Abstract
Reduced sensitivity to aversive effects of methamphetamine (MA) may increase risk for MA abuse. Studies in two replicate sets of mouse lines that were selectively bred for high and low levels of MA intake support this view. Current studies examined the extent of insensitivity to aversive MA effects of mice bred for high levels of MA drinking. Conditioning procedures in which drugs are delivered shortly after cue exposure have been used to detect aversive drug effects and, in some cases, are more sensitive to such effects. Aversive effects induced by MA injected immediately after exposure to cues from two different sensory modalities were examined. In addition, effects of higher MA doses than those used previously were examined. MA-associated place conditioning utilized tactile cues, whereas MA-induced taste conditioning utilized a novel tastant. Second replicate, MA high drinking (MAHDR-2) and low drinking (MALDR-2) mice were treated with doses of MA up to 4 mg/kg. MAHDR-2 mice were insensitive to aversive effects of MA, except after place conditioning with the 4 mg/kg dose; MALDR-2 mice exhibited sensitivity to aversive effects of MA at doses as low as 1 mg/kg. These studies show that the expression of aversion is dependent upon procedure and MA dose, and that MAHDR-2 mice have markedly reduced sensitivity to the aversive effects of MA. The current and previous results support a strong genetic relationship between level of MA intake and level of sensitivity to aversive effects of MA, a factor that could impact risk for MA use in humans.
Keywords: addiction, conditioned place aversion, conditioned taste aversion, drug reward, selective breeding
1. Introduction
Methamphetamine (MA) is a highly addictive substance. However, very little is known about individual differences in response to MA that may relate to differences in susceptibility to development of a drug problem. MA has both rewarding and aversive effects (Cruickshank and Dyer, 2009; Sheridan et al., 2009), which may contribute to risk for abuse (e.g., Davis and Riley, 2010). In the current work, we focus on aversive effects of MA, which can manifest as several symptoms in humans, such as anxiety, dysphoria and headaches (Cruickshank and Dyer, 2009; Sheridan et al., 2009). These factors may influence the probability that an individual will continue MA use beyond initial experimentation, but have been less often examined than rewarding or reinforcing effects.
A commonly used test for measuring sensitivity to aversive drug effects is drug conditioned taste aversion (CTA). In addition, place conditioning can be used to demonstrate both aversive and rewarding drug effects. The balance in sensitivity to rewarding and aversive drug effects appears to play a significant role in how fast an animal acquires operant self-administration and in how much of the drug it consumes (Davis and Riley, 2010). For example, F344 rats showed greater sensitivity than Lewis rats to aversive effects of morphine, ethanol, and nicotine in a CTA procedure and lower self-administration of these drugs (Lancellotti et al., 2001; Sanchez-Cardoso et al., 2007). In contrast, Lewis rats acquired cocaine self-administration faster than F344 rats (Kosten et al., 1997) and showed stronger cocaine CPP (Kosten et al., 1994). Although such studies suggest a possible genetic relationship between drug self-administration and sensitivity to a drug’s rewarding and aversive effects, it is difficult to draw definitive conclusions based only on comparisons between two unrelated inbred strains (i.e., this is akin to comparing two unrelated individuals). A stronger test of hypotheses about genetic relationships is derived from the study of genetic codetermination in animal lines that have been selectively bred to systematically differ with regard to only those genes that influence the trait for which they were bred (Crabbe et al., 1990). Our recent data using mice selectively bred for high and low MA drinking (MADR) indicate that genetic differences play a significant role in sensitivity to opposing rewarding and aversive drug effects (Cunningham et al., 1992; Shabani et al., 2011; Wheeler et al., 2009). However, the extent of insensitivity to aversive drug effects in the genotype with a higher propensity to consume MA is a question that remains unanswered.
In the first pair of MADR lines (replicate 1), mice selectively bred for high levels of MA drinking (MAHDR) were sensitive to rewarding effects of a low MA dose (0.5 mg/kg) as indexed by a conditioned place preference (CPP) test; but mice bred for low MA drinking (MALDR) showed no place conditioning. In that study, place preference was tested in mice that were not treated with MA prior to the test (i.e., in a drug-free state), which is the most common test condition used for this procedure. Conversely, in a CTA procedure, MALDR mice exhibited sensitivity to aversive effects of low to moderate MA doses (1–2 mg/kg), while MAHDR mice showed no taste aversion (Wheeler et al., 2009). Although these results suggested that selective breeding for MA drinking had created mouse lines with opposite sensitivities to MA’s rewarding and aversive effects, interpretation was complicated by the finding that one of the selected lines showed no learning in each of the procedures, raising the possibility that selection had differentially altered the abilities of the selected lines to learn these two tasks instead of (or in addition to) altering sensitivity to MA’s motivational effects.
This issue was partially addressed in a later CPP experiment (Shabani et al., 2011) that used a second, completely independent replicate set of selectively bred MA drinking lines (MADR-2) to examine CPP conditioned with several different MA doses (0.5, 2.0 or 4.0 mg/kg). Drug-free CPP testing in MADR-2 mice confirmed and extended the finding previously observed in the first replicate mice, i.e., significant CPP across all MA doses was seen in MAHDR-2 mice, but no CPP was seen in MALDR-2 mice. In addition, when MA was injected prior to the preference test (i.e., in a drug-present test) a significant conditioned place aversion (CPA) was seen in MALDR-2, but not MAHDR-2, mice across all doses. This outcome suggested not only that MALDR-2 mice had learned about stimulus-drug relationships during place conditioning but also that they were sensitive to MA’s aversive effects in the place conditioning procedure, whereas MAHDR-2 mice were not. These data suggest that interoceptive drug cues were an integral component of the “contextual” stimuli that had previously been associated with MA’s aversive effects in MALDR-2 mice (e.g., state-dependent learning).
Virtually complete absence of sensitivity to aversive MA effects, even at high MA doses, would likely increase risk for unlimited MA use. To examine the extent of insensitivity of MAHDR-2 mice, additional studies were performed that manipulated MA dose and cue relationships. Instead of giving MA just before exposure to the place-conditioning cue, we injected MA immediately after cue exposure in a conditioning procedure that is operationally more like that typically used in CTA procedures. On the basis of previous studies with amphetamine (Fudala and Iwamoto, 1990), nicotine (Fudala and Iwamoto, 1987) and ethanol (Cunningham et al., 2003a; Cunningham and Henderson, 2000; Cunningham et al., 1997; Cunningham et al., 2002), we predicted that post-cue drug exposure would produce CPA during drug-free tests, with high sensitivity in MALDR-2 mice, and perhaps insensitivity in MAHDR-2 mice. Prior to testing the selected lines, we tested mice of the parental genotype that served as the founding population for the replicated MADR selections (i.e., the C57BL/6J by DBA/2J F2 cross; B6D2F2). This study was performed to confirm the ability of post-cue MA to produce CPA in mice, because only amphetamine was previously tested and it was only tested in rats (Fudala and Iwamoto, 1990). It was also conducted to determine the optimal dose range for use in the selected line study. Following these determinations, we used the same methods to test for differences in post-cue MA-induced CPA between the MADR-2 lines. Previous data showed a line difference in conditioned place aversion, using a forward conditioning approach, only when MA was administered just before the place preference test. A line difference in MA-induced CPA using post-cue conditioning (and drug-free place testing) would indicate that expression of a line difference in aversive response to MA does not depend on the presence of MA drug-state cues.
