Abstract
Dendritic cells (DCs) encompass a heterogeneous population of cells capable of orchestrating innate and adaptive immune responses. The ability of DCs to act as professional antigen presenting cells has been the foundation for the development and utilization of these cells as vaccines in cancer immunotherapy. DCs are also endowed with the non-conventional property of directly killing tumor cells. The current study investigates the regulation of murine DC cytotoxic function by T lymphocytes. We provide evidence that CD4+ Th-1, but not Th-2, Th-17 cells or Treg are capable of inducing DC cytotoxic function. IFN-γ was identified as the major factor responsible for Th-1-induced DC tumoricidal activity. Tumor cell killing mediated by Th-1-activated killer DCs (Th-1 KDCs) was dependent on inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) production. Importantly, Th-1 KDCs were capable of presenting the acquired antigens from the killed tumor cells to T lymphocytes in vitro or in vivo. These observations open new possibilities for the application of KDCs in cancer immunotherapy.
Keywords: Dendritic Cells, T Cells, Tumor Immunity, Cytotoxicity, Nitric Oxide
Introduction
The cardinal property of dendritic cells (DCs) to function as professional antigen presenting cells (APCs) capable of orchestrating adaptive and innate immunity has been the basis for their implementation in vaccination strategies against cancer (1–3). However, to date, the limited efficacy of DC-based immunization in clinic has failed to spark significant enthusiasm. One possible limitation of this therapeutic approach may stem from the lack of mobilization of the full anti-tumoral potential of these cells. Indeed, the field has primarily focused on exploiting the APC function of DCs with limited consideration given to their relatively recently described potential as direct tumor cell killers.
Multiple lines of evidence have indicated that DCs, when appropriately stimulated, can acquire cytotoxic properties against tumor cells but not normal cells (4–6). The effector mechanisms underlying DC-mediated tumoricidal function are still being explored and may differ depending on the DC subtype (7). They may include the perforin/granzyme system (8), FasL (9–13), TNF-family members (TNF-α, TRAIL) (6, 8, 9, 14–25), reactive oxygen species (ROS) and/or NO (4, 5, 11, 26–28). Similarly, multiple modalities for the induction of DC cytotoxic function have been described, including CD40L (18), interferons (4, 6, 14, 17, 29, 30), LPS and other TLR agonists (4, 5, 8, 18, 31, 32). We have previously documented in the mouse (5), rat (4), and human (33) that ex-vivo generated DCs activated with LPS are capable of killing tumor cells by NO, peroxinitrites, or ROS-dependent mechanisms. However, whether DC killing function may be regulated by other immune cells has not been investigated.
In the current study, we demonstrate that mouse bone marrow-derived DC tumoricidal activity can be induced by CD4+ T helper-1 (Th-1) lymphocytes. The mechanism of induction of KDC cytotoxic activity was not dependent on cell-to-cell contact. Using DCs generated from IFN- γ receptor knockout mice and IFN-γ blocking antibodies, we identified IFN- γ as the primary factor responsible for Th-1-mediated induction of DC cytotoxic activity. Killing of tumor cells by Th-1-activated cytotoxic DCs (Th-1 KDCs) required NO production but not perforin/granzyme or members of the death receptor ligand family. Importantly, Th-1 KDCs efficiently presented antigens derived from the tumor cells they had killed in vitro. Of therapeutic relevance, Th-1 KDCs injected intratumorally migrated to draining lymph nodes where they were capable of presenting the acquired specific antigens. Thus, IFN-γ-secreting Th-1 lymphocytes promote the non-conventional cytotoxic activity of DCs which can function as efficient APCs following killing of tumor cells both in vitro and in vivo.
Materials and Methods
Animals
Mice were housed under specific pathogen-free conditions and cared for according to the guidelines of the University of Arizona Institutional Animal Care and Use Committee. Six- to 8-wk-old BALB/c (H2d) and C57BL6 (H2b) mice were obtained from the National Cancer Institute. iNOS−/− (B6.129P2-Nos2tm1Lau/J), FasL−/− (B6Smn.C3-Faslgld/J), perforin−/− (CByJ.B6-Prf1tm1Sdz/J), IFNγR−/− (B6.129S7-Ifngr1tm1Agt/J), CD11cDTRGFP (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J), OT-I and OT-II mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Cell lines
The mouse melanoma cell line B16 was obtained from the American Tissue and Cell Collection (ATCC). OVA-expressing B16 (B16-OVA) were obtained as reported (5). Mammary carcinoma tumor 4T1 were obtained from the ATCC. Cells were cultured at 37°C and 10% CO2 in RPMI media (Thermo Fisher Scientific) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific) and supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, 0.025 µg/ml amphotericin B, 0.5 × MEM nonessential amino acids and 1 mM sodium pyruvate (complete media, CM). Cells were tested routinely and found to be free of Mycoplasma contamination.
Reagents
NG-methyl-L-arginine (NMMA), LPS, and crystal violet were purchased from Sigma-Aldrich (St. Louis, MO). Murine IL-2, IL-4, IL-6, IL-12, TGF-β were obtained from (Peprotech, Rocky Hill, NJ). Anti-IFN-γ, anti-IL-4 and isotype control antibodies were obtained from eBioscience (San Diego, CA).
Generation of bone marrow-derived DCs
DC were generated from mouse bone marrow according to our previously reported procedures (4, 5, 28, 34). Briefly, total bone marrow cells were isolated from mouse femurs and tibias. Red cells were lysed in Pharm Lyse (BD Biosciences), and the cell suspension was passed through a 100-mm filter. Cells (5×105/ml) were seeded in six-well plates (3 ml/well) in RPMI 1640 medium (Thermo Fisher Scientific, Waltham MA) supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific) and GM-CSF and IL-4 (Peprotech) (10 ng/ml each) and were incubated in 5% CO2 at 37 °C. Three and 5 days after the beginning of the culture, the medium was replaced. At day 6, CD11c+ cells were selected from the culture using anti-CD11c microbeads (Miltenyi Biotec, Auburn, CA) and cultured for an additional 2 days with GM-CSF and IL-4. The phenotypical characteristics of the obtained cells post CD11c+ selection after 6 days of culture is depicted in supplemental Figure S1. DC cultures did not contain conventional cytotoxic immune cells (Figure S1). T and B lymphocytes and NK cells represented less than 1.5% of the cells.
Cytotoxic Assays
DC cytotoxic function was assessed as we previously reported (4, 5). Purified CD11c+ DC were pre-treated or not with IFN-γ (5 ng/ml) or with T lymphocyte culture supernatant, from day 6 to day 8 or as indicated. DC were then washed and plated with B16 melanoma or 4T1 carcinoma cells (tumor cells:DC ratio=1:5). LPS (1 µg/ml) - activated DCs were used as a positive control. Tumor cell killing was then evaluated as previously described (4, 5, 35–37). Briefly, the cells were rinsed with PBS and remaining adherent cells were fixed with 95% ethanol and stained with the Crystal Violet dye (100 µl in each well of a 96-well plates) for less than 10 seconds. The wells were then extensively washed with water. The dye was then eluted with acetic acid (30%). The amount of dye resuspended in the well is proportionate to the number of viable tumor cells. Plates were then read at 570 nm. Data were presented as the percentage of relative absorbance calculated from the formula: Atest/Acontrol, where Atest is the absorbance of tumor cells cultured with DCs in different conditions and Acontrol is the absorbance of tumor cells cultured alone. DCs are very poorly stained with the dye and minimally contribute to the detected absorbance (4, 5, 35–37).
Determination of nitrite concentration
Culture supernatants were collected and incubated (50 µl) with an equal volume of Griess reagent. After incubation (15 min) at room temperature, the absorbance was read at 550 nm against 690 nm following the manufacturer’s instructions (Premege, Madison, WI), and as previously reported (5).
