita, exceptional preservation, taphonomy
1. Introduction
The ca 570 Ma Ediacaran Doushantuo fossil assemblage appears to contain one of the oldest records of the animal evolutionary lineage. The Doushantuo Tianzhushania fossils have been widely interpreted as metazoan embryos, based on the complex nature of their cell structure and the similarity of their morphology to features of modern animal embryos, including reductive cell division, a bounding membrane comparable with a metazoan fertilization envelope, large size and the absence of rigid cell walls [1–3]. Other interpretations identify these fossils as representing non-metazoan holozoans [4]. These fossils may thus provide a snapshot of very early events in the evolution of animals.
However, an alternative hypothesis suggests that the fossils may not be eukaryotic cells but, rather, the remains of giant prokaryotic cells. Bailey et al. [5] argued that many of the Doushantuo globular microfossils might also be interpreted as bacteria, based on comparisons between the fossils and Thiomargarita, extant giant sulphur-oxidizing bacteria that are capable of reductive cell division [6,7]. Living Thiomargarita in reductive division stages show a remarkable resemblance to eukaryotic embryos and to the Doushantuo fossils in size and cell division boundaries (figure 2a). This interpretative model offers a solution to a number of problems with the animal embryo interpretation, including the absence of later developmental stages, the superabundance of specimens, and the bias towards one-, two- and four-cell specimens [5]. Furthermore, Thiomargarita cells are able to control phosphate mineral precipitation under both oxic and anoxic conditions [8,9], thus providing a potential mechanism for the preservation of the fossils and of other organisms in the assemblage [5].
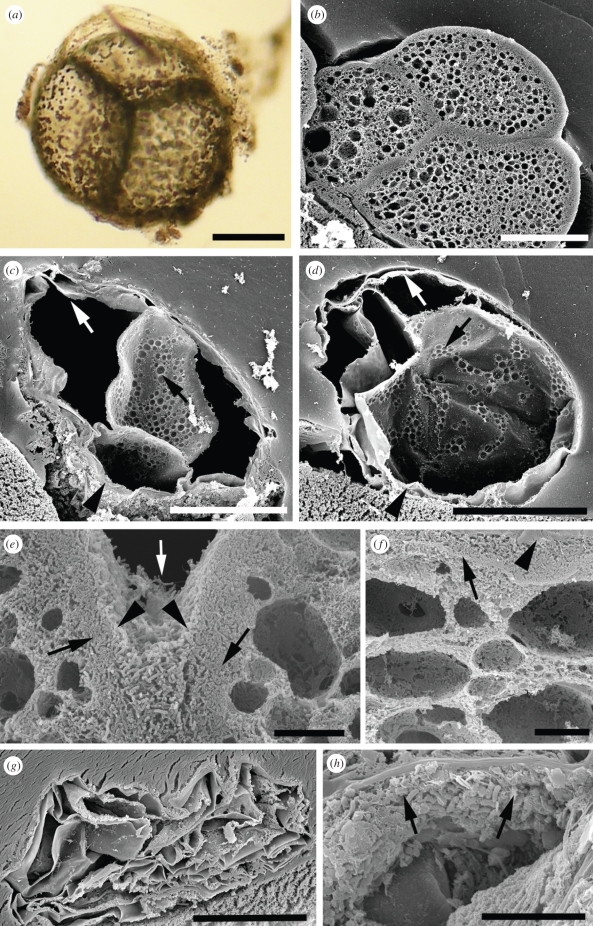
Figure 2.
Taphonomic comparison of Thiomargarita with marine invertebrate embryos. (a) Light micrograph of multi-cellular reductive division-stage Thiomargarita fixed with 2.5% glutaraldehyde. External gelatinous sheath, outer cell wall, internal cell boundaries and intracellular sulphur inclusions are visible. (b–h) Sections viewed by scanning electron microscopy. (b) Cleavage stage embryo of the sea urchin H. erythrogramma, fixed, settled on a Millipore filter and embedded in agarose. Outer fertilization envelope has contracted down onto the embryo; outer cell boundaries, internal cell boundaries, denser cortical cytoplasm and cavities of characteristic lipid droplet vesicles scattered through the internal cytoplasm are visible. (c,d) Reductive division stage Thiomargarita fixed with 2.5% glutaraldehyde at time of collection, then settled on a Millipore filter and embedded in agarose. Unlike embryos, Thiomargarita specimens collapsed. In Thiomargarita sections, external sheaths (arrowheads) are visible at bottom edges; external lamina are present under the sheath (white arrows); internal cell boundaries and sites of intracellular sulphur inclusions (black arrows) are visible. Note the vast central vacuole (black cavity). In (b–d), the Millipore filter substrate is a rough texture at lower left of the panels and embedding agarose is a smooth texture surrounding the specimen. (e,f) Microbial taphonomy of H. erythrogramma embryos. BME-stabilized embryos were incubated with Psuedoalteromanas tunicata, a marine gamma proteobacterium, for 6 days and then fixed in glutaraldehyde. (e) Internal views of a pseudomorphed two-cell embryo sectioned after fixing. The dense surface biofilm (arrowheads) and microbially replaced intracellular fabric (arrows) are visible. The white arrow indicates the pseudomorphed cell boundary between the two embryonic cells. (f) Pseudomorphed embryo embedded before sectioning, as in (b–d). Arrowhead indicates the boundary between embryo surface biofilm and the agarose; arrow shows exterior surface of the embryo. The embryo is partially compressed in the vertical plane by the embedding procedure, as indicated by the slightly flattened lipid vesicle droplets. (g,h) Microbial taphonomy of Thiomargarita stabilized in BME at the time of collection, incubated for 6 days with P. tunicata under the same conditions as in (e,f), then embedded and sectioned as in (b,c,d,f). (g) Microbial-treated Thiomargarita cell. Bacteria do not fill the vacuole, and the specimen is partly collapsed. (h) Enlarged view of part of another microbial-treated Thiomargarita cell. Bacteria do not fill the internal spaces, but partial bacterial biofilms have formed on some of the surfaces of the laminar sheets; arrows indicate bacterial bodies in the biofilm. In (g), the Millipore filter substrate is visible at the bottom right. Scale bars: (a–d,g) 100 µm; (e,f,h) 10 µm.