We also used post-cue conditioning to examine sensitivity of the MADR-2 lines to MA-induced CTA, using procedures like those used to test the first replicate MADR lines (Wheeler et al., 2009). However, we extended this examination in MAHDR-2 mice to a dose twice as high (4 mg/kg) as that tested in MALDR-2 mice. Consistent with results for the replicate 1 MADR lines, we predicted that MA would produce CTA in MALDR-2 mice, but not in MAHDR-2 mice. Finally, to determine whether there were line differences in MA absorption or metabolism, we measured blood MA levels in both lines at several time points up to 4 hours after an acute MA injection.
2. Methods
2.1. Animals
Animal care and use were approved by the Portland VA Medical Center’s Institutional Animal Care and Use Committee, and measures were taken to minimize discomfort. Mice were weaned at age 20–22 days, and then housed in same-sex groups of 2–4 per cage with littermates or mice of the same line and age (± 5 d); mice from different litters were housed together only when necessary to avoid isolate housing. Acrylic plastic shoebox cages (28.5 × 17.5 × 12 cm) with Bed-O-CobTM bedding were used, and mice had free access to rodent chow (Purina 5001™, 4.5% fat content; Animal Specialties Inc., Hubbard, OR, USA) and water except during the CTA study, as detailed below. The colony room temperature was 21 ± 1°C, and fluorescent room lighting was on a 12:12-h light:dark schedule (lights on 0600 h).
B6D2F2 mice used here (age 102 – 139 d) were derived from the F1 cross of inbred C57BL/6J (B6) and DBA/2J (D2) strain mice that were obtained from The Jackson Laboratory. A population of 120 B6D2F2 mice had served as the founding population for the MADR-2 selected lines used here. The selective breeding results for the MADR-2 mice have been fully described elsewhere (Shabani et al., 2011). The selection phenotype was based on consumption of a 40 mg/l MA in tap water solution in a two-bottle choice procedure in which the alternative fluid was pure tap water. Mice with the highest levels of consumption of the 40 mg/l MA solution were interbred to establish the MAHDR-2 line and mice with the lowest levels of consumption of the 40 mg/l MA solution were interbred to establish the MALDR-2 line. Mice used for the studies described here were male and female MA naïve MADR-2 mice that were 63 – 104 d of age. Mice used in the CPA and CTA studies were from S4–5. After only a single generation of selection, MALDR-2 mice consumed less than 1 mg/kg MA in an 18-h period, compared to consumption of about 4.5 mg/kg MA in MAHDR-2 line mice. By S4–5 MALDR-2 mice consumed less than 0.5 mg/kg MA and MAHDR-2 line mice consumed almost 6 mg/kg MA (Shabani et al., 2011).
2.2. Drugs
(+)Methamphetamine hydrochloride (MA) and sodium chloride (NaCl) were purchased from Sigma (St Louis, MO, USA). MA was dissolved in sterile physiological saline (0.9% NaCl; Baxter Healthcare Corporation, Deerfield, IL, USA) and MA and control saline injections were administered by intraperitoneal (IP) injection at a volume of 10 ml/kg body weight. NaCl was dissolved in water at a concentration of 0.2 M for the CTA drinking solution.
2.3. Conditioned place aversion (CPA)
The equipment used for conditioning and testing was the same equipment used in previous place conditioning studies (Shabani et al., 2011; Wheeler et al., 2009). Conditioning chambers consisted of clear acrylic boxes (30 × 15 × 15 cm; San Diego Instruments, San Diego, CA, USA) with removable floors. Three types of floors were used: a smooth black acrylic plastic floor during an habituation trial, and a ‘grid’ (2.3 mm stainless-steel rods mounted 6.4 mm apart), and a ‘hole’ (32 cm gauge stainless steel with 6.4 mm round holes on 9.5 mm staggered centers) floor as the tactile cues for conditioning. Black plastic panels were used to separate the chamber into two 15 × 15 × 15 cm halves. Located 2 cm above these floors were 16 evenly spaced light sources and photodetectors that were used to track animal movements and position. Data were collected and translated using Photobeam Activity System (PAS V 2.0) software (San Diego Instruments, San Diego, CA, USA). The conditioning boxes were enclosed in illuminated and ventilated, sound-attenuating chambers.
We used a conditioning procedure in which drug exposure occurred immediately after cue exposure (Cunningham and Henderson, 2000; Fudala and Iwamoto, 1987, 1990). In addition, the procedure was unbiased, as assignment of the drug conditioning floor did not depend upon initial floor preference for each individual animal. We did not assess initial (unconditioned) preference in these mice to avoid possible interference from latent inhibition (Cunningham et al., 2011) and because previous research has shown no initial bias with these cues or strain differences between the B6 and D2 mouse strains that are the inbred founders of the MADR selected lines (Cunningham et al., 1992). Importantly, previous research has shown no genetic correlation between unlearned preference and conditioned preference to these cues in a large panel of inbred BXD strains derived from the B6 and D2 strains (Cunningham, 1995).
The conditioning procedure involved three phases: habituation, conditioning and testing. All sessions occurred on week days (Monday-Friday), and mice were moved to the experiment room on all days 1 h prior to handling to allow acclimation following cage disturbance. Throughout the experiment, mice were weighed immediately before being exposed to the conditioning box. On the first day of the study, a single habituation session involved exposure to the box for 5 min with the black plastic floor inserted. Mice had access to the entire conditioning box during this session and were injected with saline immediately after removal from the box. Beginning on the next day were 12 conditioning sessions (1 per day), during which the animals were confined for 15 min to one side of the box on the grid or hole floor and were then injected with either saline or MA, immediately upon being removed from the box. Saline and MA were given on alternating days, with saline given after the 15-min trial on one floor and MA given after the 15-min trial on the other floor. Which floor was paired with MA was counterbalanced across individual animals, with genotype and sex considered. G+ group mice received MA on the grid floor and saline on the hole floor whereas G− group mice received saline on the grid floor and MA on the hole floor. The study was also counterbalanced, with regard to whether saline or MA was received on the first conditioning day, and whether the MA-paired floor was on the left or right side of the chamber.