Flow Cytometry analysis and Antibodies
Cells (~106) were washed in PBS and were first incubated with an Fc receptor blocking Ab (BD, Franklin Lakes, NJ) for 10 minutes and subsequently stained with saturating amounts of the appropriate fluorochrome-conjugated antibodies for 30 minutes. For intracellular staining, cells were fixed and permeabilized according to the manufacturer’s instructions (eBioscience) and stained with the indicated antibodies for transcription factor expression detection. Cells were then washed and analyzed using a FACS Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). A minimum of 10,000 events was collected for each sample, and data analysis was performed using FlowJo software (Treestar Inc., Ashland, OR) or CellQwest Pro 6.0 (Becton Dickinson). The following antibodies were purchased from eBiosciences (San Diego, CA): anti-CD4 FITC (GK1.5), anti-Tbet PE (eBio4B10), anti-GATA3 PE (TWAJ), anti-RORγt PE (AFKJS-9), anti-FoxP3 APC (FJK-16S), anti-F4/80 FITC (BM8), anti-CD11c FITC (N418), CD49b FITC (DX5), anti-CD86 FITC (GL1), MHC II FITC (NIMR-4), MHC I PE (SF1–1.1.1), B220 PE (RA3-6B2) and from BD: CD3 FITC (145-2C11), CD40 FITC (HM40-3), anti-H-2Kb-SIINFEKL (eBio25D1.16) and CD11b PE (M1-70).
Generation of CD4+ T lymphocyte lineages
To generate Th lymphocyte lineages, CD4+CD25−CD62L+ naïve T cells were isolated from mouse spleens using a CD4+CD62L+ T cell isolation kit (Miltenyi Biotech, Auburn, CA). The purified cells were then incubated for 3 days in 5% CO2 at 37 °C in RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific) with anti-CD3 anti-CD28 coated T cell expander beads (1:1 bead to cell ratio) (Invitrogen, Carlsbad, CA) and with the following specific cytokines and blocking antibodies: For Th-1 generation, cells were cultured with IL-2 (20 IU/ml), IL-12 (10 ng/ml) and anti-IL-4 blocking antibody (5 µg/ml). For Th-2 differentiation, cells were incubated with IL-2 (20 IU/ml), IL-4 (30 ng/ml) and anti-IFN-γ blocking antibody (5 µg/ml). Th-17 lymphocytes were generated with TGF-β1 (5 ng/ml), IL-6 (50 ng/ml), anti-IFN-γ and anti-IL-4 blocking antibodies (5 µg/mL each). Tregs were generated by culturing isolated naïve T cells in presence of IL-2 (20 IU/ml) and TGF-β1 (5 ng/ml). All cytokines were purchased from Peprotech and blocking antibodies from eBioscience. T lymphocytes were washed and re-stimulated with anti-CD3 anti-CD28 coated T cell expander beads for 8 hours and their supernatants were collected to activate DCs as described above.
Real-time PCR
Real-time RT-PCR was used to evaluate expression of different cytokines, transcription factors or chemokine receptors in different T cell lineages and in dendritic cells treated with LPS or IFN- γ. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and its integrity was confirmed by denaturing agarose gel electrophoresis and calculated densitometric 28S/18S ratio. 250 ng of total RNA were reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Subsequently, 20 µL of the PCR reactions were set up in 96-well plates containing 10 µl 2X IQ Supermix (Bio-Rad), 1 µL TaqMan® primer/probe set (ABI, Foster City, CA), 2 µL of the cDNA synthesis reaction (10% of RT reaction) and 7 µL of nuclease-free water. Reactions were run and analyzed on a Bio-Rad iCycler iQ real–time PCR detection system. Cycling parameters were determined and resulting data were analyzed by using the comparative Ct method as means of relative quantification, normalized to an endogenous reference (TATA Box Binding Protein, TBP) and relative to a calibrator (normalized Ct value obtained from control mice) and expressed as 2−ΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Western Blot
DCs were isolated, cultured and activated with LPS, IFN-γ or Th-1 supernatant as described above. Cells were collected and centrifuged at 350g for 5 minutes at 4°C. Cells were then rinsed with cold phosphate buffered saline and were lysed with radio-immunoprecipitation assay (RIPA) buffer plus a protease and phosphotase inhibitor cocktail (Thermo Scientific, Rockford, IL). The cell lysate was briefly sonicated and centrifuged at 8,000g for 8 min at 4°C. Protein concentration was determined using a BCA Protein Assay kit (Thermo Scientific). Thirty micrograms of total cell lysate was boiled for 5 minutes and resolved in 10% tris-glycine gel (Invitrogen, Carlsbad, CA) by electropheoresis at 125 V for 105 minutes. Proteins were then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked with 5% milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 30 min before overnight incubation at 4°C with the indicated primary antibodies. The blot was rinsed with TBST and incubated for 2 h at room temperature with secondary antibody. Reactive bands were visualized by exposure to film using SuperSignal Chemiluminescent Substrate (Thermo Scientific). Antibodies against iNOS, was purchased from Cell Signaling Technology (Beverly, MA). Antibodies against Actin were purchased from Sigma-Aldrich (St. Louis, MO). The secondary antibodies, peroxidase conjugated goat anti-rabbit and peroxidase conjugated goat anti-mouse, were purchased from Jackson ImmunoResearch (West Grove, PA).
Detection of cytokine production by ELISA
The concentrations of IFN-γ, IL-10, IL-17 in T lymphocyte culture supernatants were determined using ELISA kits according to the manufacturer’s procedures (eBiosciences, San Diego, CA).
Antigen presentation assay in vitro
DCs were activated or not with the supernatant of Th-1 lymphocytes, washed and co-cultured for 48 hours with B16 or B16-OVA tumor cells. Positive controls consisted of LPS-activated KDCs (5). At the end of the culture DCs were selected using anti-CD11c-microbeads and were co-cultured with B3Z cells (DC:B3Z ratio=1:10). B3Z is a mouse CD8+ T-cell hybridoma that contains an Escherichia coli lacZ reporter gene driven by nuclear factor of activated T cell (NF-AT) elements from the IL-2 promoter. The specific recognition of the SIINFEKL peptide of ovalbumin (OVA257–264) in the context of MHC class I by the TCR of B3Z results in the expression of the enzyme β-galactosidase. The activity of this enzyme is detected by evaluating the subsequent conversion of a chemoluminescent substrate measured by luminometry (Novagen kit, Madison, WI, USA) as previously documented (5).
KDC administration to B16-OVA tumor-bearing CD11c-DTR mice and antigen presentation assays
CD11c-DTR mice (H2b) (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) were injected with 1×106 B16 or B16-OVA tumor cells subcutaneously in both flanks. When tumors were palpable, diphtheria toxin (DT, 5 ng/g body weight) was administered (intraperitoneal injection) on 2 consecutive days (Sigma, St. Louis, MO). This resulted in a significant depletion of endogenous DC as verified by flow cytometry (Supplemental Figure S4A). On the day of the second DT injection, 20×106 day 8 DCs, previously activated with LPS, IFN-γ or Th-1 supernatant were injected intratumorally. Thirty-six hours later, mice were euthanized and CD11c+ cells were re-isolated from the draining lymph nodes using CD11c microbeads (Miltenyi). Approximately 2 × 105 to 2.5 × 105 CD11c+ cells can be recovered. The ability of these re-isolated DCs to induce B3Z activation was assessed as explained in the previous section. In other experiments, the capability of re-isolated CD11c+ DCs to induce MHC-I restricted proliferation of OVA-specific OT-I or MHC-II restricted proliferation of OVA-specific OT-II lymphocytes was determined using BrDU incorporation assays (Millipore) as previously reported (38).
Statistical analysis
Unless specified otherwise, all experiments were reproduced 3 times and performed in triplicate. A two-sided student’s t test with paired samples was used to determine significant differences (p<0.05) between groups. For real time PCR experiments, statistical significance was determined by the analysis of variance (ANOVA) followed by Fisher protected least significant difference (PLSD) post-hoc test with StatView software package v.4.53 (SAS Institute, Cary, NC). Data are expressed as mean ± standard deviation of mean.