Nonetheless, there are inherent problems with the giant-sulphur-bacteria interpretation. There is no structure in Thiomargarita comparable with the multi-layered ornate envelope observed in the fossils [10], though Bailey et al. [11] compared the ornamentation of the fossil envelope walls with the surface features seen in Achromatium (a close relative of Thiomargarita) that reflect the shape of calcite and sulphur inclusions in the cell [12]. Nor is there in the bacteria anything resembling the highly ornamented organic vesicles that surround the dividing cells in silicified Doushantuo specimens [13]. Thiomargarita also differs from the fossils in having a very large central vacuole in each cell, rather than having subcellular structures distributed throughout the cell volume, as seen in many of the Doushantuo globular microfossils [14]. Furthermore, reports of nucleus-like bodies in the fossils [15–17] are seemingly at odds with a prokaryote hypothesis. In spite of these problems, giant sulphur bacteria offer a potential explanation of the Doushantuo fossils, whose kingdom-level identification remains uncertain. Moreover, giant sulphur bacteria might also account for other fossils interpreted as eukaryotes mostly on the basis of their large size.
Debate over the interpretation of the embryo-like Doushantuo fossils has hitherto relied on comparisons between living organisms and fossilized remains. However, as fossils have invariably undergone death, post-mortem decay and diagenetic alteration, it is not meaningful to compare them directly with living organisms [18]. Although patterns of decay in animal embryos are now being studied [17,19–22], nothing is known about decay processes or preservation potential in giant sulphur-oxidizing bacteria. Previous studies on decay of bacteria (see earlier studies [23,24] for reviews) are of limited relevance because of the comparatively small size, lack of central vacuole and lack of successive reductive division in the species studied. In order to address this issue, we experimentally infer a decay sequence for Thiomargarita, and compare the results with the patterns observed in the Doushantuo fossils and other putative fossil eukaryotes. We use the data to assess whether the inferred decay pathways of giant sulphur bacteria are compatible with the patterns observed in the fossil remains.
We also assess the potential of giant sulphur bacteria for stabilization and preservation by biofilm-forming bacteria. We have shown that animal embryo tissues can be replaced and replicated by bacterial biofilms and hypothesized that this process is key to their fossilization [17]. Here, we report the results of applying to Thiomargarita the same experimental microbial taphonomic conditions that we have previously applied to animal embryos.
2. Material and methods
(a). Sequence of decay in giant sulphur bacteria
Populations of Thiomargarita namibiensis collected off the Namibian coast between 24° S and 26° S at less than 100 m depth were obtained from Heide N. Schulz-Vogt and stored in a container with the original sea water and host sediment. This container was sealed and maintained at a temperature of 15°C continuously before the experiments. Attempts to kill the bacteria in a consistent manner using strongly reducing conditions induced with beta-mercaptoethanol (BME) [17,19–21] were ineffective. Preliminary trials placing Thiomargarita in oxic, anoxic and reducing conditions to assess preservation potential under different environmental conditions using standard protocols [19] produced no discernible difference between samples. Consequently, we relied on a decay pathway from the natural taphonomic spectrum in the population.
Batches of the bacteria–sediment mixture were fixed in 4 per cent paraformaldehyde, then viewed with differential interference contrast on a Zeiss Photomicroscope III and photographed using Q-Imaging QCapture Pro v. 6.0 software. In other cases, colonies were isolated from the mixture using a Pasteur pipette and transferred to sea water. These were then fixed in 4 per cent paraformaldehyde, stained with streptavidin-Alexa 488, washed in sea water and mounted in Mowiol on a slide with a coverslip. These specimens were analysed using a Leica SPS confocal microscope.
(b). Experimental microbial taphonomy
Sea urchins, Heliocidaris erythrogramma (egg diameters approx. 400 µm), were collected in Sydney, Australia. Embryos were raised to early cleavage stages, then killed and stabilized with 100 mM BME in sea water as previously described [17,19]. BME-stabilized embryos do not undergo autolysis and can be stored for more than a year at 4°C. Embryos were fixed with 2.5 per cent glutaraldehyde in sea water at 4°C for study using scanning electron microscopy (SEM), or washed in sterile sea water for use in microbial taphonomy experiments (figure 2). For SEM, embryos were fractured and processed as described previously [17,19], or embedded and processed as described below for Thiomargarita.