On the test day, mice were given access to the entire chamber for 30 min with both floor types present. The relative floor positions on the test day were consistent with those used for each mouse on the conditioning days. This maintained consistency of cues other than floor cues that may have impacted conditioning. Durations of habituation, conditioning and test sessions were chosen to be the same as those for previous place conditioning studies in these lines (Wheeler et al., 2009) and similar work for ethanol using the same apparatus (Cunningham and Henderson, 2000).
Mice from the F2 cross were used in two initial studies. These studies were performed to determine the dose range for testing the MADR-2 lines and to assess the sensitivity of the founding population to aversive post-cue conditioning effects of MA for comparison to the sensitivities of the MADR-2 lines. We expected to see conditioned place aversion (CPA) using the post-cue drug conditioning procedure, consistent with results for amphetamine in rats (Fudala and Iwamoto, 1990). The first experiment in B6D2F2 mice (n = 7–9 per sex and dose) tested higher MA doses (4, 8 and 12 mg/kg MA), some of which had been shown to induce CPA in DBA/2J mice in a standard CPP procedure (Cunningham and Noble, 1992). When results from the first experiment were examined, we found that all doses induced robust CPA. Therefore, a second experiment (n = 6–8 per sex and dose) was planned that included lower MA doses (0.5, 1 and 2 mg/kg MA) to determine if CPA would be seen. These doses have been used in standard CPP studies (i.e., drug injection before context exposure) and did not induce aversion in drug-free tests using DBA/2J mice (Cunningham and Noble, 1992) or any of the MADR selected lines (Shabani et al., 2011; Wheeler et al., 2009).
The place conditioning experiment in MADR-2 mice (n = 10–14 per line, sex and dose) was completed in three passes using a combination of MA doses from the two studies in F2 mice (0.5, 2 and 4 mg/kg). These doses were chosen to include the lowest dose that has been shown to induce a place preference in a standard CPP procedure using pre-cue drug injections (Cunningham and Noble, 1992; Shabani et al., 2011; Wheeler et al., 2009), and two doses that produced CPA in F2 mice, but that we thought might differentiate the sensitivities of the MAHDR-2 and MALDR-2 lines. The experimental passes had equal numbers of mice from each line, sex and MA dose.
2.4. Conditioned taste aversion (CTA)
Previous work in replicate 1 MADR mice examined doses of MA up to 2 mg/kg and found robust CTA in MALDR mice, but no CTA in MAHDR mice (Wheeler et al., 2009). In the current study, MALDR-2 mice were conditioned with saline, 1 or 2 mg/kg MA (n = 6–7 per line, sex and dose) to replicate previous results, and MAHDR-2 mice were conditioned with saline, 2 and 4 mg/kg MA (n = 6–7 per line, sex and dose) to replicate the finding for the largest dose used previously, and extend the examination to a higher dose of MA.
Mice were isolate-housed and acclimated for 2 days to graduated drinking tubes filled with water (days −1 and 0). They were then water-restricted (water access for 2 h per day) to increase motivation to drink during a restricted drinking period, as is standard for CTA procedures. Acclimation to water restriction occurred on 4 consecutive days (days 1–4). Water-access and conditioning sessions were always conducted 4 h into the light cycle. On each conditioning day, animals had their body weight measured, were given access to 0.2 M NaCl for 1 h, and were then immediately injected with saline or MA. To ensure that animals received adequate fluids, they were given access to water for a 30-min period, beginning 4 h after the conditioning session. The first NaCl access session measured consumption of NaCl without subsequent injection of saline or MA (day 5). This served to reduce possible neophobia for the NaCl solution, prior to consumption on conditioning days. NaCl access occurred on days 7, 9, 11, 13, and 15, and mice were injected with saline or MA on each of these days except the last. We used a one-bottle procedure to assess CTA instead of a two-bottle choice procedure, because the one-bottle procedure is more easily used to monitor genetic differences in the rate of CTA acquisition in a multi-trial conditioning procedure. Moreover, the one-bottle procedure was previously found to be sensitive to genetic differences in the first replicate MADR lines (Wheeler et al., 2009), as well as many other inbred and selectively bred mouse lines (Cunningham et al., 2009). Conditioning sessions were alternated with 2 h water-access sessions, which occurred on days 6, 8, 10, 12 and 14. Body weight was measured immediately before every water-access session. None of the animals lost more than 10% of their body weight.
2.5. Pharmacokinetics
To identify possible line differences in MA levels and clearance after treatment with a fixed dose, male MAHDR-2 and MALDR-2 mice were injected with 2 mg/kg MA and MA levels in blood were determined immediately (time 0), 15, 30, 60, 120 and 240 min after treatment in independent groups of mice (n = 5 per line and time). This dose of MA was chosen because it differentiated the two lines in the current CPA and CTA studies and in previous work (Shabani et al., 2011), and is also a dose we have used to examine gene expression differences in these lines (Wheeler et al., 2009). Trunk blood was collected to provide 500 µl samples from which plasma was extracted. Each blood sample was immediately placed in a chilled microcentrifuge tube that contained 20 µl of 0.2 M ethylenediaminetetraacetic acid (EDTA) to avoid clotting. Tubes were centrifuged (Beckman Coulter INC, Allegra 6R centrifuge, Brea, CA, USA) at 4°C for 20 min at 3750 rpm and then plasma was collected and stored at −20°C until shipped on dry ice to University of Utah (Salt Lake City, UT), where samples were analyzed by liquid chromotography-tandem mass spectrometry (LC-MSMS) for MA levels (Slawson et al., 2002).
2.6. Statistical analysis
All data were analyzed by factorial analysis of variance (ANOVA), with repeated measures when appropriate (Statistica; StatSoft, Tulsa, OK, USA). Possible independent variables were sex, MA dose, line, time, and conditioning floor type. For all studies, since initial analyses did not identify any significant main or interaction effects of sex, subsequent analyses considered data collapsed on sex. For the CPA study, both time on the grid floor (sec/min) and percent time on the MA-paired floor were examined. The former provided a variable that can be statistically evaluated to determine whether significant conditioned aversion was expressed (Cunningham et al., 2003a). The expression of aversion would be detected as less time spent on the grid floor by G+ animals than by G− animals. That is, G+ mice would avoid the MA-paired grid floor (and spend more time on the saline-paired hole floor), whereas the G− animals would avoid the MA-paired hole floor (and spend more time on the saline-paired grid floor). This outcome was reflected statistically as a main effect of conditioning group (G+ vs. G−). Drug dose effects in this analysis were indicated by a significant interaction effect with conditioning group. However, an a priori decision was made to examine results for the 0.5 mg/kg MA dose data separately from other doses for comparison to other studies using this dose (Shabani et al., 2011; Wheeler et al., 2009).