Results
Activated T lymphocytes induce DC tumor killing function
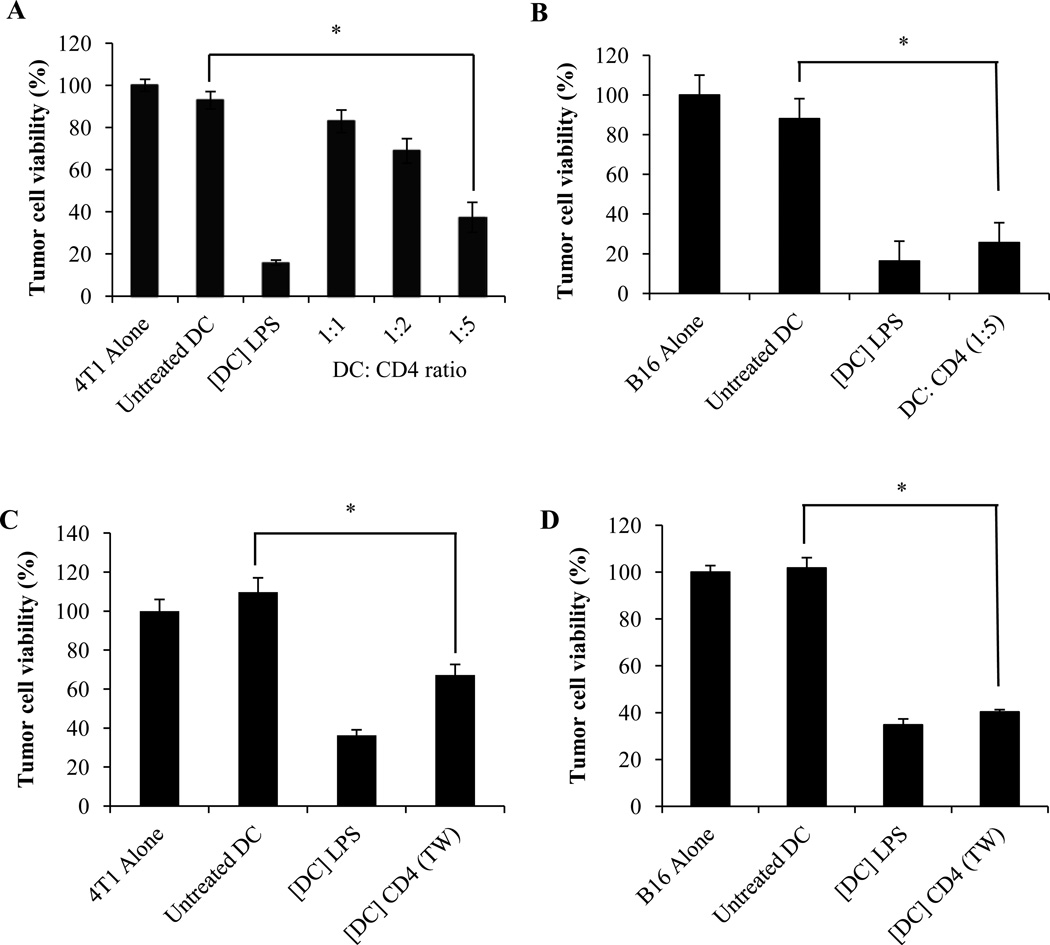
The cytotoxic function of ex-vivo generated DC may be triggered by different approaches. We and others have previously reported on the induction of the tumoricidal activity of these cells by the TLR-4 ligand LPS (4, 5, 28, 39). However, the possible modulation of DC cytotoxic potential by T lymphocytes had not been elucidated. To assess whether DC killing function may be regulated by helper T cells, day 6 CD11c+ bone marrow-derived DCs were incubated for 48 hours with anti-CD3 and anti-CD28 - activated CD4+ T lymphocytes. DCs were then separated from T cells using CD11c microbeads and their ability to kill tumor cells was determined as previously described (4, 5). The results depicted in Figure 1A indicate that CD4+ T cells triggered the ability of DCs to kill 4T1 mammary carcinoma cells. Induction of DC killing function was dependent on the DC:T cell ratio, with a significant cytotoxic activity obtained at 1:5 ratio (Figure 1A). Identical results were obtained using B16 melanoma as targets (Figure 1B). Similar to our previous reports (5) DC-mediated tumor cell killing depended on the effector DC: target tumor cell ratio and was prominent at 5:1 effector to target ratios (data not shown). The induction of DC cytotoxic activity did not require direct cell to cell contact and therefore involved soluble factor(s) produced by activated CD4+ T lymphocytes (Fig. 1C and 1D).
Figure 1. Activated CD4+ T cells induce DC tumoricidal activity.
(A, B) Day 6 CD11c+ DCs were cultured for 48 hours with activated CD4+ T lymphocytes at the indicated ratios. CD11c+ DCs were then separated from T cells, washed and subsequently incubated for an additional 48 hours with (A) 4T1 or (B) B16 tumor cells. Tumor cell survival was determined using a crystal violet assay. LPS-activated (1 µg/ml) DCs ([DC] LPS) were used as positive controls and untreated DCs (Untreated DCs) as negative controls. The data represent the mean +/− SD from triplicate wells. * Significant difference when compared with tumor cells cultured with control untreated DCs (p<0.001). (C) 4T1 or (D) B16 tumor cells were cultured for 48 hours with untreated DCs (Untreated DCs), with LPS-activated (1 µg/ml) KDCs ([DC] LPS), or with DCs that had first been co-cultured for 48 hours separated from activated CD4+ T cells by a micro-porous membrane ([DC] CD4 (TW)) as described in material and methods. Tumor cell viability was determined as described above. Mean +/− SD of triplicate cultures. (*p<0.0001).
Th-1 lymphocytes trigger DC cytotoxic activity
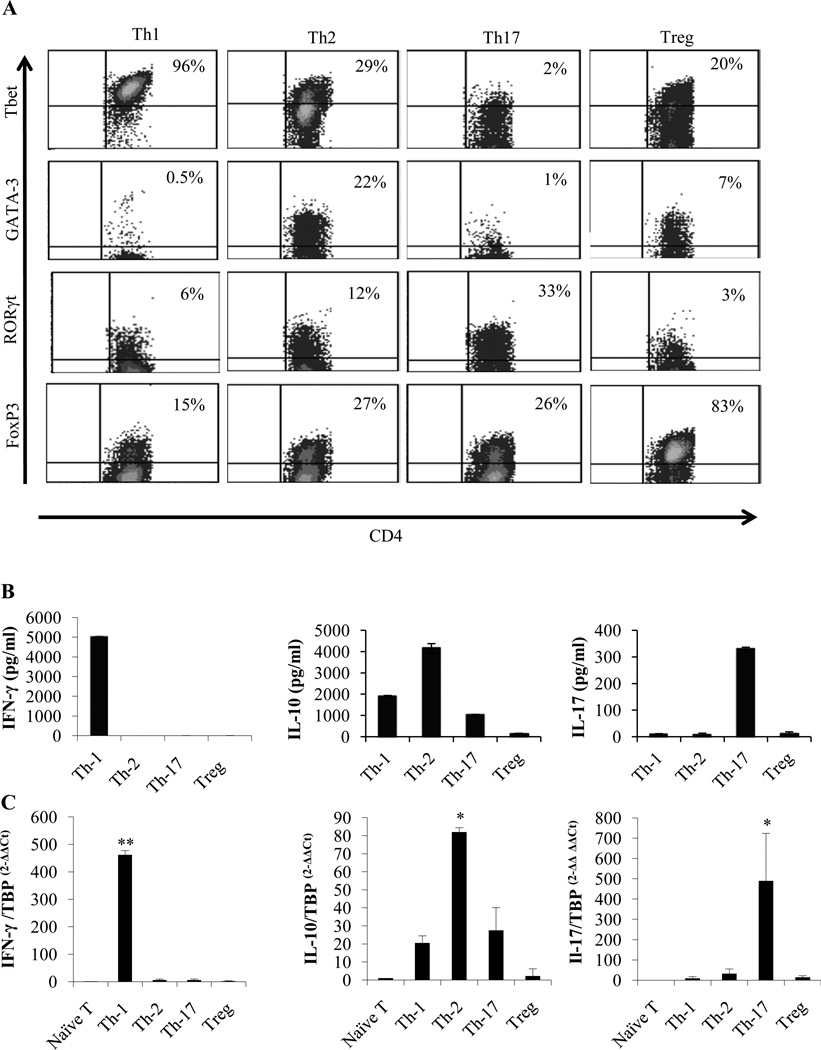
CD4+ T lymphocytes represent a heterogeneous cell population comprised of functionally and phenotypically distinct subsets primarily characterized as T helper 1 (Th-1), T helper 2 (Th-2), IL-17-producing T helper cells (Th-17) and immunosuppressive FoxP3+ regulatory T cells (Treg) (40). We further evaluated the respective contribution of these CD4+ T cell lineages in the induction of DC tumoricidal function. Naïve T cells were cultured for 3 days in different conditions to generate Th-1, Th-2, Th-17 and Treg lymphocytes as described in materials and methods. Cell lineage identity was confirmed by flow cytometry analysis of specific transcription factor expression (Tbet for Th-1; GATA3 for Th-2; RORγt for Th-17 and FoxP3 for Treg) (Figure. 2A), and by ELISA and real-time PCR for detection of specific cytokine production (IFN-γ, IL-10, IL-17) (Figure. 2B and 2C).
