Abstract
The regulation of ATP metabolism by inorganic phosphate (Pi) was examined in five normal volunteers through measurements of ATP degradation during relative Pi depletion and repletion states. Relative Pi depletion was achieved through dietary restriction and phosphate binders, whereas a Pi-repleted state was produced by oral Pi supplementation. ATP was radioactively labeled by the infusion of [8(14)C]adenine. Fructose infusion was used to produce rapid ATP degradation during Pi depletion and repletion states. Baseline measurements indicated a significant decrease of Pi levels during phosphate depletion and no change in serum or urinary purines. Serum values of Pi declined 20 to 26% within 15 min after fructose infusion in all states. Urine measurements of ATP degradation products showed an eightfold increase within 15 min after fructose infusion in both Pi-depleted and -supplemented states. Urinary radioactive ATP degradation products were fourfold higher and urinary purine specific activity was more than threefold higher during Pi depletion as compared with Pi repletion. Our data indicate that there is decreased ATP degradation to purine end products during a relative phosphate repletion state as compared to a relative phosphate depletion state. These data show that ATP metabolism can be altered through manipulation of the relative Pi state in humans.
Full text
PDF
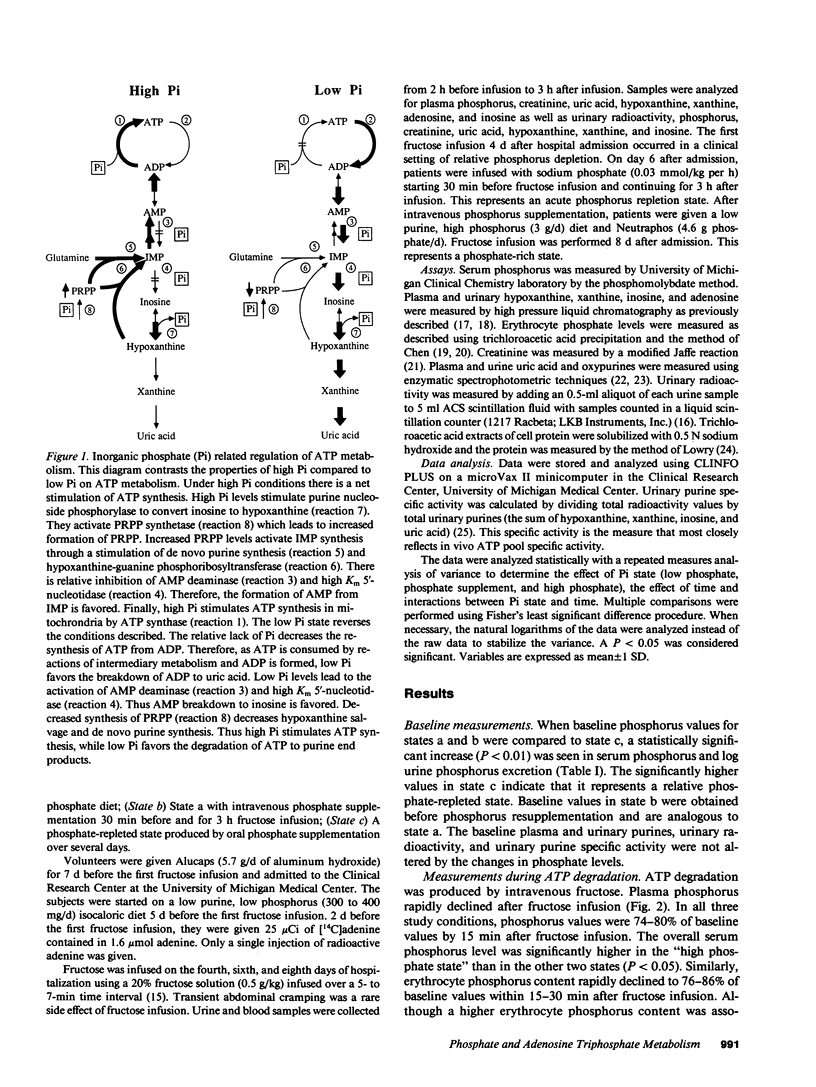
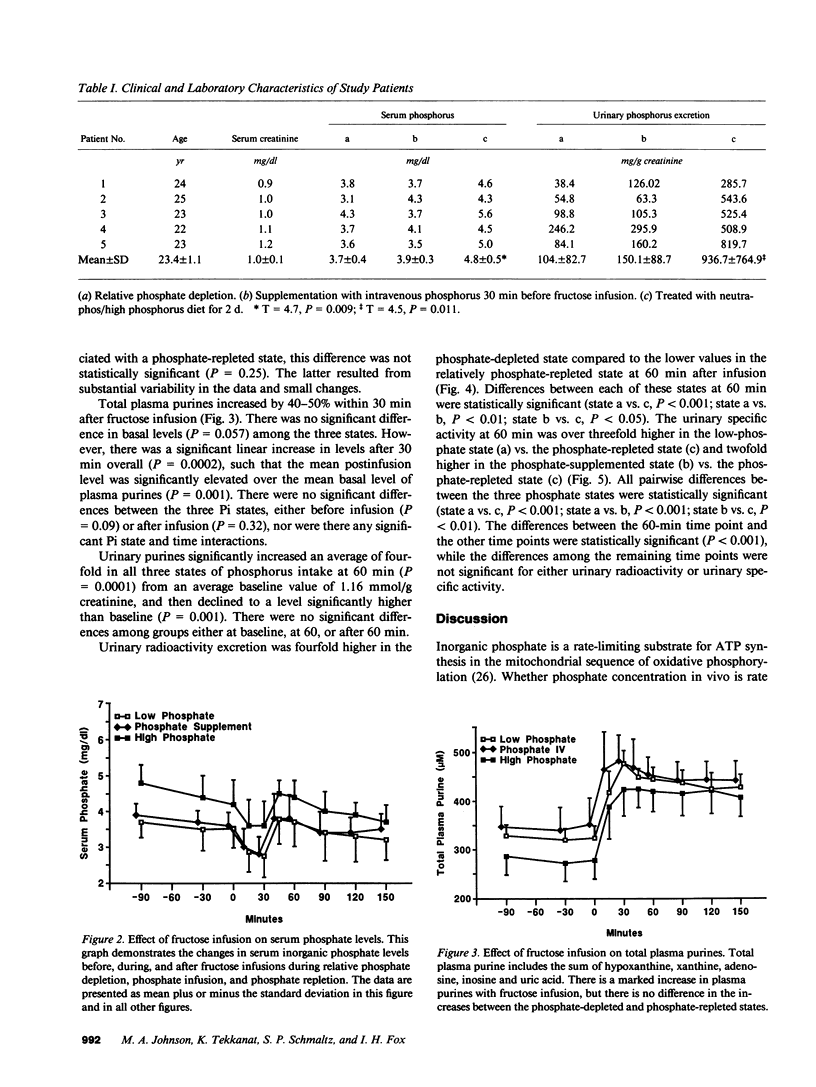
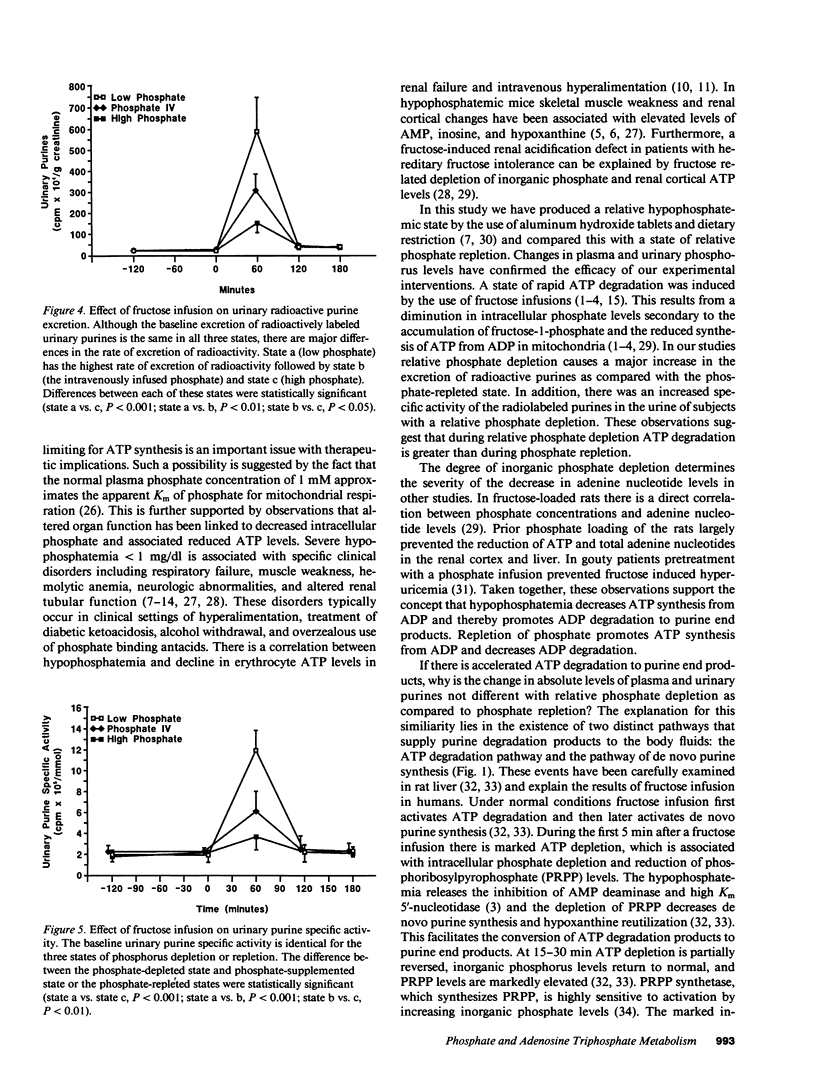


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubier M., Murciano D., Lecocguic Y., Viires N., Jacquens Y., Squara P., Pariente R. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med. 1985 Aug 15;313(7):420–424. doi: 10.1056/NEJM198508153130705. [DOI] [PubMed] [Google Scholar]
- Brautbar N., Carpenter C., Baczynski R., Kohan R., Massry S. G. Impaired energy metabolism in skeletal muscle during phosphate depletion. Kidney Int. 1983 Jul;24(1):53–57. doi: 10.1038/ki.1983.125. [DOI] [PubMed] [Google Scholar]