As discussed in detail previously (Cunningham, 1993; Cunningham et al., 2003a), the between-groups approach provides an appropriate control for drug-induced learning and exposes performance in each of the counterbalanced subgroups. However, data were also expressed as percent time on the MA-paired floor, without regard to conditioning sub-group, as this variable may be easier to understand when considering pharmacological effects. Test day data from the place conditioning study were examined for both the first 15-min of the test, a time period comparable to the duration of conditioning trials, and for the entire 30-min period of the test. Because outcomes were virtually identical, data are only shown for the entire 30-min test, consistent with our previous examinations (Shabani et al., 2011; Wheeler et al., 2009). Three-way interactions were further examined by two-way ANOVA within each level of a relevant factor (e.g., for effects of dose and conditioning group within each selected line). Simple main effect analyses were performed to examine significant two-way interactions and post hoc mean comparisons were performed when appropriate, using the Newman-Keuls test. Type 1 error was reduced by restricting mean comparisons to those appropriate for evaluating specific hypotheses.
3. Results
3.1. Post-cue MA place conditioning in B6D2F2 mice
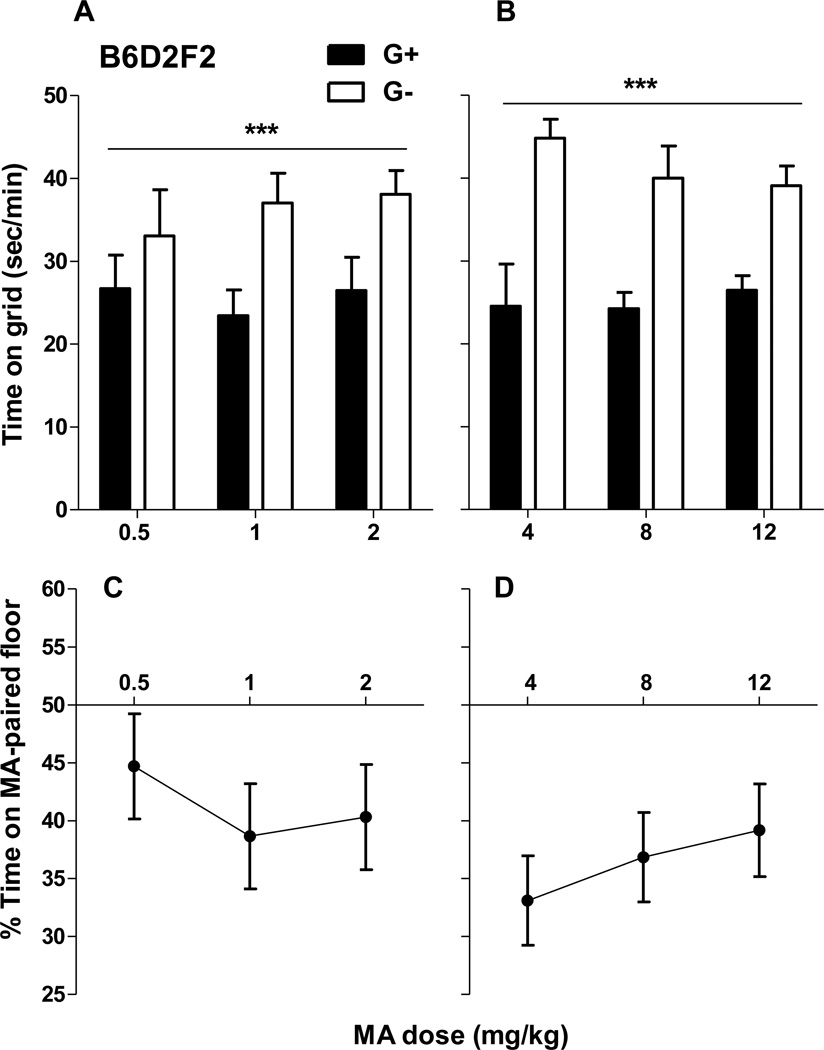
B6D2F2 mice developed robust CPA that did not vary with dose within each experiment, although CPA magnitude was generally greater in the experiment with higher doses. Figure 1 depicts time on grid floor for the two conditioning groups (G+ and G−) in ascending dose order, but data from the lower dose study (Figure 1A) and higher dose study (Figure 1B) were analyzed separately because they were collected in independent experiments. Data for the higher set of doses were collected first. Analysis of these data identified significant CPA, as indicated by significantly less time spent on the grid floor by the G+ group (which received MA paired with grid) than the G− group (which received saline paired with grid) (F1,41 = 33.6, p < 0.001). There were no significant effects of dose, sex or interactions of conditioning group with these variables. For the experiment including the lower range of doses (Figure 1A), G+ mice again spent less time on the grid floor, compared to G− mice, indicating significant aversion (F1,36 = 10.5, p < 0.005), and there were no significant effects of dose, sex or interactions of conditioning group with these variables. We had an a priori interest in the 0.5 mg/kg MA dose, because this dose has been consistently examined across previous place conditioning studies of F2 and the two replicate sets of MADR mice (Shabani et al., 2011; Wheeler et al., 2009). A t-test comparing the G+ and G− groups at this dose found that 0.5 mg/kg MA did not induce a significant CPA (p = 0.265). Data for percent time on the MA-paired floor corroborated the results for the between-groups analysis by showing that the mice spent less than half of their time in the MA-paired location, suggesting aversion for the drug-paired cues (Figure 1C and D). There were no significant effects of dose or sex, or interactions of these variables. Locomotor activity during the place test was not significantly affected by prior exposure to different doses of MA or by sex or conditioning group (data not shown). Overall, the data across these two studies indicate that the post-cue drug conditioning procedure produces robust place aversion across a wide range of MA doses.
Figure 1.
B6D2F2 mice display conditioned place aversion (CPA) to a wide range of methamphetamine (MA) doses. (A and B) Time in sec/min on the grid floor during a 30-min test for mice previously conditioned with MA on the grid (G+) or on the hole (G−) floor (n = 7–8 per group and dose). (C and D) Percent (%) time spent on the MA-paired floor by the same animals for the same 30-min test as for the data shown in A and B. Data are collapsed on conditioning group (G+ vs G−) and sex, due to the absence of significant effects of these factors; n = 14–16 per dose. Shown are means ± SEM. ***p<.001 for the main effect of conditioning group.