Figure 2. Generation and characterization of T-helper lymphocyte lineages.
Splenic CD4+CD25−CD62L+ T cells were isolated by magnetic cell sorting and cultured for 3 days in different polarization conditions as described in materials and methods to generate Th-1, Th-2, Th-17 and Treg cells. (A) Phenotypical analysis of the obtained T lymphocytes. Cells were stained with the indicated Ab and were analyzed by flow cytometry. (B) On day 3, cells were washed and re-stimulated with anti-CD3/CD28 antibody coated beads for eight hours and the culture supernatants were collected and analyzed by ELISA. (C) Real time PCR analysis of the cells obtained in (B) for the expression of the indicated cytokine mRNAs. Significant difference when compared to naïve T cell groups (* p<0.05, * p<0.0001).
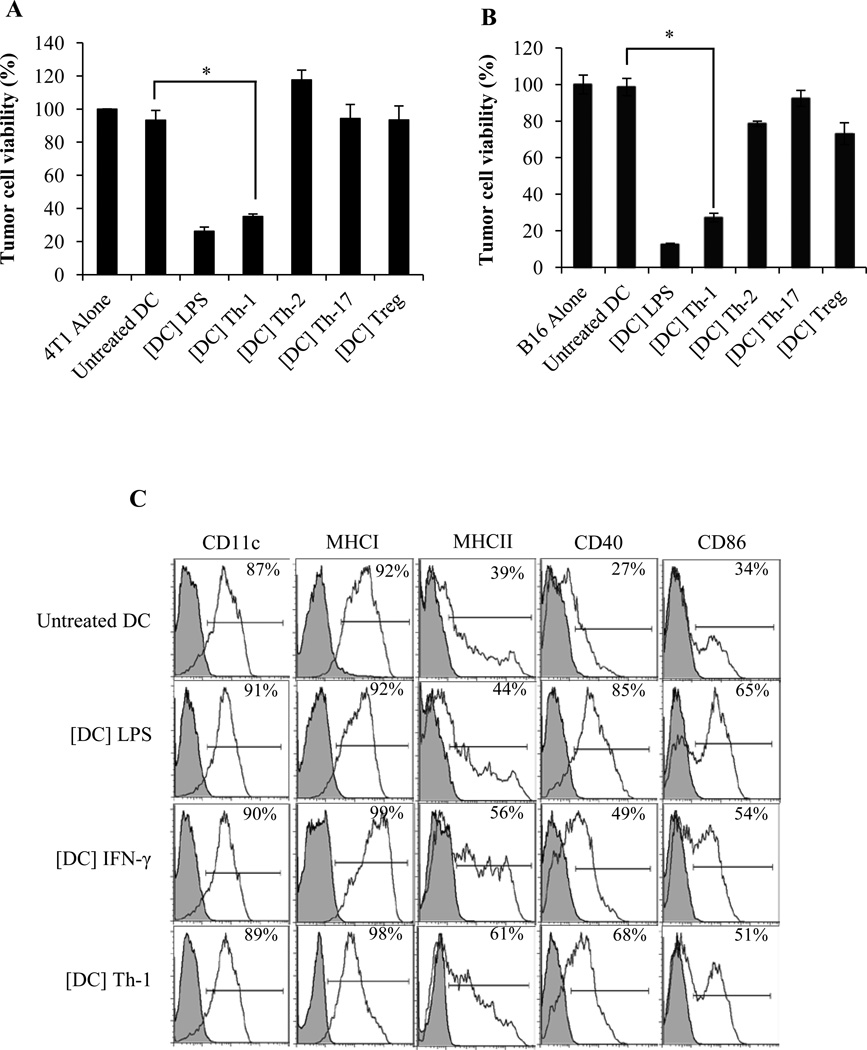
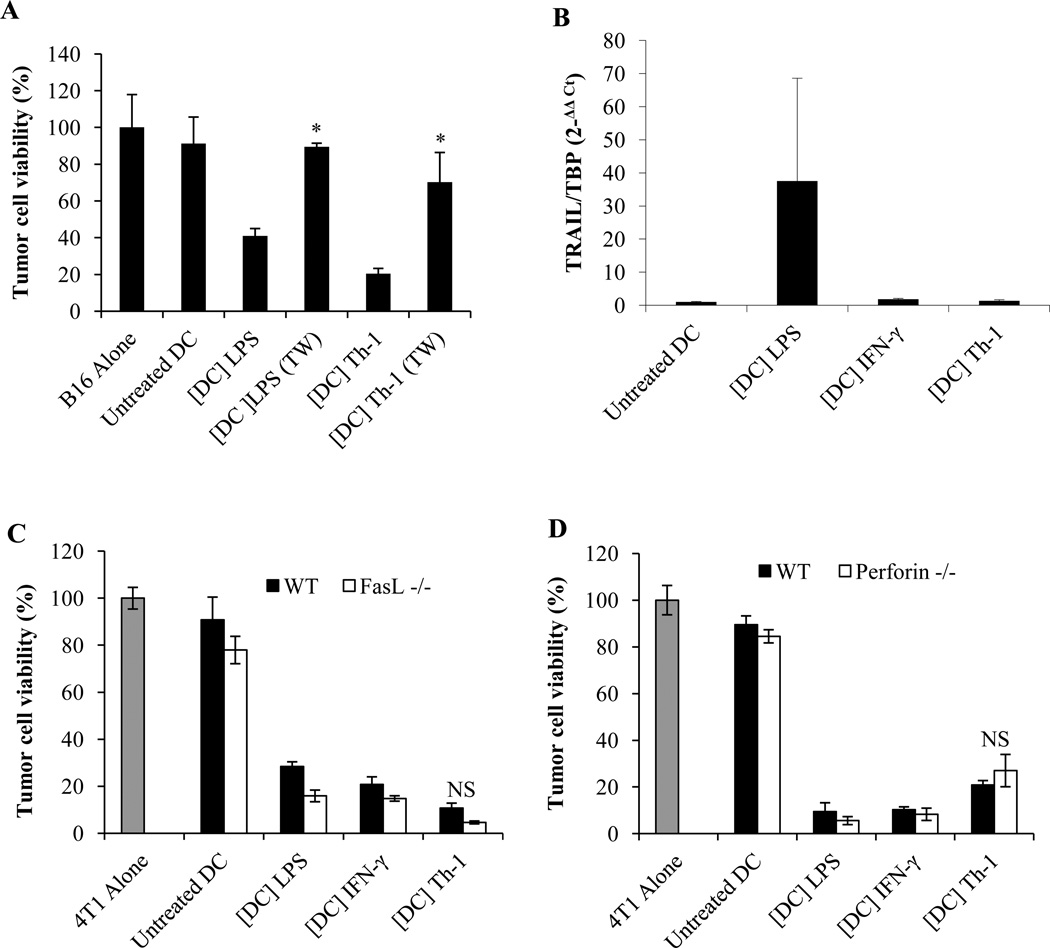
Day 6 CD11c+ DCs generated from BALB/c mice were exposed for 48 hours to the supernatant of Th-1, Th-2, Th-17 or Treg cultures and their ability to kill 4T1 tumor cells was evaluated. Our results indicate that only Th-1-derived factor(s) were capable of inducing DC cytotoxic function (Figure 3A). Similar results were obtained when B16 melanoma cells were used as targets (Figure 3B), indicating that Th-1-activated KDCs (Th-1 KDCs) are capable of killing both syngeneic and allogeneic tumor cells. Similar results were obtained with DCs and T cells generated from C57BL/6 mice (Supplemental Figure S2). No new cancer cell colony was observed when detached cells from tumor cell and Th-1 KDC co-cultures were incubated for a week in complete medium. Phenotypically, these Th-1 KDCs demonstrated increased expression of MHC II, CD40 and CD86 compared to untreated DCs (Figure 3C).