Multicellular reductive division stages of Thiomargarita bacteria that are morphologically similar to Thiomargarita nelsonii (Candidatus Thiomargarita nelsonii [25]) used for microbial taphonomy were collected from Hydrate Ridge, Oregon, USA, by Gregory W. Rouse, and were provided courtesy of the Scripps Institution of Oceanography, San Diego, California, USA. At the time of collection, Thiomargarita samples were either fixed in 2.5 per cent glutaraldehyde or stabilized with 100 mM BME, and stored at 4°C. BME-stabilized specimens were used for taphonomy experiments. For SEM, Thiomargarita fixed at the time of collection or following microbial incubation were collected on a Millipore filter, embedded in agarose, and then fractured and processed as previously described for embryos.
For experimental bacterial taphonomy [17], cultures of the aerobic marine bacterium Pseudoalteromonas tunicata were introduced to embryos or Thiomargarita in sterile sea water (sea water filtered through a 0.22 µm Millipore filter). Pseudoalteromonas tunicata was used for microbial taphonomy based on the prevalence of Pseudoalteromonas spp. in natural sea water bacteria populations that resulted in pseudomorphing [17]. Incubations were done for 6 days at 23°C in Nitex mesh-bottomed baskets (mesh pore size 150 µm for embryos; 70 µm for Thiomargarita) in Corning six-well cell culture plates. Following bacterial taphonomy, specimens were fixed in 2.5 per cent glutaraldehyde and processed as described.
(c). Processing and imaging of fossil material
Figured fossil specimens originate from the upper Doushantuo Formation in Guizhou and Hubei Provinces, China. Fossils were extracted from carbonate host rocks using 10 per cent acetic acid. Figured specimens are deposited in the Swedish Museum of Natural History (NRM). The synchrotron radiation X-ray Tomographic Microscopy (srXTM) investigations were conducted at the X04SA and X02DA (TOMCAT) beamlines of the Swiss Light Source (SLS) using methods outlined previously [26]. The data were visualized and analysed using Avizo software.
3. Results
(a). Sequence of decay in giant sulphur bacteria
In life, Thiomargarita specimens have a large central membrane-bound vacuole that occupies 98 per cent of the cell volume [6]. Surrounding the vacuole is a thin layer of cytoplasm in which vesicles of elemental sulphur are stored alongside granules of glycogen and polyphosphate (figure 1a,e). When examined under confocal microscopy with fluorescein isothiocyanate, the vacuole does not take up the stain as the vacuole contains aqueous nitrate, not biomass [6]. The sulphur globules are distributed throughout the cytoplasm layer but are never found within the central vacuole in intact cells [6]. The vacuole and outer cytoplasm are surrounded by a multi-layered envelope [5], and the entire organism is frequently enveloped in a mucous sheath, which commonly plays host to epibiont bacteria of various morphologies. In T. namibiensis, the sheath is typically thick and contains chains of cells in comparison with other Thiomargarita-like sulphur bacteria in which the sheath can be thin or absent. Cells within a single chain can exhibit a broad taphonomic spectrum.
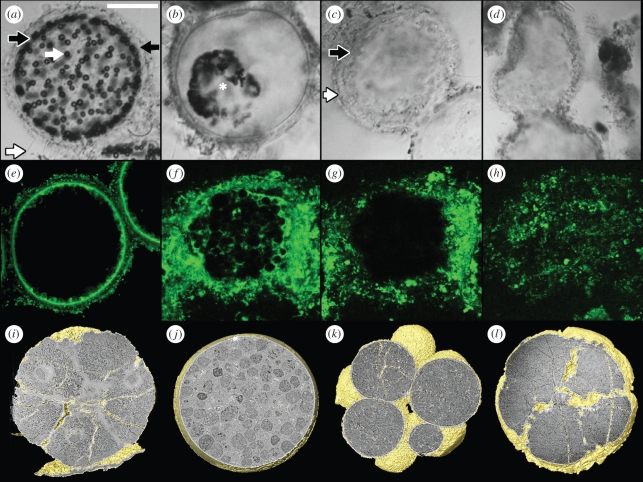
Figure 1.
(a–h) Decay in the giant sulphur bacterium Thiomargarita namibiensis and (i–l) comparison with Doushantuo microfossils. (a–d) Differential interference contrast (DIC) microscopy images of Thiomargarita. (a) Living Thiomargarita cell with diffuse sulphur vesicles surrounding the central vacuole (black arrow, mucous sheath; white arrow, sulphur vesicle; white-lined black arrow, laminar sheets; black-lined white arrow, microbial process). (b) Cell showing sulphur vesicles (region around asterisk) coalescing on the surface of the vacuole. (c) Internal contents have decayed but cell wall (white-lined black arrow) and mucous sheath (black-lined white arrow) remain. (d) Chain of cells where only the distorted mucous sheath remains. (e–h) Confocal microscopy of Thiomargarita. (e) Living cell showing the large central vacuole (image courtesy of V. Salman, Max Planck Institute, Bremen). (f) Cell showing clustered vesicles on the surface of the vacuole. (g) The same cell as in (f), showing the vacuole. (h) Decayed cell showing diffuse organic material. (i–l) Synchrotron radiation X-ray tomographic microscopy images of Tianzhushania specimens from the Ediacaran Doushantuo formation. (i) Specimen showing putative nuclei (NRM-PZ X 4469); (j) single-celled specimen showing putative lipid droplets (NRM-PZ X 4470); (k) cell morphology, but not internal structure, is preserved (NRM-PZ X 4471); (l) only surface morphology is preserved (NRM-PZ X 4472). Scale bars: (a) 100 µm; (b) 95 µm; (c,d) 120 µm; (e) 60 µm; (f,g) 25 µm; (h) 65 µm; (i) 180 µm; (j) 160 µm; (k) 170 µm; (l) 115 µm.