3.2. Post-cue MA place conditioning in MADR-2 mice
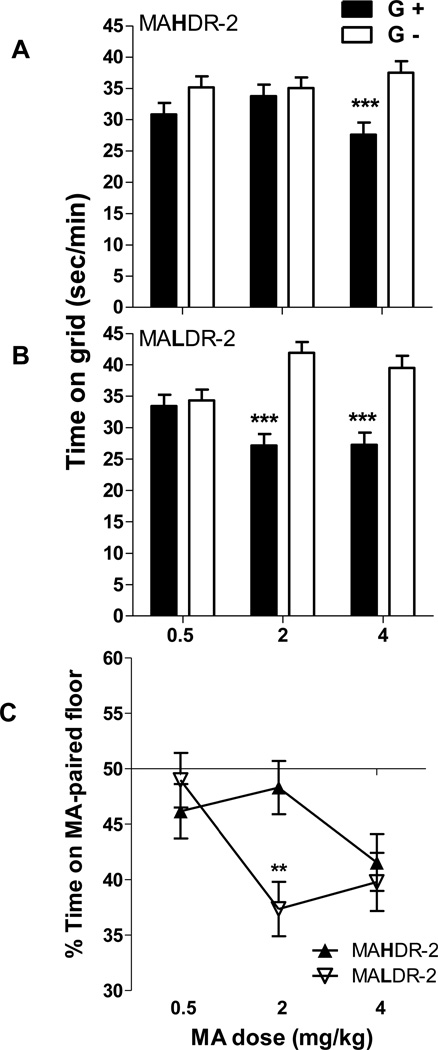
MALDR-2 mice were sensitive to aversive effects of MA at a lower dose than MAHDR-2 mice (Figure 2A and B). There was a significant interaction of conditioning group, dose, and line for time on the grid floor (F2,122 = 5.8, p < 0.005). Data were next examined for each line separately. There was a significant interaction of conditioning group and dose for both lines (F(2,62) = 3.7, p < 0.05 and F(2,60) = 6.7, p < 0.005 for MAHDR-2 and MALDR-2, respectively). Mean comparisons identified a significant difference between the G+ and G− group only at the 4 mg/kg MA dose in MAHDR-2 mice (p < 0.001) (Figure 2A), indicating significant CPA. MALDR-2 mice showed CPA at MA doses of 2 (p < 0.001) and 4 mg/kg (p < 0.001) (Figure 2B). Analysis of percent time on the MA-paired floor (Figure 2C) revealed a significant effect of dose (F2,128 = 4.0, p < 0.05), and an interaction of dose and line (F2,128 = 4.1, p < 0.05). A significant line difference was found only at the 2 mg/kg MA dose (p<.005), indicating that MALDR-2 mice spent significantly less time on the MA-paired floor than MAHDR-2 mice. We found no effects of conditioning group, line, or dose on locomotor activity during the test session (data not shown).
Figure 2.
MALDR-2 mice display MA-induced conditioned place aversion (CPA) at a lower MA dose than MAHDR-2 mice. (A and B) Time in sec/min on the grid floor during a 30-min test for mice previously conditioned with MA on the grid (G+) or on the hole (G−) floor (n = 10–13 per line, group and dose). (C) Percent (%) time on the MA-paired floor by the same animals for the same 30-min test as for the data shown in A and B. Data are collapsed on conditioning group (G+ vs G−) and sex, due to the absence of significant effects of these factors; n = 20–23 per line and dose. Shown are means ± SEM. ***p<.001 for the comparison of G+ and G− at the specific MA dose. **p<.005 for the line comparison at the specific MA dose. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
3.3. Conditioned taste aversion in MADR-2 mice
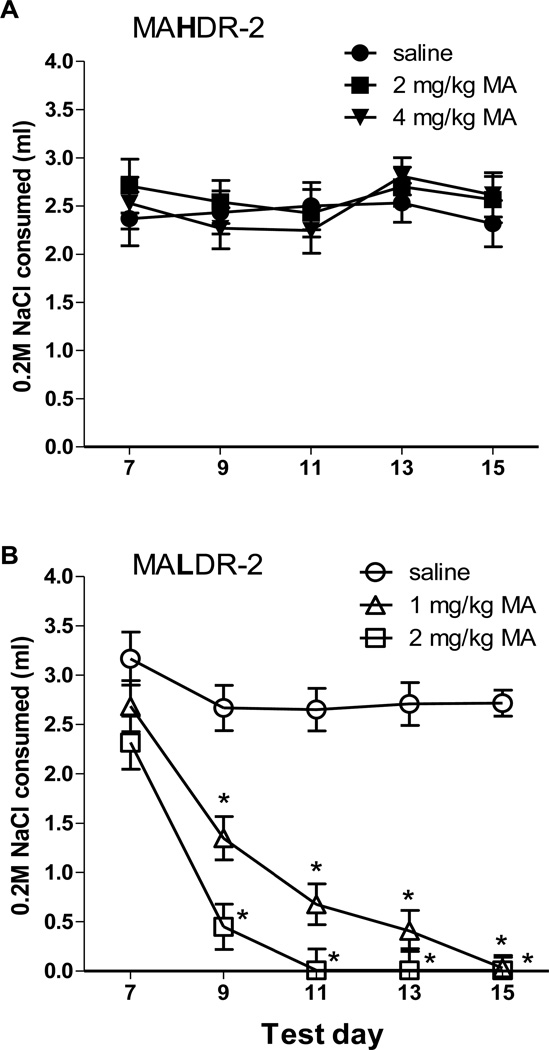
Results for the first replicate MADR lines, showing no MA-induced CTA in MAHDR mice, led us to examine a higher MA dose (4 mg/kg) in MAHDR-2 mice. However, due to dose-dependent sensitivity to MA-induced CTA in the first replicate MALDR line after treatment with 1 and 2 mg/kg MA (Wheeler et al., 2009), this lower dose range was used here to test MALDR-2 mice. The current data were first analyzed by including data for the doses tested in common in the two lines (0 and 2 mg/kg MA), and then by coding the doses as 0, low and high, to allow inclusion of all data. The outcome of both analyses led to the same conclusion that MAHDR-2 mice are insensitive to MA-induced CTA. Data are shown in Figure 3. Repeated measures ANOVA for just the 0 and 2 mg/kg MA dose group data revealed a significant three-way interaction of line, dose, and test day (F4,176 = 5.5, p < 0.001). Follow-up examination of data for MAHDR-2 mice alone identified no significant effects of dose or test day. However, examination of data from MALDR-2 mice revealed a significant dose × test day interaction (F8,136 = 10.5, p < 0.001). Simple main effects analyses revealed no significant change in NaCl intake across days for the saline group, but significant changes for both the 1 and 2 mg/kg MA treated mice (ps < 0.001). Newman-Keuls mean comparisons for consumption values on test day 7 (prior to conditioning) and all following sessions (after conditioning) revealed significant reductions in intake (ps < 0.001) for every day for both MA doses.
Figure 3.