- Cohen J. L., Vinik A., Faller J., Fox I. H. Hyperuricemia in glycogen storage disease type I. Contributions by hypoglycemia and hyperglucagonemia to increased urate production. J Clin Invest. 1985 Jan;75(1):251–257. doi: 10.1172/JCI111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. L., Recker D., Fox I. H. Overproduction of uric acid in hypoxanthine-guanine phosphoribosyltransferase deficiency. Contribution by impaired purine salvage. J Clin Invest. 1979 May;63(5):922–930. doi: 10.1172/JCI109392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. H. Adenosine triphosphate degradation in specific disease. J Lab Clin Med. 1985 Aug;106(2):101–110. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971 Sep 25;246(18):5739–5748. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972 Aug;21(8):713–721. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- Fox I. H. Metabolic basis for disorders of purine nucleotide degradation. Metabolism. 1981 Jun;30(6):616–634. doi: 10.1016/0026-0495(81)90142-6. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Palella T. D., Kelley W. N. Hyperuricemia: a marker for cell energy crisis. N Engl J Med. 1987 Jul 9;317(2):111–112. doi: 10.1056/NEJM198707093170209. [DOI] [PubMed] [Google Scholar]
- Fuller T. J., Carter N. W., Barcenas C., Knochel J. P. Reversible changes of the muscle cell in experimental phosphorus deficiency. J Clin Invest. 1976 Apr;57(4):1019–1024. doi: 10.1172/JCI108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene H. L., Wilson F. A., Hefferan P., Terry A. B., Moran J. R., Slonim A. E., Claus T. H., Burr I. M. ATP depletion, a possible role in the pathogenesis of hyperuricemia in glycogen storage disease type I. J Clin Invest. 1978 Aug;62(2):321–328. doi: 10.1172/JCI109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen I. E. Mitochondrial respiratory control in the myocardium. Biochim Biophys Acta. 1986;853(2):135–151. doi: 10.1016/0304-4173(86)90008-x. [DOI] [PubMed] [Google Scholar]