Shortly before death, sulphur globules begin to amass on the surface of the vacuole as a response to stress (H. N. Schulz-Vogt 2007, personal communication; figure 1b). This progresses until globules become polarized on the vacuole and tightly aggregated (figure 1b), although in the specimens we observed they did not tend to coalesce to form a single body (figure 1b). In figure 1f–g, the aggregate is situated on the upper surface of the vacuole; these figures show confocal slices through the clustered vesicles (figure 1f) as well as through the vacuole below (figure 1g). The region immediately surrounding the sulphur globules is stained strongly, suggesting that the cytoplasm is concentrated in this region. The remainder of the region outside the vacuole is filled with diffuse amorphous organic material, possibly representing more strongly degraded cytoplasm.
As decay progresses, sulphur globules begin to disappear from the cytoplasm. The remaining cytoplasmic material contains no other subcellular structures visible under light microscopy. At the same time, the cytoplasm continues to collapse, the vacuole shrinks and the internal membrane loses integrity, allowing material that has taken up fluorescent stain to penetrate into the vacuole, which is ultimately lost. The outer laminar sheets and mucous sheath, along with attached microbial filaments, show no signs of physical degradation at this stage (figure 1c).
Subsequently, the laminar envelope is lost and the mucous sheath is all that remains. At this stage, the sheath begins to show evidence of physical distortion (figure 1d). The interior contains diffuse and entirely amorphous material in which no structures are discernible (figure 1h). At this stage, individual T. namibiensis cells frequently remain joined to form chains bound by the outer mucous sheath, in which microbial filaments are still present. Eventually, the sheath disintegrates and diffuses into the surrounding sea water, though sheaths can remain intact for months or years before degradation.
(b). Experimental exposure of giant sulphur bacteria to biofilm-forming heterotrophic bacteria
Embryos of the sea urchin H. erythrogramma, which approximate the size and morphology of the Doushantuo fossils, have been used previously as experimental models for taphonomy of soft tissues [17,19]. The key initial step in the preservation of organic substrates for potential mineral replication is stabilization of the embryos under conditions that block the rapid autolysis (e.g. reducing conditions [17]) that would otherwise rapidly ensue after death. The second step in long-term preservation is a microbial process, in which the soft tissues are consumed and replaced by bacteria, producing a stable three-dimensional replica of the tissue. In the resulting ‘pseudomorph’, the external morphology and internal cellular structure of the entire embryo are replaced by bacterial biofilm components [17].
We found that although fixed H. erythrogramma embryos can be directly fractured for SEM observation [17,19], Thiomargarita are too fragile to section this way. We sectioned samples successfully by collection on Millipore filters and embedding in agarose. Sections of embedded embryos and Thiomargarita were compared by SEM. Heliocidaris erythrogramma embryos show the typical cellular morphology, including external and internal cell boundaries, the sub-surface cortical cytoplasm and the internal cytoplasm containing many lipid vesicles (figure 2b). The internal structures of Thiomargarita are distinct from those of embryos, with Thiomargarita cell volume being nearly filled by a huge vacuole rather than containing a dense cytoplasm (figure 2c,d). In these sectioned Thiomargarita samples, the external sheaths (arrowheads) are visible at the bottom edges; an external lamina (composed of the outer cell wall and thin layer of cytoplasm) was present under the sheath (white arrows); internal cell boundaries and sites of intracellular sulphur inclusions (black arrows) are visible.
For experimental bacterial taphonomy, BME-stabilized H. erythrogramma embryos and Thiomargarita were incubated for 6 days with P. tunicata, a marine gamma proteobacterium, then fixed in glutaraldehyde and observed by SEM. Embryo tissues were replaced by a dense, three-dimensional biofilm composed of bacterial bodies and secreted material, yielding a robust and stable microbial pseudomorph that replicates both overall morphology and cell structure [17]. Figure 2e,f shows internal views of pseudomorphed embryos revealing the dense surface biofilm (arrowheads) and the microbially replaced intracellular fabric (arrows). In figure 2e, the pseudomorphed cell boundary and other cellular features of a two-cell embryo can be seen, preserved as bacterial cells surrounded by a tight three-dimensional biofilm (white arrow). Figure 2f shows the bacterially replaced internal structure of a pseudomorphed embryo that was embedded in agarose and processed like the Thiomargarita samples.