MAHDR-2 mice are insensitive to MA-induced conditioned taste aversion (CTA) at doses that produce profound CTA in MALDR-2 mice. MALDR-2 mice (n = 12 per sex and dose) received injections of saline, 1 or 2 mg/kg MA, whereas MAHDR-2 mice (n = 12 per sex and dose) received injections of saline, 2 or 4 mg/kg MA, immediately following consumption of 0.2 M NaCl on days 7, 9, 11 and 13. (A) Consumption of 0.2 M NaCl in MAHDR-2 mice. (B) Consumption of 0.2 M NaCl in MALDR-2 mice. Shown are means ± SEM for data collapsed on sex because there was no significant effect of this factor. *p<.01 for indicated day vs day 7. MAHDR-2, MA high drinking replicate 2; MALDR-2, MA low drinking replicate 2.
3.4. MA clearance
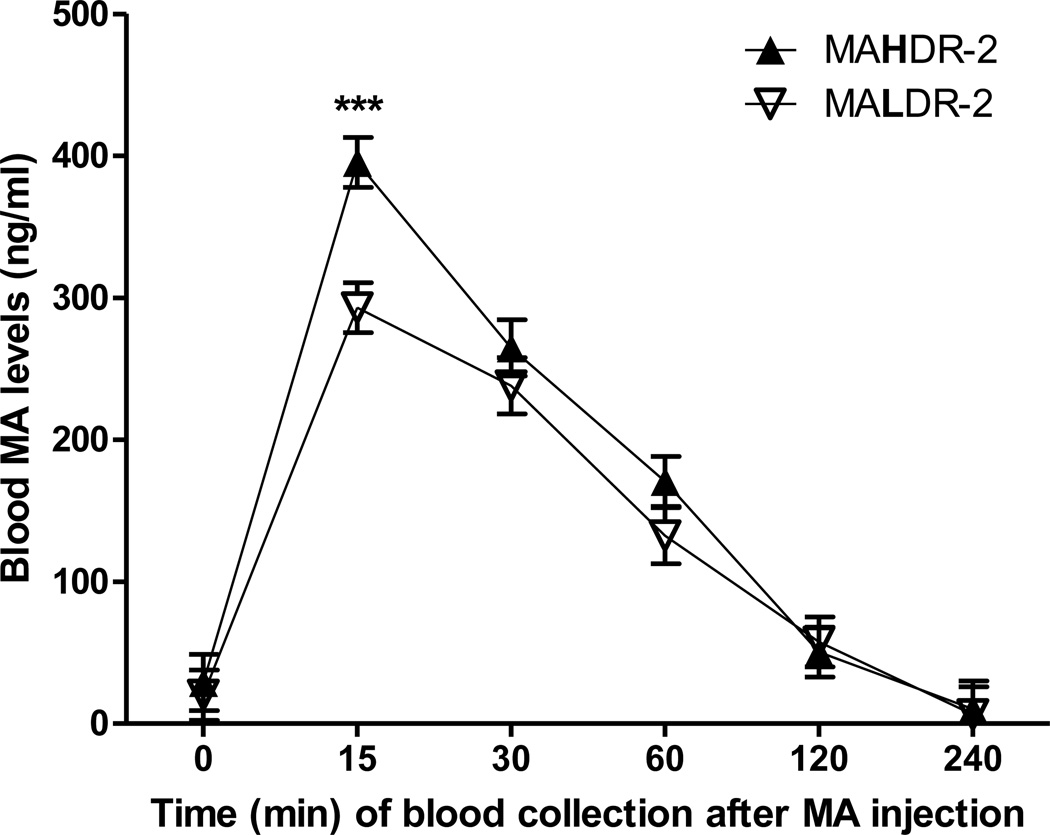
As shown in Figure 4, the time required for MA to clear from the blood was similar for the two lines, but peak MA levels were higher in MAHDR-2 than in MALDR-2 mice. Two-way ANOVA for MA levels revealed a significant interaction of line and time (F5,42 = 2.4, p < 0.05). Simple effect analyses identified a significant line difference at only the 15-min time point (p < 0.001), which was when peak levels of MA occurred.
Figure 4.
Plasma MA levels in male MAHDR-2 and MALDR-2 mice at several time points following ip administration of 2 mg/kg MA. N=4–5 mice per line and time point. *** p<.001 for the line comparison at the 15-min time point.
4. Discussion
These studies are the first to show that post-cue MA injection in a place conditioning procedure can produce robust conditioned place aversion in mice, and that higher sensitivity to this effect of MA is related to lower genetic risk for MA intake. Further, the results shown here indicate that mice that have high genetic risk for MA consumption are insensitive to some aversive effects of even high doses of MA. The mice tested here were not exposed to MA prior to the CPA or CTA tests. Rather, they were the offspring of parents that had been tested for level of MA consumption prior to breeding. Thus, the difference between MALDR-2 and MAHDR-2 mice in MA-induced CPA cannot be attributed to a difference in their own MA pre-exposure. MALDR-2 mice displayed significant MA-induced CPA after post-cue conditioning with both 2 and 4 mg/kg MA, whereas MAHDR-2 mice displayed aversion only after post-cue conditioning with 4 mg/kg MA, supporting the hypothesis that reduced sensitivity to the aversive effects of MA is associated with higher genetic susceptibility to MA intake. The responses of MALDR-2 mice for this trait were more similar to those of the founding F2 population than were those of MAHDR-2 mice. The hypothesis that reduced sensitivity to aversive effects of MA increases susceptibility to MA intake was also supported by the CTA data. MALDR-2 mice showed significant and strong MA-induced CTA after conditioning with 1 and 2 mg/kg MA, whereas MAHDR-2 mice failed to show CTA even after conditioning with a 4 mg/kg MA dose. Finally, MAHDR-2 mice had higher peak MA levels after administration of 2 mg/kg MA, compared to MALDR-2 mice, but this line difference was limited to a single time point. Higher levels of MA in the blood seem unlikely to explain the reduced aversion to MA of MAHDR-2 mice.
