Abstract
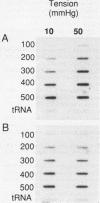
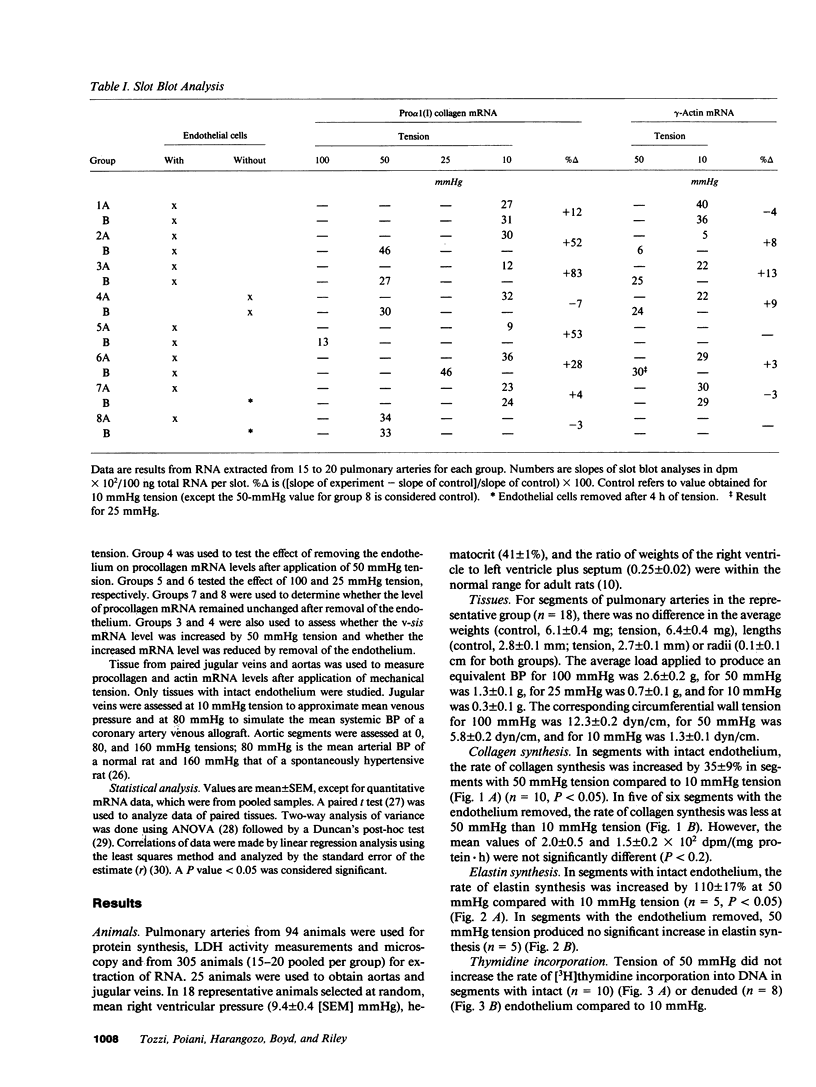
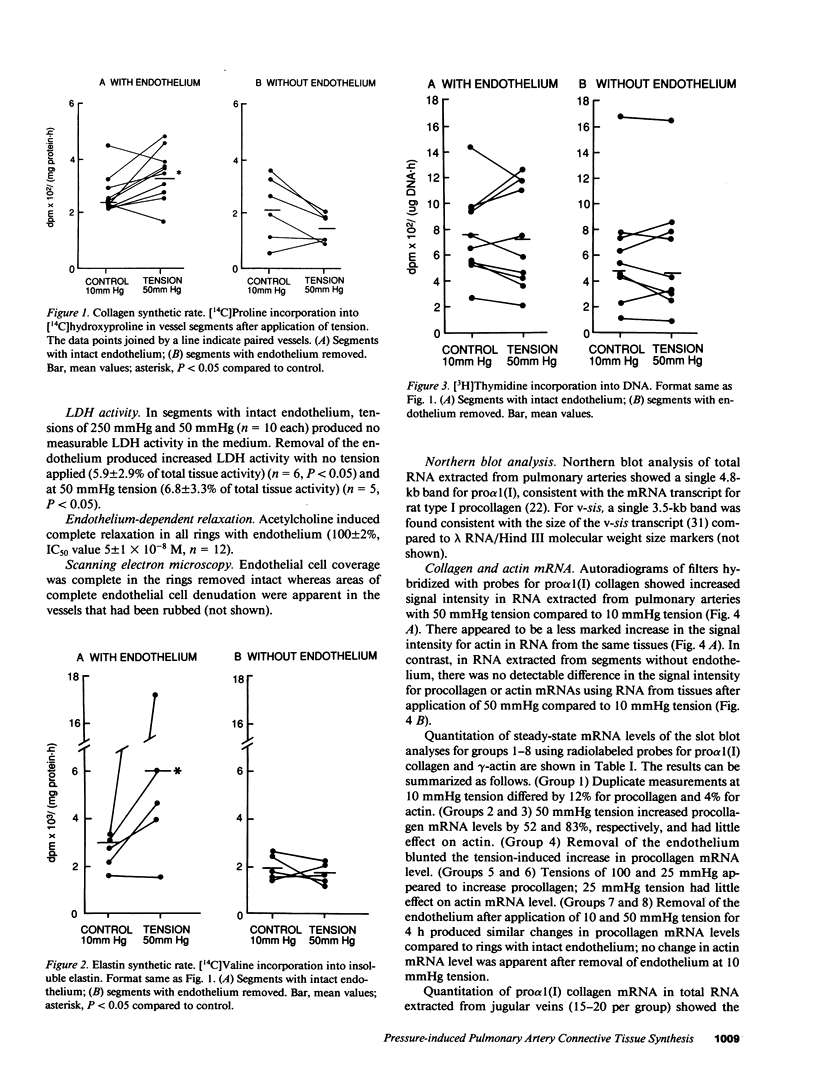
Physiologic stimuli of connective tissue accumulation in pulmonary vascular remodeling are poorly defined. We postulated that increased pressure within central pulmonary arteries is a stimulus for connective tissue synthesis and the response is dependent on an intact endothelium. Mechanical tension equivalent to 50 mmHg pressure was applied for 4 h to isolated rat main pulmonary arteries (endothelium intact or removed), and incorporation of [14C]proline into collagen, [14C]valine into elastin, [3H]thymidine into DNA and pro alpha 1 (I) collagen mRNA levels were measured. In intact vessels, tension induced synthesis of collagen (3.1 +/- 0.4 vs. 2.3 +/- 0.5 [SEM] dpm X 10(2) [14C]-hydroxyproline/[mg protein.h]) (n = 10) and elastin (6.1 +/- 2.4 vs. 2.9 +/- 0.4 dpm X 10(3) [14C]valine/[mg protein.h]) (n = 5) (both P less than 0.05). Steady state mRNA levels of pro alpha 1 (I) collagen were also increased by tension (46 vs. 30 X 10(2) dpm hybridized/100 ng total RNA). However, the stimulus did not increase [3H]thymidine incorporation into DNA. In denuded vessels, tension had no effect on connective tissue synthesis or mRNA level of pro alpha 1 (I) collagen. Messenger RNA levels for v-sis were induced by tension in intact but not denuded vessels. Our findings establish that induction of vascular connective tissue synthesis by mechanical tension is dependent on an intact endothelium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON A. C. On the physical equilibrium of small blood vessels. Am J Physiol. 1951 Feb;164(2):319–329. doi: 10.1152/ajplegacy.1951.164.2.319. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Cox R. H. Comparison of mechanical and chemical properties of extra- and intralobar canine pulmonary arteries. Am J Physiol. 1982 Feb;242(2):H245–H253. doi: 10.1152/ajpheart.1982.242.2.H245. [DOI] [PubMed] [Google Scholar]