Figure 2g,h shows Thiomargarita cells after the same bacterial taphonomy process. Under the conditions used, Thiomargarita cells gave distinctly different taphonomic outcomes than H. erythrogramma embryos. Embedded pseudomorphed embryos deformed slightly (figure 2f), indicated by the oval shape of the lipid vesicles, as opposed to rounder vesicle shapes in unembedded pseudomorphs (figure 2e). However, both embryos and their microbial pseudomorphs retained their overall morphology (figure 2b,f). In contrast, the bacterially treated Thiomargarita ruptured easily and collapsed into the central vacuole as a set of laminar sheets (figure 2g). Bacteria do not fill the vacuole, consistent with the absence of a cytoplasmic mass that can be consumed and replaced by biofilm-forming bacteria. However, partial bacterial biofilms formed on some of the surfaces of the laminar sheets (figure 2h, arrows).
4. Discussion
(a). Comparison with fossil data
The decay sequence presented for Thiomargarita and the evaluation of its interaction with biofilm-forming bacteria allow certain comparisons to be drawn between the decayed giant sulphur bacteria specimens and the embryo-like fossils that have undoubtedly undergone post-mortem decay, phosphatization and diagenetic alteration [17,20,27]. This allows a taphonomically based test of the hypothesis that the Doushantuo fossils represent the remains of giant sulphur bacteria [5,11]. Any phylogenetic interpretation of the Doushantuo embryo-like fossils must account for the various stages of decay recorded in the assemblage. These range from specimens that preserve putative nuclei (figure 1i) or putative lipid vesicles (figure 1j), through those in which the cell surface but no internal features are preserved (figure 1k), to those in which only the surface morphology of the specimen is preserved (figure 1l) [17]. Bailey et al. [5,11] draw comparison between Thiomargarita and a subset of the Doushantuo embryo-like fossils. The available evidence suggests that there are no more than two, very similar embryo-like genera in the Doushantuo assemblage [4,10]. It has been argued that the vast majority of these globular microfossils are taphonomic variants and developmental stages of Tianzhushania, a senior synonym of Megasphaera (single-celled specimens with a smooth—M. inornata—or ornamented—M. ornata—envelope), Parapandorina (specimens with multiple polygonal cells) and Megaclonophycus (specimens with hundreds of spheroidal cells) [10,13,28]. ‘Megasphaera inornata’ specimens were interpreted by Xiao et al. [10] as taphomorphs of Tianzhushania ornata in which the ornate envelope has been lost.
In life, the sulphur globules of Thiomargarita occupy a thin band of cytoplasm surrounding the large central vacuole. This is in marked contrast to the pattern observed in mineralized Tianzhushania, where structures interpreted as vesicles are sub-evenly distributed throughout the cell [14]. Prior to death, Thiomargarita individuals undergo a significant change in morphology, where the sulphur vesicles aggregate in one area on the surface of the vacuole. Bailey et al. [5,11] argued that these aggregations might be a better interpretation of the structures reported in a relatively small number of ‘Parapandorina’-stage fossils and interpreted as possible nuclei [15–17]. However, when the bacteria are considered in three dimensions, the structures are not compatible. The vesicles form sheet-like aggregations in the thin layer of cytoplasm that surrounds the large central vacuole. Conversely, the structures observed in the fossils are consistently close to spherical and often lie in the centre of each cell of a specimen. The structures documented in Tianzhushania are not composed of many smaller, rounded entities, and aggregates such as those seen in the decay of Thiomargarita have not been documented in the Doushantuo assemblage. The vacuole itself and the surrounding cytoplasm are also distinct from the putative nuclei: the vacuole shrinks from occupying almost the entire cell as decay progresses, and it becomes irregular in morphology, whereas the structures observed in the fossils are consistent in both shape and size.
As decay progresses in Thiomargarita, the sulphur vesicles degrade and disappear, and there is concomitant collapse of the vacuole; eventually only the laminar envelope and mucous sheath remain, with the space filled with diffuse amorphous organic matter. At this stage, the cells have an overall resemblance to those of Tianzhushania ornata fossils in which subcellular features are absent. Bailey et al. [11] considered the multi-layered envelope and surrounding mucous sheath of Thiomargarita, if phosphatized, would make an ‘excellent textural match’ to the Doushantuo envelopes. They showed that Achromatium, a genus in the same family as Thiomargarita, bears a simple polygonal surface texture that reflects the shape of mineral inclusions [12]. However, there are important differences between the unmineralized envelopes of Thiomargarita and Tianzhushania. The sheath in Thiomargarita bears small microbial filaments [5] and appears to carry small inclusions and attachments of particulate matter and other debris, but in its unmineralized state, it bears no resemblance to the polygonal, cerebral or fractal-like structures with associated dimples corresponding to the position of processes in the outer organic vesicle, which are present in the envelopes of Tianzhushania ornata [10,28]. Critically, there is also no analogue in bacteria or other prokaryotes for the highly ornate organic outer wall in which rare silicified fossil specimens can be found when examined in thin section [13]. In summary, while there is an overall similarity between the structure of the walls of Thiomargarita and Tianzhushania, the latter has a level of complexity that is incompatible with that of known giant sulphur bacteria and is found only in eukaryotes (e.g. invertebrate resting stages [1]).