Although other data have suggested that sensitivity to both rewarding and aversive drug effects play important roles in drug intake, our investigations in the MADR selected lines specifically explored the genetic relationships between these factors. When a pair of selected lines differs for a trait other than the selection trait, the new and selected traits are described as genetically correlated. This correlation indicates that some of the same genes influence both traits, i.e., that there is genetic codetermination. This conclusion is strengthened when similar findings are obtained in replicated sets of selected lines (Crabbe et al., 1990). A major strength of the selected line approach is that genetic correlation can be examined without measuring both traits in the same animals, thus avoiding potential confounds due to test-order effects. Genetic relationships between MA intake and sensitivity to aversive and rewarding effects of MA are supported by our previous (Shabani et al., 2011; Wheeler et al., 2009) and current work using CPP, CPA, and CTA procedures. Interestingly, our preliminary data from ongoing experiments examining anxiogenic effects of acute MA using the elevated zero maze indicate greater sensitivity in MALDR-2 than MAHDR-2 mice. Such heightened sensitivity to anxiogenic effects of MA may play a role in the increased aversive responses and decreased MA intake of MALDR mice. Our results for MA are similar to some previous results for ethanol. D2 mice show stronger ethanol-induced CTA than B6 mice (Broadbent et al., 2002; Risinger and Cunningham, 1995), and D2 mice consume little ethanol, while B6 consume relatively more of their fluid from an ethanol-containing bottle, in home cage two-bottle choice studies (Lessov et al., 2001; Meliska et al., 1995). Unlike our study, however, D2 mice show stronger ethanol-induced CPP than B6 mice (Cunningham et al., 1992). A strong negative genetic correlation between severity of ethanol-induced CTA and home-cage ethanol preference was found in a panel of 15 inbred mouse strains (Broadbent et al., 2002), and similar results have been found for mouse and rat lines bred for high and low ethanol preference or consumption (Chester et al., 2003; Cunningham et al., 2009; Froehlich et al., 1988).
The sensitivity of conditioning procedures for detecting rewarding and aversive drug effects can be affected by temporal factors (Cunningham et al., 2006; Shabani et al., 2011; Sherman et al., 1980). Our previous results confirm those of others, showing that when MA is given just before cue exposure, a CPP is more likely to be detected in a drug-free test. Our results are also consistent with results for amphetamine in rats and for other drugs, showing that when MA is given shortly after cue exposure, a CPA is more likely to be detected. Similar to the CTA procedure, it has been suggested that in the post-cue drug conditioning procedure, animals respond only to the contingency that occurs first, in this case, the rapid initial aversive drug effects occurring immediately after tactile cue exposure (perhaps the transition from a sober to a drugged state), whereas, in a standard conditioning procedure, the longer duration of drug exposure provides greater opportunity for longer lasting positive effects of the drug to be experienced and overlap with the tactile cues. This line of thought has been extensively examined in ethanol research (Cunningham et al., 2003b), but little examined for psychostimulants, and is consistent with conditioning theories which conceptualize unconditioned stimuli as events that can activate more than one representational component (Wagner and Brandon, 1989).
MAHDR-2 mice expressed an aversive response after conditioning with 4 mg/kg MA in the CPA, but not CTA, procedure. It is possible that conditioning procedures for different modalities activate different brain pathways and that selective breeding had different effects on each of these pathways in the low and high MA drinking lines. For example, others have shown disruption of morphine-induced CTA, but sparing of place conditioning, by lesions of the parabrachial nucleus (Bechara et al., 1993; Reilly et al., 1993). It has been suggested that CTA learning has more evolutionary importance and involves caudal regions of the brain, whereas place conditioning involves phylogenetically younger regions (Mosher et al., 2006). However, data support at least some overlap in brain regions mediating taste and place conditioning. For example, the nucleus accumbens (NAc) appears to have a role in both (Aujla and Beninger, 2003; Roitman et al., 2010). In addition, the heightened sensitivity of MAHDR mice to MA reward might contribute to the difference in aversion expressed by MAHDR-2 mice in the two procedures. This factor would not affect MALDR-2 mice because of their insensitivity to MA reward (Shabani et al., 2011; Wheeler et al., 2009). Greater sensitivity to MA reward in MAHDR-2 mice would be expected to offset CTA more effectively than CPA if one assumed that flavor cues are more readily associated with delayed rewarding effects of MA than are contextual cues.
Reduced consumption of a novel tastant induced by conditioned drug effects using the current procedures has sometimes been described as taste avoidance, rather than aversion (Parker et al., 2008; Dwyer et al., 2008). This distinction has been made to distinguish between reductions in flavor intake that reflect a negative shift in taste palatability (“taste aversion”) and reductions whose basis is unknown or is not based on a learned change in palatability (“taste avoidance”). Measurement of taste reactivity responses (Grill and Norgren, 1978) have shown, for example, that rats will avoid an amphetamine-paired tastant, but do not necessarily show aversive taste reactivity responses, which are readily induced by emetic compounds and are thought to reflect conditioned nausea (Dwyer et al., 2008; Zalaquett and Parker, 1989). Whether disgust reactions (e.g., oral gaping) would be seen in either of the MADR lines using a taste reactivity test after conditioning with MA, is not known. However, whether reduced NaCl consumption in these lines was due to a shift in taste palatability or other factors (e.g., drug novelty), there is a clear difference between the lines in the effects of MA.
5. Conclusions
Results from our selective breeding projects suggest that genetic factors have a fundamental role in vulnerability to MA abuse. Table 1 summarizes the existing data for the MADR lines that support the notion that animals with high genetic sensitivity to rewarding, and lower sensitivity to aversive, effects of a drug are more likely to self-administer the drug. The converse also appears to be true. These data support a model wherein the balance between genetically determined sensitivity to rewarding and aversive drug effects determines the overall hedonic effect (whether largely positive or largely negative) and the potential for MA intake and abuse. One set of potentially influential factors not examined here are epigenetic factors. Given recent demonstrations that drugs of abuse can have epigenetic effects that can occur in germ cells and have the potential for being passed on to subsequent generations (Robison and Nestler, 2011), such factors cannot be ruled out as influencing differences found in offspring of drug-exposed selected line parents. This would be an interesting question for future investigation. The selection for high and low MA intake resulted in extreme phenotypes for sensitivity to reward and aversion. In both replicates of the MADR selected lines, high drinking line mice consumed three times more MA than the founding population, whereas low drinking line mice consumed virtually no MA (Shabani et al., 2011; Wheeler et al., 2009). Furthermore, data being prepared for publication, using operant self-administration procedures (both for intracerebroventricular self-infusion and oral self-administration) indicate that MALDR-2 mice not only self-administer very little MA solution but also show reduced MA seeking (Shabani et al., unpublished).
Table 1.
Compiled results for B6D2F2 and MADR mice in tests of MA conditioned reward and aversion.
| Sensitivity to rewarding and aversive effects of MA at the indicated dose |
||||
|---|---|---|---|---|
| Mice | Procedure | 0.5 mg/kg | 2 mg/kg | 4 mg/kg |
| B6D2F2 | drug-free CPP | rewarding | neutral | neutral |
| MALDR | drug-free CPP + | neutral | not tested | not tested |
| MALDR-2 | drug-free CPP * | neutral | neutral | neutral |
| MAHDR | drug-free CPP + | rewarding | not tested | not tested |
| MAHDR-2 | drug-free CPP* | rewarding | rewarding | rewarding |
| MALDR-2 | drug-present CPP* | aversive | aversive | aversive |
| MAHDR-2 | drug-present CPP* | rewarding | neutral | neutral |
| B6D2F2 | CPA | neutral | aversive | aversive |
| MALDR-2 | CPA | neutral | aversive | aversive |
| MAHDR-2 | CPA | neutral | neutral | aversive |
| MALDR | CTA+ | not tested | aversive | not tested |
| MALDR-2 | CTA | not tested | aversive | not tested |
| MAHDR | CTA+ | not tested | neutral | not tested |
| MAHDR-2 | CTA | not tested | neutral | neutral |
see Shabani et al., 2011.