- DUNCAN L. E., Jr, BUCK K., LYNCH A. THE EFFECT OF PRESSURE AND STRETCHING ON THE PASSAGE OF LABELED ALBUMIN INTO CANINE AORTIC WALL. J Atheroscler Res. 1965 Jan-Feb;5(1):69–79. doi: 10.1016/s0368-1319(65)80010-2. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterly J. A., Glagov S., Ferguson D. J. Morphogenesis of intimal obliterative hyperplasia of small arteries in experimental pulmonary hypertension. An ultrastructural study of the role of smooth-muscle cells. Am J Pathol. 1968 Feb;52(2):325–347. [PMC free article] [PubMed] [Google Scholar]
- Fischer G. M., Llaurado J. G. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966 Aug;19(2):394–399. doi: 10.1161/01.res.19.2.394. [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Swain M. L., Cherian K. Pulsatile distention and vascular collagen synthesis in the rabbit. Blood Vessels. 1980;17(4):216–220. doi: 10.1159/000158251. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Jennische E., Skottner A. IGF-I expression in blood vessels varies with vascular load. Acta Physiol Scand. 1987 Feb;129(2):165–169. doi: 10.1111/j.1748-1716.1987.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hume W. R. Proline and thymidine uptake in rabbit ear artery segments in vitro increased by chronic tangential load. Hypertension. 1980 Nov-Dec;2(6):738–743. doi: 10.1161/01.hyp.2.6.738. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K., Cardinale G. J., Spector S., Udenfriend S. Hypertension: increase of collagen biosynthesis in arteries but not in veins. Science. 1977 Oct 28;198(4315):403–405. doi: 10.1126/science.198877. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Guo C., Ratner L., Wong-Staal F. Human-proto-oncogene nucleotide sequences corresponding to the transforming region of simian sarcoma virus. Science. 1984 Feb 3;223(4635):487–491. doi: 10.1126/science.6318322. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Keeley F. W., Johnson D. J. The effect of developing hypertension on the synthesis and accumulation of elastin in the aorta of the rat. Biochem Cell Biol. 1986 Jan;64(1):38–43. doi: 10.1139/o86-006. [DOI] [PubMed] [Google Scholar]