The final structure to decay in Thiomargarita is the mucous sheath, which breaks down slowly until little but amorphous organic matter remains. This process can take a number of years, indicating that there is a much longer temporal window for potential mineralization than there is, for example, in metazoan embryos that have not been pseudomorphed by bacterial biofilms; these typically decay to amorphous material in a matter of weeks [19,20]. The exopolymeric substances present in bacterial sheaths have, in some circumstances, been shown to have a relatively high potential to become mineralized [29] and to enhance the preservation of organic matter [30]. Given the high preservation potential relative to other structures, the likelihood of finding such specimens in the fossil record should be high. These specimens, if infilled with void filling phosphate, would resemble ‘M. inornata’ specimens that preserve no internal features. However, this comparison cannot account for the evidence discussed above that ‘M. inornata’ is a taphonomic variant of Tianzhushania, which has a number of features that are incompatible with Thiomargarita. Nor can it account for the fact that fossils comparable with Thiomargarita at earlier decay stages (e.g. with sulphur vesicles or the collapsing vacuole preserved) or later decay stages (e.g. with flaccid sheaths) are not known from the Doushantuo assemblage.
Overall, the decay sequence of Thiomargarita has much in common with those of other bacteria [24]. As in other bacteria, the Thiomargarita sheath is the most resistant structure to decay. Within the sheath, adjacent Thiomargarita and other bacterial cells can exhibit the entire taphonomic spectrum, whereas adjacent cells in Doushantuo fossil specimens invariably show an approximately equal grade of decay.
The fact that there was only limited interaction between Thiomargarita and biofilm-forming bacteria, and that these bacteria did not pseudomorph the cell interior, has potentially important implications for understanding the preservation potential of Thiomargarita. Our taphonomy experiments show that Thiomargarita and animal embryos have distinctly different properties that might affect taphonomic outcomes. If the conditions of our bacterial taphonomy experiments are relevant to producing a Doushantuo-like mode of preservation, we would expect large eukaryotic cells to be preserved in the round, similar to our embryo taphonomic models that are completely pseudomorphed by biofilm-forming bacteria. On the other hand, giant sulphur bacteria—which collapse easily and have only patchy biofilms that are limited to the multi-layered envelope—would more probably be represented as layers of laminar structures. Bacterial biofilm pseudomorphosis is therefore not a potential vector for fossilization of three-dimensionally preserved giant sulphur bacteria. If other modes of preservation in which apatite is secreted into the giant cell vacuole are feasible (perhaps associated with their ability to mediate phosphate precipitation [8,9]), then Thiomargarita-like forms could yield uncrushed fossils; there is, however, no available evidence to support this scenario. The combination of the lack of a known preservation mechanism and the morphological incompatibilities between decayed Thiomargarita and the fossils provides evidence against the interpretation of Tianzhushania as the remains of Thiomargarita-like giant sulphur bacteria or of other bacteria that achieve large size by means of a vacuole that takes up the majority of the cell volume.
5. Conclusions
Interpretations of the Doushantuo Tianzhushania fossils as giant sulphur bacteria, which seem plausible based on gross morphological comparisons with living organisms and their demonstrated mediation of phosphorite formation, are not supported by our observations of taphonomic processes in Thiomargarita. In life, Thiomargarita differs from Tianzhushania in having a large central vacuole occupying up to 98 per cent of the cell volume, rather than having subcellular structures throughout the cell. A number of key structures observed in Tianzhushania cannot be explained by any of the features exhibited by Thiomargarita as it undergoes death and decay. These include the putative nuclei, the putative lipid vesicles, the complex fractal ornament of the Tianzhushania ornata envelope and the ornate outer vesicles of silicified Tianzhushania fossils. Moreover, bacteria that readily replicate animal embryos do not pseudomorph Thiomargarita; it seems unlikely that the vacuole could be preserved in this way.
However, Thiomargarita does have important implications for understanding the Precambrian fossil record as it provides an alternative interpretative model for other Precambrian fossils that have been identified as eukaryotes primarily or partially on the basis of their large size [31,32]. Furthermore, in light of the fact that some prokaryotes like Thiomargarita can reach large size, identifications of eukaryotes should now rely on other features, such as complex wall ultrastructure or surface ornament, in addition to size alone [33–35]. Bacterial interpretations should be considered for fossil cells that are of large size but lack any definitively diagnostic eukaryote features [31,36,37].
Acknowledgements
We thank H. N. Schulz-Vogt (Max Planck Institute, Bremen) and G. W. Rouse (University of California, San Diego) for generously supplying Thiomargarita material; H. N. Schulz-Vogt for valuable discussion; V. Salman (Max Planck Institute, Bremen) for providing figure 1e; M. E. Andrews (Indiana University) for able technical assistance with experimental taphonomy; and P. Verkade (University of Bristol) for assistance with confocal microscopy. J.A.C. and P.C.J.D. were supported by NERC grant NE/F00348X/1 to P.C.J.D.; C.-W.T. was supported by a NERC studentship; J.V.B. was supported by NSF grant EAR-1057119.