Figure 3. Th-1 but not Th-2, Th-17 or Treg are capable of inducing DC tumoricidal activity.
Day 6 CD11c+ DCs were cultured for 48 hours with the supernatants from Th-1, Th-2, Th-17 and Treg cultures. DCs were then washed extensively and incubated for 48 hours with (A) 4T1 or (B) B16 tumor cells. KDCs activated with LPS (1 µg/ml) ([DC] LPS) were used as positive controls. Tumor cell survival was then determined. Mean +/− SD from triplicate cultures. (*p < 0.001). (C) Phenotype of DCs obtained after 48 hours of culture with the culture supernatant from Th-1, Th-2, Th-17 and Treg cultures. Cells were stained with the indicated Ab and analyzed by flow cytometry.
Th-1 mediated induction of DC cytotoxic function depends on IFN-γ
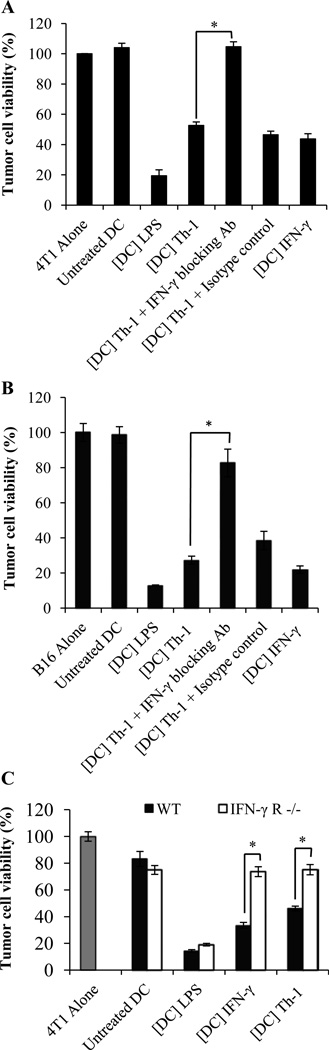
We next sought to determine the mechanism(s) underlying Th-1-mediated induction of DC killing activity. Since the data depicted in Figure 1C and 1D indicated that induction of DC killing activity required soluble factor(s) produced by activated CD4+ T lymphocytes, we investigated the role of the prominent Th-1 cytokine, IFN-γ ((41) and Figure 2B), in the triggering of DC cytotoxic function. CD11c+ DCs were exposed to Th-1 supernatant for 48 hours in the presence or absence of anti-IFN-γ blocking Ab. DC were then washed and co-cultured for another 48 hours with tumor cells. As illustrated in Figure 4A and 4B, IFN-γ neutralization prevented Th-1 lymphocyte-mediated induction of DC cytotoxic activity. Consistent with this result, recombinant IFN-γ triggered DC killing activity (Figure. 4A and 4B). We further confirmed the central role of IFN-γ by demonstrating that Th-1 cell supernatant failed to trigger tumor-killing activity of DCs generated from IFN-γ receptor knockout mice (Figure 4C).
Figure 4. The induction of DC killing activity by Th-1 lymphocytes depends on IFN-γ.
Day 6 DCs were treated with Th-1 supernatant ([DC] Th-1) with or without anti-IFN-γ blocking antibody (+IFN-γ blocking Ab) or isotype control antibody (+Isotype control) or were treated with recombinant IFN-γ (5 ng/ml) ([DC] IFN-γ) for 48 hours. DCs were then washed extensively and plated at 5:1 DC: tumor cell ratio with (A) 4T1 or (B) B16 tumor cells. (C) 4T1 tumor cells were cultured with DC generated from wild-type (WT) or from IFN-γ receptor −/− mice (IFN-γ R−/−) and treated with Th-1 culture supernatant ([DC] Th-1) or IFN-γ ([DC] IFN-γ). (A–C) Tumor cell viability was then evaluated. Mean +/− standard deviation from triplicate cultures. Untreated DCs (Untreated DC) and DCs treated with LPS (1 µg/ml) ([DC] LPS) were used as negative and positive controls respectively. (*p<0.001)
Th-1 KDC cytotoxic activity depends on Nitric Oxide, but not on perforin/granzyme or death receptor ligands
We next investigated the mechanisms underlying Th-1 KDC cytotoxic function. Th-1 KDC killing activity was dependant on a direct cell-cell contact as separation of target and effector cells by a microporous membrane prevented tumor cell death (Figure 5A). Death receptor ligands (TRAIL or Fas-L) and perforin and granzyme have been described as effector molecules responsible for DC cytotoxic activity (6, 8, 16, 18, 20, 21, 42–46). We therefore evaluated whether Th-1 KDC tumoricidal function was mediated by any of these molecules. TRAIL was not detected in Th-1 KDCs by real time PCR (Figure 5B). Fas-L, perforin and granzyme expression were also not expressed at significant levels (data not shown). Using knock-out mice, we further confirmed that Fas-L and TRAIL did not participate in Th-1 KDC killing activity (Figure 5C and data not shown). Of note, DCs from Fas-L−/− mice were slightly more effective at killing tumor cells than DCs from wild-type mice. Similarly, Th-1-activated KDCs from perforin knockout mice were not impaired in their capability to induce tumor cell death (Figure 5D).
Figure 5. Th-1-induced KDC tumoricidal activity requires a direct cell-cell contact but does not depend on death receptor ligands.
(A) 4T1 or B16 tumor cells were cultured separated or not by a transwell insert (TW), with untreated DCs (Untreated DC), or DCs treated with IFN-γ ([DC] IFN-γ) or Th-1 supernatant-treated DCs ([DC] Th-1). * Significant difference compared to the corresponding group without transwell insert (p<0.0001). (B) Expression of TRAIL detected by RT-PCR analysis of RNA isolated from day 8 DCs treated for 24 hours with LPS (1 µg/ml), IFN-γ (5ng/ml) or Th-1 supernatant. (C) DCs generated from FasL−/− or (D) perforin−/− mice were treated with IFN-γ or Th-1 supernatant and cultured for 48 hours with 4T1 tumor cells. * Significant difference compared to wild-type DCs (p<0.01). (A,C,D) Tumor cell killing was determined after 48 hours. Mean +/− standard deviation from triplicate cultures. Untreated DCs (Untreated DC) and DCs treated with LPS (1 µg/ml) ([DC] LPS) were used as negative and positive controls respectively.