- Hettleman B. D., Sabina R. L., Drezner M. K., Holmes E. W., Swain J. L. Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J Clin Invest. 1983 Aug;72(2):582–589. doi: 10.1172/JCI111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura M., Sabina R. L., Heald P. W., Holmes E. W. Basis for the control of purine biosynthesis by purine ribonucleotides. J Clin Invest. 1981 Apr;67(4):994–1002. doi: 10.1172/JCI110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Amsden T. Acute hemolytic anemia with rigid red cells in hypophosphatemia. N Engl J Med. 1971 Dec 23;285(26):1446–1450. doi: 10.1056/NEJM197112232852602. [DOI] [PubMed] [Google Scholar]
- Klinenberg J. R., Goldfinger S., Bradley K. H., Seegmiller J. E. An enzymatic spectrophotometric method for the determination of xanthine and hypoxanthine. Clin Chem. 1967 Oct;13(10):834–846. [PubMed] [Google Scholar]
- Knochel J. P. The clinical status of hypophosphatemia: an update. N Engl J Med. 1985 Aug 15;313(7):447–449. doi: 10.1056/NEJM198508153130711. [DOI] [PubMed] [Google Scholar]
- Knochel J. P. The clinical status of hypophosphatemia: an update. N Engl J Med. 1985 Aug 15;313(7):447–449. doi: 10.1056/NEJM198508153130711. [DOI] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtman M. A., Miller D. R., Cohen J., Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med. 1971 Apr;74(4):562–568. doi: 10.7326/0003-4819-74-4-562. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Mateos F. A., Puig J. G., Jiménez M. L., Fox I. H. Hereditary xanthinuria. Evidence for enhanced hypoxanthine salvage. J Clin Invest. 1987 Mar;79(3):847–852. doi: 10.1172/JCI112893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozzi G., Berti C., Pellegrini R., Lalumera M., Marcolongo R. Inibizione dell'iperuricemia da fruttoso mediante pretrattamento con fosfati. Boll Soc Ital Biol Sper. 1982 Apr 30;58(8):491–496. [PubMed] [Google Scholar]