The differential conditioned responses to MA between the high and low line mice could be associated with a difference in response or neuroadaptation of striatal neurons. In fact, multiple genes in the NAc are differentially expressed in drug naïve and MA-exposed MADR line mice, which could contribute to differential neuroadaptation. Drug naïve high and low line mice, for example, show differential expression of the gene that codes for the noradrenergic transporter, which is thought to contribute to the negative effects of psychostimulants (Jones et al., 2009; Schank et al., 2008). However, how this gene, or others that are differentially expressed in the MADR lines, contributes to sensitivity to anxiogenic and aversive effects of MA remains to be determined. Expression of the gene that codes for the vesicular monoamine transporter type 2 (VMAT-2) was down-regulated in MALDR, but not MAHDR, mice by administration of 2 mg/kg MA (Wheeler et al., 2009). Heterozygous VMAT-2 knockout mice, which express half the amount of the VMAT-2 protein, compared to matched wild type mice, show lower sensitivity to the rewarding effects of amphetamine in a CPP procedure (Takahashi et al., 1997). Furthermore, heterozygous VMAT-2 knockout mice were shown to be more sensitive to the neurotoxic effects of MA than wild type mice (Fumagalli et al., 1999). Therefore, down regulation of VMAT-2 expression could contribute to lower DA levels in the NAc and hence lower sensitivity to rewarding effects of MA in the MALDR-2 mice. Future studies, including utilization of in vivo microdialysis to examine level of DA response to MA in the two selected lines have the potential to confirm this hypothesis.
Highlights.
Genetics have a fundamental role in vulnerability to methamphetamine addiction.
High risk for methamphetamine (MA) intake reduces MA aversion.
Post-cue conditioning with MA produces place and taste aversion.
Sensitivity to aversive effects of methamphetamine (MA) protects from MA intake.
Acknowledgments
We thank David E. Moody and Jerdravee Morrison for conducting plasma MA level assessments. This work was supported by a grant from the Department of Veterans Affairs, NIDA T32 DA07262, N01 DA97767, and NIDA Center grant P50 DA018165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
All authors report no conflicts of interest or biomedical financial interests
Contributor Information
Shkelzen Shabani, Email: shabanis@scripps.edu.
Carrie S. McKinnon, Email: mckinnon@ohsu.edu.
Christopher L. Cunningham, Email: cunningh@ohsu.edu.
References
- Aujla H, Beninger RJ. Intra-accumbens protein kinase C inhibitor NPC 15437 blocks amphetamine-produced conditioned place preference in rats. Behav. Brain Res. 2003;147:41–48. doi: 10.1016/s0166-4328(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin GM, Pridgar A, van der Kooy D. The parabrachial nucleus: a brain stem substrate critical for mediating the aversive motivational effects of morphine. Behav. Neurosci. 1993;107:147–160. doi: 10.1037//0735-7044.107.1.147. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav. Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol. Clin. Exp. Res. 2003;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol. Clin. Exp. Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: van Haaran F, editor. Methods in behavioral pharmacology. Amsterdam: Elsevier; 1993. pp. 349–381. [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003a;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 387–421. [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Olmstead MC. Animal models of drug addiction. Totowa, NJ: Humana Press; 2011. Place conditioning; pp. 167–189. [Google Scholar]
- Cunningham CL, Henderson CM. Ethanol-induced conditioned place aversion in mice. Behav. Pharmacol. 2000;11:591–602. doi: 10.1097/00008877-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann. N. Y. Acad. Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Okorn DM, Howard CE. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Anim. Learn. Behav. 1997;25:31–42. [Google Scholar]
- Cunningham CL, Smith R, McMullin C. Competition between ethanol-induced reward and aversion in place conditioning. Learn. Behav. 2003b;31:273–280. doi: 10.3758/bf03195988. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology. 2002;160:414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Davis CM, Riley AL. Conditioned taste aversion learning: implications for animal models of drug abuse. Ann. N. Y. Acad. Sci. 2010;1187:247–275. doi: 10.1111/j.1749-6632.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Boakes RA, Hayward AJ. Reduced palatability in lithium- and activity-based, but not in amphetamine-based, taste aversion learning. Behav. Neurosci. 2008;122:1051–1060. doi: 10.1037/a0012703. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol. Biochem. Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology. 1987;92:376–381. doi: 10.1007/BF00210847. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with amphetamine. Pharmacol. Biochem. Behav. 1990;35:89–92. doi: 10.1016/0091-3057(90)90209-z. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J. Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Rice K, Riley AL. Differential involvement of the norepinephrine, serotonin and dopamine reuptake transporter proteins in cocaine-induced taste aversion. Pharmacol. Biochem. Behav. 2009;93:75–81. doi: 10.1016/j.pbb.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J. Pharmacol. Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol. Biochem. Behav. 2001;68:603–610. doi: 10.1016/s0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology. 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol. Biochem. Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Mosher TM, Smith JG, Greenshaw AJ. Aversive stimulus properties of the 5-HT2C receptor agonist WAY 161503 in rats. Neuropharmacology. 2006;51:641–650. doi: 10.1016/j.neuropharm.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can. J. Exp. Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav. Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn. Mem. 2010;17:539–546. doi: 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–948. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol. Psychiatry. 2008;63:1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon C, Cheryl R, Cunningham CL, Phillips T. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J, Butler R, Wheeler A. Initiation into methamphetamine use: qualitative findings from an exploration of first time use among a group of New Zealand users. J. Psychoactive Drugs. 2009;41:11–17. doi: 10.1080/02791072.2009.10400670. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Roberts T, Roskam SE, Holman EW. Temporal properties of the rewarding and aversive effects of amphetamine in rats. Pharmacol. Biochem. Behav. 1980;13:597–599. doi: 10.1016/0091-3057(80)90288-9. [DOI] [PubMed] [Google Scholar]
- Slawson MH, Taccogno JL, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Anal. Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Klein SB, Mowrer RR. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) Hillsdale, NJ: Erlbaum; 1989. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory; p. 149. [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalaquett CP, Parker LA. Further evidence that CTAs produced by lithium and amphetamine are qualitatively different. Learn. Motiv. 1989;20:413–427. [Google Scholar]