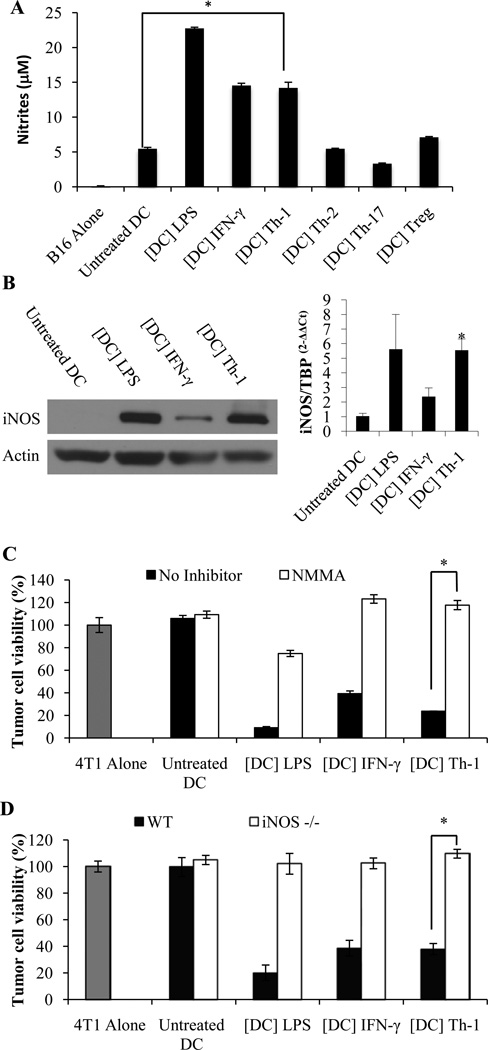
We and others have previously reported that nitric oxide (NO) or peroxynitrites are essential short half-life effector molecules that contribute to the tumoricidal activity of rat, mouse and human DCs and that require close proximity with the target cells (4, 5, 26, 28, 47). We therefore assessed whether these cytotoxic products may also be involved in Th-1 KDC-mediated killing of tumor cells. The concentration of nitrites (the main metabolites of NO) was significantly increased in the cultures of DCs pre-incubated with Th-1 supernatant (Figure 6A). In line with this result, iNOS expression was significantly increased in DCs treated with Th-1 supernatant (Figure 6B). Moreover, NMMA, an inhibitor or iNOS, abrogated Th-1 KDC-mediated tumor killing activity (Figure 6C). Further confirming these data, the cytotoxic potential of Th-1 supernatant-activated DCs generated from iNOS−/− mice was significantly impaired (Figure 6D). These findings were reproduced using the B16 model in C57BL/6 mice (data not shown). Taken together these data demonstrate that NO is essential for Th-1 KDC-mediated tumor cell killing.
Figure 6. Th-1 KDC cytotoxic activity depends on Nitric Oxide.
(A) Detection of nitrites in the supernatants of DCs treated for 48 hours with the supernatants of Th-1, Th-2, Th-17 and Treg cell cultures, or with LPS or IFN-γ. (B) iNOS expression was determined by Western blot (left panel) and RT-PCR (right panel) in day 8 DCs treated for 24 hours with LPS (1 µg/ml) ([DC] LPS), IFN-γ (5 ng/ml) ([DC] IFN-γ) or Th-1 supernatant ([DC] Th-1). * Significant compared to untreated DCs (p<0.05). (C) DCs activated with LPS, IFN-γ or Th-1 culture supernatant were incubated for 48 hours with 4T1 tumor cells with or without the iNOS inhibitor NMMA (1 mM). (D) DCs generated from iNOS−/− or wild type mice were treated with IFN-γ or Th-1 supernatant and cultured for 48 hours with 4T1 tumor cells. (C,D) Tumor cell killing was determined after 48 hours. Mean +/− standard deviation from triplicate cultures (A,C,D *p<0.001). Untreated DCs and DCs treated with LPS were used as negative and positive controls respectively.
Th-1 KDCs process and present to specific T cells the antigens from the tumor cells they kill in vitro
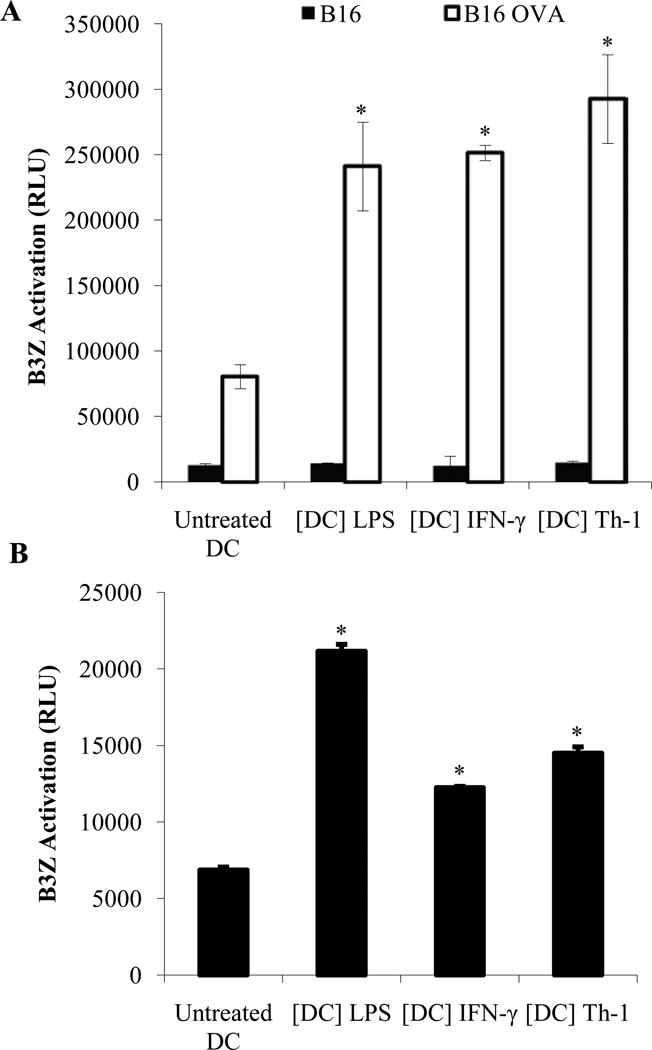
To investigate the ability of Th-1 KDCs to process and present antigens from the tumor cells they kill, Th-1-activated DCs were co-cultured for 48 hours with B16 melanoma cells expressing the model antigen ovalbumin (B16-OVA). DCs were then re-isolated using CD11c microbeads and either stained with anti-CD11c and an antibody recognizing MHC Class I-SIINFEKL complexes (Kb/SIINFEKL) or cultured for 24 hours with B3Z (a CD8+ T cell line expressing a TCR which specifically recognizes the SIINFEKL peptide of OVA in the context of MHC Class I) (5). We did not detect CD11c− H-2Kb/SIINFEKL+ cells (contaminating tumor cells) following isolation, but MHC Class I–SIINFEKL complexes were detected at the surface of isolated CD11c+ DCs (supplemental Figure S3). In addition, purified Th-1 DCs were capable of activating B3Z cells (Figure 7A). These results therefore indicate that Th-1 KDC are able to acquire, process and present antigens from the OVA-expressing B16 cells they had killed.
Figure 7. Th-1 KDCs are capable of presenting tumor antigens from the tumor cells they have killed to tumor-specific T cells.
CD11c+ DCs were treated with LPS (1 µg/ml) ([DC] LPS), IFN-γ (5 ng/ml) ([DC] IFN-γ) or Th-1 supernatant ([DC] Th-1) for 48 hours. (A) DCs were then washed and cultured for 24 hours with B16 or B16-OVA melanoma cells. DCs were selected from the culture using CD11c microbeads and incubated for an additional 24 hours with B3Z cells (DC:B3Z ratio = 1:10) as outlined in material and methods. The activity of β-galactosidase was measured by evaluating the conversion of its substrate into a chemoluminescent product (RLU, Relative Luminescence Unit). (B) CD11c-GFP-DTR mice were injected with B16-OVA melanoma cells. When tumors become palpable, diphtheria toxin (DT) was administered to deplete host CD11c+ cells. CD11c+ DCs generated in vitro and treated with LPS or IFN-γ or Th-1 supernatant were then injected intratumorally (20×106 cells/tumor). After 36 hours, draining lymph nodes were harvested and CD11c+ cells were isolated using CD11c microbeads. The obtained DCs were then cultured with the OVA-specific T cell line B3Z. Specific recognition of tumor-derived OVA peptide was measured as outlined above. * Significant difference when compared to untreated DC group (p<0.01).