- Morris R. C., Jr An experimental renal acidification defect in patients with hereditary fructose intolerance. I. Its resemblance to renal tubular acidosis. J Clin Invest. 1968 Jun;47(6):1389–1398. doi: 10.1172/JCI105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr, Nigon K., Reed E. B. Evidence that the severity of depletion of inorganic phosphate determines the severity of the disturbance of adenine nucleotide metabolism in the liver and renal cortex of the fructose-loaded rat. J Clin Invest. 1978 Jan;61(1):209–220. doi: 10.1172/JCI108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planet G., Fox I. H. Inhibition of phosphoribosylpyrophosphate synthesis by purine nucleosides in human erythrocytes. J Biol Chem. 1976 Oct 10;251(19):5839–5844. [PubMed] [Google Scholar]
- Puig J. G., Fox I. H. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. 1984 Sep;74(3):936–941. doi: 10.1172/JCI111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid M., Robson M. Proximal myopathy caused by latrogenic phosphate depletion. JAMA. 1976 Sep 20;236(12):1380–1381. [PubMed] [Google Scholar]
- Sabina R. L., Drezner M. K., Holmes E. W. Reduced renal cortical ribonucleoside triphosphate pools in three different hypophosphatemic animal models. Biochem Biophys Res Commun. 1982 Dec 15;109(3):649–655. doi: 10.1016/0006-291x(82)91989-1. [DOI] [PubMed] [Google Scholar]
- Shields H. M. Rapid fall of serum phosphorus secondary to antacid therapy. Gastroenterology. 1978 Dec;75(6):1137–1141. [PubMed] [Google Scholar]
- Tekkanat K. K., Fox I. H. Isocratic separation of ATP and its degradation products from biological fluids by automated liquid chromatography. Clin Chem. 1988 May;34(5):925–932. [PubMed] [Google Scholar]
- Travis S. F., Sugerman H. J., Ruberg R. L., Dudrick S. J., Delivoria-Papadopoulos M., Miller L. D., Oski F. A. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N Engl J Med. 1971 Sep 30;285(14):763–768. doi: 10.1056/NEJM197109302851402. [DOI] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. Effect of fructose on the concentration of phosphoribosylpyrophosphate in isolated hepatocytes. Adv Exp Med Biol. 1986;195(Pt B):615–621. doi: 10.1007/978-1-4684-1248-2_96. [DOI] [PubMed] [Google Scholar]