- Kerr J. S., Riley D. J., Frank M. M., Trelstad R. L., Frankel H. M. Reduction of chronic hypoxic pulmonary hypertension in the rat by beta-aminopropionitrile. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1760–1766. doi: 10.1152/jappl.1984.57.6.1760. [DOI] [PubMed] [Google Scholar]
- Langille B. L., O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986 Jan 24;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. Y., Glagov S., Mathews M. B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976 Feb 6;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L. A., Wrenn D. S., Parks W. C., Griffin G. L., Senior R. M., Crouch E. C., Stenmark K. R., Voelkel N. F. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987 Jul 24;237(4813):423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963 Mar;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Pierce R. A., Glaug M. R., Greco R. S., Mackenzie J. W., Boyd C. D., Deak S. B. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Konstam M. A., Gamble W. J., Papanicolaou N., Aronovitz M. J., Treves S., Reid L. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ Res. 1983 Apr;52(4):432–441. doi: 10.1161/01.res.52.4.432. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S. Regulation of human fibroblast growth rate by both noncycling cell fraction transition probability is shown by growth in 5-bromodeoxyuridine followed by Hoechst 33258 flow cytometry. Proc Natl Acad Sci U S A. 1983 May;80(10):2951–2955. doi: 10.1073/pnas.80.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard S. Negative feedback mechanisms in the architecture and function of the connective and cardiovascular tissues. Perspect Biol Med. 1970 Summer;13(4):507–527. doi: 10.1353/pbm.1970.0054. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]