Th-1 KDCs injected into established tumors migrate to the lymph nodes and are capable of presenting tumor specific antigens in vivo
We next assessed the anti-tumoral potential of Th-1 KDCs in vivo. B16-OVA tumors were established in CD11c-GFP-DTR mice. Animals were then treated with diphtheria toxin (DT) to eliminate host CD11c+ DCs when tumors became palpable. This approach prevented possible interference by endogenous DCs. Th-1 activated killer DCs were then injected into the tumor beds and CD11c+ cells were isolated from the tumor draining lymph nodes after 36 hours. These purified CD11c+ DCs were GFP-negative and therefore entirely of donor origin (supplemental Figure S4A). Their ability to activate the OVA-specific B3Z hybridoma was then evaluated. Our results indicate that only DCs activated with LPS or IFN-γ, and Th-1 KDCs (e.g. killer DCs), but not untreated non-killer DCs were capable of inducing B3Z activation (Figure 7B). Th-1 KDC were also capable of inducing the proliferation of lymphocytes isolated from OT-II or OT-I or mice (Supplemental Figure S4B and data not shown). These results therefore demonstrate that Th-1-activated killer DCs are capable of migrating from the tumor site to the lymph nodes, and that functionally these killer DC retained their ability to process and present in a MHC Class I and Class II-restricted manner the in vivo acquired antigens as demonstrated by ex vivo assays.
Discussion
DCs play an essential role in the initiation and regulation of tumor-specific immune responses, as they are endowed with the unique potential to efficiently activate anticancer effector cells such as T helper and cytotoxic T cells (1). This capacity has been extensively exploited, leading to the development of DC-based cancer immunotherapies. However, the initial evidence that protective anti-tumor immunity can be successfully generated by vaccination with tumor antigen-loaded DCs has been undermined by the limited clinical responses observed in cancer patients. Therefore, the prospect of exploiting the non-conventional direct tumor killing function of DCs represents an important step forward for the advancement of these cells in cancer therapies. KDCs are indeed not only endowed with the capacity of directly killing tumor cells (and thereby they participate to the effector mechanisms of immune responses), but they can also generate their own source of released tumor antigens immediately available for uptake, processing and presentation to specific T lymphocytes (5, 28).
Several agents have been reported to trigger DC killing potential, including cytokines such as IFNs, TNF-α, IL-12, IL-18, IL-2, and IL-15 (14, 17, 19, 24, 30, 48) or TLR ligands (8, 18, 31) such as the TLR4 ligand LPS as we and others have previously reported (4, 5). However, the optimal mode of activation of DC cytotoxic activity remains to be determined. DC-T helper lymphocyte cross-talk critically contributes to the regulation of conventional DC function (49), but the putative effects exerted by T cells on the cytotoxic activity of DCs had not been previously delineated. In the current report we provide evidence that only activated pro-inflammatory Th-1 lymphocytes can drive the activation of bone-marrow-derived DCs into potent tumor killers.
The mechanism of induction of DC killing activity by these Tbet+ Th-1 cells does not depend on direct cell contact but requires the Th-1-related cytokine IFN-γ. The possibility that IFN-γ may induce DC cytotoxic activity has been controversial. This observation has been documented primarily in the case of human DC and only when high and less physiologically achievable concentrations of this cytokine were used (4, 6, 14, 17, 29, 30), In addition, only recombinant IFN-γ was tested. We provide novel evidence that IFN-γ produced by Th-1 at physiological levels can activate mouse bone marrow-derived DC tumoricidal potential. In our study, the concentration of IFN-γ detected in Th-1 supernatants capable of inducing DC killing activity was significantly lower than that tested in previous reports (4, 6, 14, 17, 29, 30). Additionally, in most of these studies cytotoxic effects were detected only when significantly higher effector KDC to target tumor cell ratios were used (4, 6, 14, 17, 29, 30).
The mode of tumor cell killing by DCs may involve the death receptor family and their ligands (6, 8, 10–13, 15, 17–20, 25, 31), perforin and granzyme (8), or as we previously reported NO or peroxynitrites (4, 11, 23, 26, 27). NO has been shown to sensitize tumor cells to Fas-ligand-mediated killing by immature or spontaneously matured DC (9). A NO donor was used at low (and non-cytotoxic concentrations) to modulate anti-apoptotic pathways (9). In the same study the NO donor alone was however capable of inducing tumor cell death when used at higher concentrations (9). We demonstrated that iNOS expression is significantly increased in Th-1 KDCs and identified NO as the primary cytotoxic effector molecule implicated in Th-1 KDC-mediated tumor cell elimination as DCs from iNOS−/− mice were not capable of killing cancer cells. The levels of NO produced by activated DCs were sufficient to trigger the death of tumor cells, and ligands of the TNF superfamily were not involved in this process. The short half-life of these cytotoxic molecules is also consistent with a cell to cell contact dependent mechanism of tumor cell killing.
Typically, only immature DCs are efficient at taking up antigens (50). The observation that activated mature Th-1 KDCs, following killing of cancer cells, are still capable of capturing tumor antigens is therefore of importance. This property allows them to subsequently process and present the derived antigenic peptides to T cells and therefore to initiate adaptive immune responses. Of therapeutic relevance, Th-1 KDCs injected into the tumor beds that migrated to the draining lymph nodes were capable of activating T lymphocytes, indicating that they acquired antigens and have the ability of presenting them in vivo. This capacity of Th-1 KDCs to efficiently present antigens is contingent upon induction of their killing function, as non-killer DCs were significantly less potent APCs. Further supporting this observation, DC from iNOS−/− mice were less potent at activating specific T cells following culture with tumor cells compared to their wild-type counterparts (data not shown).
The potential for DCs to act not only as antigen presenting cells but also as tumor cell killers has revitalized their attractiveness as immunotherapeutic agents against cancer. Our results further advocate for the implementation of KDCs in immunotherapy strategies and highlight new possibilities related to the mode of induction of their killing and antigen presenting function, and underline the need for a rethinking of DC-based vaccine approaches.
Supplementary Material
Acknowledgments
We thank Anthony Pilutti for his assistance.
Grant support: This work was supported by the NIH grant R01 CA104926 (E.K. and N.L.), the Cancer Biology Training Grant T32CA009213 (SC), the AZ Cancer Center Support Grant CA023074 (EK and NL), the Tee Up for Tots and the PANDA Funds (E.K. and N.L.).