References
- 1.Xiao S. H., Knoll A. H. 2000. Phosphatized animal embryos from the Neoproterozoic Doushantuo formation at Weng'an, Guizhou, South China. J. Paleontol. 74, 767–788 (doi:10.1666/0022-3360(2000)074<0767:PAEFTN>2.0.CO;2) [DOI] [Google Scholar]
- 2.Xiao S. H., Zhang Y., Knoll A. H. 1998. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 391, 553–558 10.1038/35318 (doi:10.1038/35318) [DOI] [Google Scholar]
- 3.Xiao S. H. 2002. Mitotic topologies and mechanics of Neoproterozoic algae and animal embryos. Paleobiology 28, 244–250 (doi:10.1666/0094-8373(2002)028<0244:MTAMON>2.0.CO;2) [DOI] [Google Scholar]
- 4.Bengtson S., Cunningham J. A., Donoghue P. C. J., Huldtgren T., Yin C. 2010. A critical view of ‘animal embryos’ in the Ediacaran Doushantuo biota. Geol. Soc. Am. Programs Abstr. 42, 409 [Google Scholar]
- 5.Bailey J. V., Joye S. B., Kalanetra K. M., Flood B. E., Corsetti F. A. 2007. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature 445, 198–201 10.1038/nature05457 (doi:10.1038/nature05457) [DOI] [PubMed] [Google Scholar]
- 6.Schulz H. N., Brinkhoff T., Ferdelman T. G., Marine M. H., Teske A., Jorgensen B. B. 1999. Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science 284, 493–495 10.1126/science.284.5413.493 (doi:10.1126/science.284.5413.493) [DOI] [PubMed] [Google Scholar]
- 7.Kalanetra K. M., Joye S. B., Sunseri N. R., Nelson D. C. 2005. Novel vacuolate sulfur bacteria from the Gulf of Mexico reproduce by reductive division in three dimensions. Environ. Microbiol. 7, 1451–1460 10.1111/j.1462-2920.2005.00832.x (doi:10.1111/j.1462-2920.2005.00832.x) [DOI] [PubMed] [Google Scholar]
- 8.Schulz H. N., Schulz H. D. 2005. Large sulfur bacteria and the formation of phosphorite. Science 307, 416–418 10.1126/science.1103096 (doi:10.1126/science.1103096) [DOI] [PubMed] [Google Scholar]
- 9.Goldhammer T., Bruchert V., Ferdelman T. G., Zabel M. 2010. Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 3, 557–561 10.1038/ngeo913 (doi:10.1038/ngeo913) [DOI] [Google Scholar]
- 10.Xiao S. H., Zhou C. M., Yuan X. L. 2007. Undressing and redressing Ediacaran embryos. Nature 446, E9–E10 10.1038/nature05753 (doi:10.1038/nature05753) [DOI] [PubMed] [Google Scholar]
- 11.Bailey J. V., Joye S. B., Kalanetra K. M., Flood B. E., Corsetti F. A. 2007. Undressing and redressing Ediacaran embryos—reply. Nature 446, E10–E11 10.1038/nature05754 (doi:10.1038/nature05754) [DOI] [PubMed] [Google Scholar]
- 12.Head I. M., Gray N. D., Clarke K. J., Pickup R. W., Jones J. G. 1996. The phylogenetic position and ultrastructure of the uncultured bacterium Achromatium oxaliferum. Microbiology 142, 2341–2354 10.1099/00221287-142-9-2341 (doi:10.1099/00221287-142-9-2341) [DOI] [PubMed] [Google Scholar]
- 13.Yin L. M., Zhu M. Y., Knoll A. H., Yuan X. L., Zhang J. M., Hu J. 2007. Doushantuo embryos preserved inside diapause egg cysts. Nature 446, 661–663 10.1038/nature05682 (doi:10.1038/nature05682) [DOI] [PubMed] [Google Scholar]
- 14.Donoghue P. C. J. 2007. Embryonic identity crisis. Nature 445, 155–156 10.1038/nature05520 (doi:10.1038/nature05520) [DOI] [PubMed] [Google Scholar]
- 15.Chen J. Y., et al. 2009. Complex embryos displaying bilaterian characters from Precambrian Doushantuo phosphate deposits, Weng'an, Guizhou, China. Proc. Natl Acad. Sci. USA 106, 19 056–19 060 10.1073/pnas.0904805106 (doi:10.1073/pnas.0904805106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagadorn J. W., et al. 2006. Cellular and subcellular structure of Neoproterozoic animal embryos. Science 314, 291–294 10.1126/science.1133129 (doi:10.1126/science.1133129) [DOI] [PubMed] [Google Scholar]
- 17.Raff E. C., et al. 2008. Embryo fossilization is a biological process mediated by microbial biofilms. Proc. Natl Acad. Sci. USA 105, 19 360–19 365 10.1073/pnas.0810106105 (doi:10.1073/pnas.0810106105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoghue P. C. J., Purnell M. A. 2009. Distinguishing heat from light in debate over controversial fossils. BioEssays 31, 178–189 10.1002/bies.200800128 (doi:10.1002/bies.200800128) [DOI] [PubMed] [Google Scholar]
- 19.Raff E. C., Villinski J. T., Turner F. R., Donoghue P. C. J., Raff R. A. 2006. Experimental taphonomy shows the feasibility of fossil embryos. Proc. Natl Acad. Sci. USA 103, 5846–5851 10.1073/pnas.0601536103 (doi:10.1073/pnas.0601536103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gostling N. J., et al. 2008. Deciphering the fossil record of early bilaterian embryonic development in light of experimental taphonomy. Evol. Dev. 10, 339–349 10.1111/j.1525-142X.2008.00242.x (doi:10.1111/j.1525-142X.2008.00242.x) [DOI] [PubMed] [Google Scholar]