Abbreviations
- APC
antigen presenting cell
- DC
dendritic cell
- DT
diphtheria toxin
- iNOS
inducible nitric oxide synthase
- KDC
killer dendritic cell
- NMMA
NG-methyl-L-arginine
- NO
nitric oxide
- Treg
regulatory T cell
References
- 1.Palucka K, Ueno H, Zurawski G, Fay J, Banchereau J. Building on dendritic cell subsets to improve cancer vaccines. Current opinion in immunology. 2010;22:258–263. doi: 10.1016/j.coi.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Current opinion in immunology. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Klechevsky E, Schmitt N, Morita R, Palucka K, Ueno H. Harnessing human dendritic cell subsets to design novel vaccines. Annals of the New York Academy of Sciences. 2009;1174:24–32. doi: 10.1111/j.1749-6632.2009.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolas A, Cathelin D, Larmonier N, Fraszczak J, Puig PE, Bouchot A, Bateman A, Solary E, Bonnotte B. Dendritic cells trigger tumor cell death by a nitric oxide-dependent mechanism. J Immunol. 2007;179:812–818. doi: 10.4049/jimmunol.179.2.812. [DOI] [PubMed] [Google Scholar]
- 5.Fraszczak J, Trad M, Janikashvili N, Cathelin D, Lakomy D, Granci V, Morizot A, Audia S, Micheau O, Lagrost L, Katsanis E, Solary E, Bonnotte B, Larmonier N. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J Immunol. 2010;184:1876–1884. doi: 10.4049/jimmunol.0900831. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Ikeda K, Fujii N, Kondo E, Shinagawa K, Ishimaru F, Kaneda K, Tanimoto M, Li X, Pu Q. Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer science. 2005;96:127–133. doi: 10.1111/j.1349-7006.2005.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josien R, Heslan M, Soulillou JP, Cuturi MC. Rat spleen dendritic cells express natural killer cell receptor protein 1 (NKR-P1) and have cytotoxic activity to select targets via a Ca2+-dependent mechanism. J Exp Med. 1997;186:467–472. doi: 10.1084/jem.186.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Tatsumi T, Pizzoferrato E, Vujanovic N, Storkus WJ. Nitric oxide sensitizes tumor cells to dendritic cell-mediated apoptosis, uptake, and cross-presentation. Cancer research. 2005;65:8461–8470. doi: 10.1158/0008-5472.CAN-05-0654. [DOI] [PubMed] [Google Scholar]
- 10.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. Journal of immunology. 1997;158:5676–5684. [PubMed] [Google Scholar]
- 12.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Shibaki A, Katz SI. Activation through CD40 ligation induces functional Fas ligand expression by Langerhans cells. Eur J Immunol. 2001;31:3006–3015. doi: 10.1002/1521-4141(2001010)31:10<3006::aid-immu3006>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Manna PP, Mohanakumar T. Human dendritic cell mediated cytotoxicity against breast carcinoma cells in vitro. J Leukoc Biol. 2002;72:312–320. [PubMed] [Google Scholar]
- 15.Ayres FM, Narita M, Takahashi M, Yano T, Liu A, Toba K, Furukawa T, Aizawa Y. Human dendritic cells mediate anti-tumor activity against hematopoietic tumor cells without direct contact and Fas/FasL killing pathway. Oncology reports. 2004;11:1017–1023. [PubMed] [Google Scholar]
- 16.Lu G, Janjic BM, Janjic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha(1)beta(2), Fas ligand, and TNF-related apoptosis-inducing ligand. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 17.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. The Journal of experimental medicine. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidalain PO, Azocar O, Yagita H, Rabourdin-Combe C, Servet-Delprat C. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167:3765–3772. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. Journal of immunology. 2001;166:5407–5415. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 20.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 21.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 22.Alli R, Savithri B, Das S, Varalakshmi C, Rangaraj N, Khar A. Involvement of NKR-P2/NKG2D in DC-mediated killing of tumor targets: indicative of a common, innate, target-recognition paradigm? Eur J Immunol. 2004;34:1119–1126. doi: 10.1002/eji.200324793. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava RM, Varalakshmi C, Khar A. Cross-linking a mAb to NKR-P2/NKG2D on dendritic cells induces their activation and maturation leading to enhanced anti-tumor immune response. Int Immunol. 2007;19:591–607. doi: 10.1093/intimm/dxm024. [DOI] [PubMed] [Google Scholar]
- 24.Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, Alber SM, Watkins SC, Okada H, Storkus WJ. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)- 12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer research. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 25.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura H, Cumberland R, Hiroishi K, Watkins SC, Lotze MT, Baar J. Murine dendritic cell-induced tumor apoptosis is partially mediated by nitric oxide. J Immunother. 2002;25:226–234. doi: 10.1097/00002371-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Aiello S, Noris M, Piccinini G, Tomasoni S, Casiraghi F, Bonazzola S, Mister M, Sayegh MH, Remuzzi G. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–4658. doi: 10.4049/jimmunol.164.9.4649. [DOI] [PubMed] [Google Scholar]
- 28.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2010;59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz M, Zhao S, Deuse Y, Schakel K, Wehner R, Wohner H, Holig K, Wienforth F, Kiessling A, Bornhauser M, Temme A, Rieger MA, Weigle B, Bachmann M, Rieber EP. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174:4127–4134. doi: 10.4049/jimmunol.174.7.4127. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Liu S, Wang W, Song W, Zhang M, Zhang W, Qin Z, Cao X. Involvement of tumour necrosis factor-alpha-related apoptosis-inducing ligand in enhanced cytotoxicity of lipopolysaccharide-stimulated dendritic cells to activated T cells. Immunology. 2002;106:308–315. doi: 10.1046/j.1365-2567.2002.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 33.Lakomy D, Janikashvili N, Fraszczak J, Trad M, Audia S, Samson M, Ciudad M, Vinit J, Vergely C, Caillot D, Foucher P, Lagrost L, Chouaib S, Katsanis E, Larmonier N, Bonnotte B. Cytotoxic Dendritic Cells Generated from Cancer Patients. Journal of immunology. 2011 doi: 10.4049/jimmunol.1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantrell J, Larmonier C, Janikashvili N, Bustamante S, Fraszczak J, Herrell A, Lundeen T, C JL, Situ E, Katsanis E, Larmonier N. Signaling pathways induced by a tumor-derived vaccine in antigen presenting cells. Immunobiology. 2010;215:535–544. doi: 10.1016/j.imbio.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnotte B, Larmonier N, Favre N, Fromentin A, Moutet M, Martin M, Gurbuxani S, Solary E, Chauffert B, Martin F. Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J Immunol. 2001;167:5077–5083. doi: 10.4049/jimmunol.167.9.5077. [DOI] [PubMed] [Google Scholar]
- 36.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 37.Larmonier N, Billerey C, Rebe C, Parcellier A, Moutet M, Fromentin A, Kroemer G, Garrido C, Solary E, Martin F, Bonnotte B. An atypical caspase-independent death pathway for an immunogenic cancer cell line. Oncogene. 2002;21:6091–6100. doi: 10.1038/sj.onc.1205738. [DOI] [PubMed] [Google Scholar]
- 38.Janikashvili N, Lacasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, Har-Noy M, Larmonier N, Katsanis E. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood. 2011;117:1555–1564. doi: 10.1182/blood-2010-06-288621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesa AK, Storkus WJ. Killer dendritic cells: mechanisms of action and therapeutic implications for cancer. Cell Death Differ. 2008;15:51–57. doi: 10.1038/sj.cdd.4402243. [DOI] [PubMed] [Google Scholar]
- 40.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. Journal of clinical immunology. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–5415. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 43.Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- 44.Vanderheyde N, Aksoy E, Amraoui Z, Vandenabeele P, Goldman M, Willems F. Tumoricidal activity of monocyte-derived dendritic cells: evidence for a caspase-8-dependent, Fas-associated death domain-independent mechanism. J Immunol. 2001;167:3565–3569. doi: 10.4049/jimmunol.167.7.3565. [DOI] [PubMed] [Google Scholar]
- 45.Vanderheyde N, Vandenabeele P, Goldman M, Willems F. Distinct mechanisms are involved in tumoristatic and tumoricidal activities of monocyte-derived dendritic cells. Immunol Lett. 2004;91:99–101. doi: 10.1016/j.imlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Yang R, Xu D, Zhang A, Gruber A. Immature dendritic cells kill ovarian carcinoma cells by a FAS/FASL pathway, enabling them to sensitize tumor-specific CTLs. Int J Cancer. 2001;94:407–413. doi: 10.1002/ijc.1484. [DOI] [PubMed] [Google Scholar]
- 47.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhry UI, Kingham TP, Plitas G, Katz SC, Raab JR, DeMatteo RP. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006;66:10497–10504. doi: 10.1158/0008-5472.CAN-06-1908. [DOI] [PubMed] [Google Scholar]
- 49.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Seminars in immunology. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunological reviews. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.