- 21.Gostling N. J., Dong X. P., Donoghue P. C. J. 2009. Ontogeny and taphonomy: an experimental taphonomy study of the development of the brine shrimp Artemia salina. Palaeontology 52, 169–186 10.1111/j.1475-4983.2008.00834.x (doi:10.1111/j.1475-4983.2008.00834.x) [DOI] [Google Scholar]
- 22.Martin D., Briggs D. E. G., Parkes R. J. 2005. Decay and mineralization of invertebrate eggs. Palaios 20, 562–572 10.2110/palo.2004.p04-67 (doi:10.2110/palo.2004.p04-67) [DOI] [Google Scholar]
- 23.Westall F. 1999. The nature of fossil bacteria: a guide to the search for extraterrestrial life. J. Geophys. Res. 104, 16 437–16 451 10.1029/1998JE900051 (doi:10.1029/1998JE900051) [DOI] [Google Scholar]
- 24.Bartley J. K. 1996. Actualistic taphonomy of cyanobacteria: implications for the Precambrian fossil record. Palaios 11, 571–586 10.2307/3515192 (doi:10.2307/3515192) [DOI] [Google Scholar]
- 25.Salman V., Amann R., Girnth A. C., Polerecky L., Bailey J. V., Hogslund S., Jessen G., Pantoja S., Schulz-Vogt H. N. 2011. A single-cell sequencing approach to the classification of large, vacuolated sulfur bacteria. Syst. Appl. Microbiol. 34, 243–259 10.1016/j.syapm.2011.02.001 (doi:10.1016/j.syapm.2011.02.001) [DOI] [PubMed] [Google Scholar]
- 26.Donoghue P. C. J., et al. 2006. Synchrotron X-ray tomographic microscopy of fossil embryos. Nature 442, 680–683 10.1038/Nature04890 (doi:10.1038/Nature04890) [DOI] [PubMed] [Google Scholar]
- 27.Xiao S. H., Knoll A. H. 1999. Fossil preservation in the Neoproterozoic Doushantuo phosphorite Lagerstätte, South China. Lethaia 32, 219–240 10.1111/j.1502-3931.1999.tb00541.x (doi:10.1111/j.1502-3931.1999.tb00541.x) [DOI] [PubMed] [Google Scholar]
- 28.Yin C. Y., Bengtson S., Yue Z. 2004. Silicified and phosphatized Tianzhushania, spheroidal microfossils of possible animal origin from the Neoproterozoic of South China. Acta Palaeontol. Pol. 49, 1–12 [Google Scholar]
- 29.Oehler J. H., Schopf J. W. 1971. Artificial microfossils: experimental studies of permineralization of blue-green algae in silica. Science 174, 1229–1231 10.1126/science.174.4015.1229 (doi:10.1126/science.174.4015.1229) [DOI] [PubMed] [Google Scholar]
- 30.Pacton M., Fiet N., Gorin G. E. 2007. Bacterial activity and preservation of sedimentary organic matter: the role of exopolymeric substances. Geomicrobiol. J. 24, 571–581 10.1080/01490450701672042 (doi:10.1080/01490450701672042) [DOI] [Google Scholar]
- 31.Han T. M., Runnegar B. 1992. Megascopic eukaryotic algae from the 2.1-billion-year-old Negaunee iron-formation, Michigan. Science 257, 232–235 10.1126/science.1631544 (doi:10.1126/science.1631544) [DOI] [PubMed] [Google Scholar]
- 32.Schopf J. W. 1992. Proterozoic prokaryotes: affinities, geologic distribution, and evolutionary trends. In The Proterozoic biosphere: a multidisciplinary study (eds Schopf J. W., Klein C.), pp. 195–218 Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Javaux E. J., Knoll A. H., Walter M. 2003. Recognizing and interpreting the fossils of early eukaryotes. Orig. Life Evol. Biosph. 33, 75–94 10.1023/A:1023992712071 (doi:10.1023/A:1023992712071) [DOI] [PubMed] [Google Scholar]
- 34.Javaux E. J., Knoll A. H., Walter M. R. 2004. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans. Geobiology 2, 121–132 10.1111/j.1472-4677.2004.00027.x (doi:10.1111/j.1472-4677.2004.00027.x) [DOI] [Google Scholar]
- 35.Knoll A. H., Javaux E. J., Hewitt D., Cohen P. 2006. Eukaryotic organisms in Proterozoic oceans. Phil. Trans. R. Soc. B 361, 1023–1038 10.1098/rstb.2006.1843 (doi:10.1098/rstb.2006.1843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javaux E. J., Marshall C. P., Bekker A. 2010. Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463, 934–938 10.1038/nature08793 (doi:10.1038/nature08793) [DOI] [PubMed] [Google Scholar]
- 37.Dong L., Xiao S., Shen B., Zhou C. 2008. Silicified Horodyskia and Palaeopascichnus from upper Ediacaran cherts in South China: tentative phylogenetic interpretation and implications for evolutionary stasis. J. Geol. Soc. Lond. 165, 367–378 10.1144/0016-76492007-074 (doi:10.1144/0016-76492007-074) [DOI] [Google Scholar]